Abstract
Purpose
Upper limb dysfunction and sleep disturbance are common and serious health problems in women with breast cancer. Yoga is a mind-body intervention which is shown to improve physical and psychological health. The aim of this study is to evaluate the effectiveness of a tailor-made yoga program on upper limb function and sleep quality in women with breast cancer.
Methods
A pilot randomized controlled trial (RCT) study design was used. Participants were randomly allocated to either the yoga intervention group (YG; eight weekly 60-min group-based yoga sessions) or the wait-list control group (CG). The primary outcome measures were upper limb function and sleep quality, which were assessed by the self-reported questionnaires – the shortened version of the Disabilities of the Arm, Shoulder and Hand (QuickDASH) and the Pittsburgh Sleep Quality Index (PSQI), respectively. The secondary outcome measures were upper limb muscle strength and mobility, heart rate variability (HRV), anxiety and depression, fatigue, and health-related quality of life. All participants underwent assessment at four time-points (baseline, mid-intervention, post-intervention, and 1-month follow-up). The effectiveness of the intervention was tested by two-way mixed-design repeated-measures analysis of covariance.
Results
For the primary outcomes, there was no significant between-group difference in the upper limb function. The YG demonstrated significantly shorter sleep latency and higher HRV, and less sleep disturbance than the CG at post-intervention, and 1-month follow-up, respectively. For the secondary outcomes, the YG demonstrated significantly improved shoulder muscle strength and arm symptoms compared to the CG from mid-intervention until the 1-month follow-up.
Conclusion
This pilot trial revealed that the yoga program was feasible to be implemented for women with primary stage breast cancer. Although yoga was not found to be effective in improving the upper limb function, it improved sleep latency, HRV, shoulder muscle strength and arm symptoms of women with breast cancer.
Keywords: Breast cancer, Yoga therapy, Upper limb function, Sleep quality, Clinical trial
Trial registration number and date of registration
ClinicalTrials.gov Identifier: NCT05869721; May 22, 2023.
1. Introduction
Breast cancer is the most commonly diagnosed cancer worldwide [1]. Current treatments for breast cancer include surgery with radiotherapy and chemotherapy, and these can reduce the residual and recurrence of the disease and improve survival [2]. However, more than half of breast cancer survivors suffered from disease- or treatment-related adverse effects, such as upper limb dysfunction, sleep disturbance, persistent fatigue, and psychological distress [3,4].
Over 60 % of women with breast cancer have reported experiencing impairments in upper limb function immediately post-surgery and/or post-radiotherapy that have led to chronic symptoms or permanent disability and disorders, such as shoulder pain, decreased joint mobility and muscle strength, altered sensory perception, neuropathies, and lymphedema [5,6]. Sleep disturbance, such as difficulty falling or staying sleep, is another prevalent and persistent complication of cancer and cancer treatment [7]. A recent review found that the prevalence of sleep disturbance in women with breast cancer ranged from 14 % to 90 % (pooled estimated = 0.4, 95 % confidence interval [CI]: 0.29 to 0.52) [8], and its persistence rates were 51 % and 56 % in those with a mean survivorship of 8.9 and 5.6 years, respectively [9,10]. All of these complications can lead to suffering, economic burdens [11], and compromised quality of life in women with breast cancer [12]. Thus, it is crucial to reduce impairments in upper limb function and decrease sleep disturbance in women with breast cancer following cancer treatment.
Yoga is a form of mind–body training based on ancient Indian philosophy that emphasizes the integration of postures, breathing, and meditation [13]. In particular, yoga combines joint movements and breathing exercises that involve lung expansion and muscle stretching, which increase lymphatic circulation and improve upper limb function [14]. Saraswathi et al. [15] systematically reviewed seven studies and found that yoga was effective in improving arm symptoms, including shoulder range of motion (ROM) and hand-grip strength, in breast cancer survivors with lymphedema. Moreover, as yoga combines physical activity with mindful elements consisting of breathing and meditative maneuvers, it is a potential intervention for decreasing sleep disturbance in women with breast cancer [16]. For example, meta-analyses have revealed that yoga interventions compared to no active control have resulted in short-term decreases in sleep disturbance in women with breast cancer, with a standard mean difference (SMD) of −0.25 (95 % CI: −0.40 to −0.09, p = 0.0018; six randomized controlled trials [RCTs] comprising a total of 657 participants) [17] and an SMD of −0.34 (95 % CI: −0.55 to −0.12, p = 0.002; three RCTs comprising a total of 343 participants) [18].
Upper limb dysfunction and sleep disturbance are common and serious health problems in women with breast cancer. Deconditioning of upper limb function can lead to a decrease in physical activity and an increase in sleep disturbance. Therefore, improvement in sleep quality can help to increase physiological reserves and thereby facilitate upper limb function. Yoga is regarded as “meditation in motion” as it comprises both physical and mental training, which are believed to simultaneously improve upper limb function and sleep quality in women with breast cancer.
Nevertheless, despite the popularity of yoga, there is only limited evidence comprehensively examined the efficacy of yoga on the physiological and psychological health conditions of women with breast cancer. Thus, this study aimed to investigate the effects of a tailor-made 8-week yoga program on upper limb function, sleep quality, and quality of life in women with breast cancer. The research hypothesis was that the intervention group should have better upper limb function and sleep quality than the control group immediately after yoga intervention and at the 1-month follow-up.
2. Material and methods
2.1. Study design
This pilot RCT (Trial registration: ClinicalTrials.gov Identifier: NCT05869721) was conducted in the Wellness and Exercise Laboratory of The Hong Kong Polytechnic University from May to July 2023.
2.2. Sample size calculation
A previous RCT investigating the effects of mind–body training on reducing joint pain in comparison with a control treatment reported that such training exhibited an effect size of r = 0.53 in improving upper limb function (assessed using the Disabilities of the Arm, Shoulder and Hand [DASH] Questionnaire) and an effect size of r = 0.74 in improving sleep (assessed using the Pittsburgh Sleep Quality Index [PSQI]) in breast cancer survivors, respectively [19]. The sample size required to detect differences in our primary outcomes would be assumed a moderate effect size (Cohen's d = 0.5) in the present study. Using G*Power software (version 3.1.9.7) with F-test (repeated measures ANOVA), an estimated total sample size of 30 participants was needed. Thus, allowing for a 15 % attrition rate, the required total sample size was 34.
2.3. Participants
Women with breast cancer were recruited from local breast cancer self-help associations through poster advertisements. Potential participants were included if they (1) were aged 18 or older; (2) were female; (3) had a normal cognitive function; (4) had been diagnosed with stage I–III primary breast cancer; (5) had finished cancer treatment(s) (i.e., operations and adjuvant therapies) no less than 1 month before enrollment, may or may not have been currently receiving conventional medical care (e.g., hormonal therapy). Potential participants were not recruited for the study if they (1) had a metastatic stage of cancer; (2) had a major disease or disorder, such as a pulmonary, cardiovascular, neurological, musculoskeletal (except upper-extremity problems secondary to breast cancer), endocrine, or metabolic disease, or a psychological disorder; (3) were pregnant; (4) had prior experience of practicing yoga; or (5) were involved in a drug study or other clinical trial.
2.4. Procedure
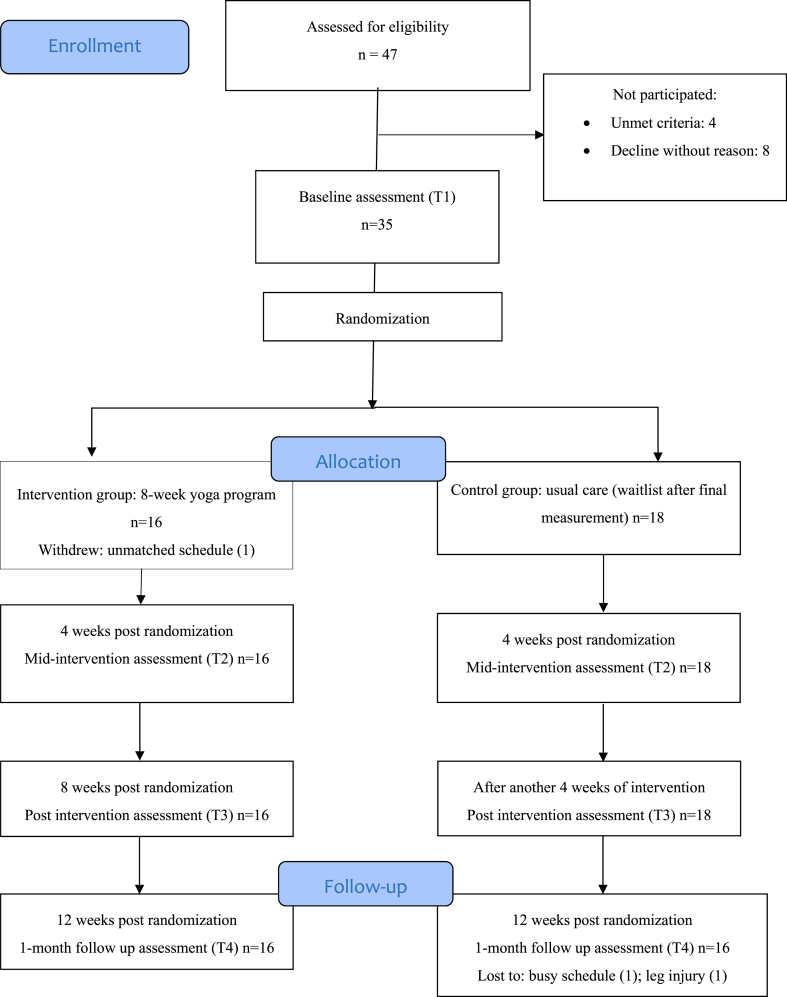
Potential participants were screened by research personnel for eligibility. The participants were allocated randomly using a computer software-generated randomization list to an intervention group or a wait-list control group in a 1:1 ratio by a research assistant who was not involved in the data collection or analysis. To prevent the occurrence of bias that might have affected measurements, the participants were requested to not begin any new exercise regime during the trial period and to not disclose their group allocation to the assessor. All of the assessments were performed by the same assessor, who was a registered nurse. The assessor was blinded to the group allocation and not involved in the delivery of the intervention. During the assessment, the participants completed the standardized self-reported questionnaires and physiological assessments. The assessments lasted for about 120 min. Rest was allowed between assessments. The participants in both groups underwent assessment at four time-points (baseline, mid-intervention, post-intervention, and 1-month follow-up) (Fig. 1). Besides, another research assistant would record participants’ attendance at yoga sessions and any adverse events that occurred during the intervention and assessments.
Fig. 1.
Flowchart of the pilot randomized controlled trial.
2.5. Yoga intervention group
The yoga intervention group (YG) received a 60-min face-to-face group yoga session once per week for 8 weeks. Each group comprised 6–8 participants, and the intervention therapist who delivered the intervention was a qualified yoga instructor with more than 10 years of yoga-coaching experience. Yoga sessions for different groups were conducted by the same yoga instructor and followed with the yoga intervention protocol (Supplementary material). The protocol was developed based on modified traditional Hatha yoga and consisted of yogic breathing (pranayama), postures (asana), and meditation (dhyana) with relaxation elements.
Some of the poses (e.g., “Triangle,” “Warriors,” “Locust,” and “Bridge”) require the participants in the YG to deeply stretch their shoulders, arms, and upper back, as the poses specifically engage the deltoids, triceps, trapezii, and rhomboids. In addition, these poses help to build core stability and strengthen the chest and back muscles. Other poses (e.g., “Cow Face” and “Thread the Needle”) require the participants in the YG to perform a gentle twist that helps to release tightness in the shoulders, chest, and upper back and stretch the rotator cuff, thereby increasing the flexibility of the shoulder complex. Moreover, poses involving twists and inversion (e.g., “Humble Warrior”) help to stimulate lymphatic circulation. Lymphatic drainage is crucial for preventing arm swelling and thus is particularly important for those who have undergone lymph node removal.
It is believed that yoga poses performed in synchrony with breathing exercises reinforce the benefits of limb stretching. In addition, the combination of relaxation elements with guided meditative practice in yoga helps practitioners to restore body homeostasis, including disrupted circadian rhythms, which is associated with sleep problems [20,21].
2.6. Control group
The participants in the control group (CG) received usual care (i.e., routine medical follow-up) and underwent assessment on the same timeline as the YG. The CG participants were requested to maintain their usual activities throughout the intervention and measurement periods and were informed that, after the final assessments had been performed, they would be offered the same yoga program as the YG.
2.7. Outcome measures
2.7.1. Primary outcomes
Upper limb function Upper limb function was determined by the Chinese (Hong Kong) shortened version of the DASH (QuickDASH) [22], which measures physical functioning and disability of the upper limbs. A higher score represents greater disability (11 items, score range: 0%–100 %). The Chinese version of QuickDASH was found strongly correlated with DASH (r = 0.82) and demonstrated good internal consistency (Cronbach's α = 0.818) and test–retest reliability (intraclass correlation coefficient [ICC] = 0.907) in people suffering upper limb disorders [23].
Sleep quality Sleep was evaluated by the Pittsburgh Sleep Quality Index in Chinese version (PSQI-C) [24], which assesses the seven components: sleep quality, disturbance, duration, latency, efficiency, daytime dysfunction, and use of sleep medication. A higher score represents poorer sleep quality (19 items, score range: 0–21). The Cantonese version of PSQI demonstrated acceptable internal consistency (Cronbach's α = 0.75) in Chinese adult [25].
2.7.2. Secondary outcomes
Shoulder muscle strength Shoulder muscle strength for flexion and abduction was assessed by a handheld dynamometer (HHD) (Lafayette Instrument Company, USA), which demonstrated intra-tester reliability with ICC3,1 of 0.72–0.99 in breast cancer survivors [26]. The participants elevated arm to 90° while the assessor supported the device with one hand, in a contrary direction to the arm elevated movement and stabilize the proximal joint with another hand. The participants were instructed to increase their effort to their maximum counterforce and then hold it for 5 s. The average of the three measurements was taken as the score for analysis. Each test was separated by at least 1-min rest. Body-weight-adjusted muscle strength was adopted in our analysis, i.e., muscle strength was normalized to body weight.
Shoulder joint flexibility The active ROM of shoulder flexion and abduction were measured using a goniometer according to a standard protocol [27]. The participant rotated shoulder joint externally to its maximum extent without compensatory trunk movement. The hand behind the back (HBB) motion was determined with the length between the cervical vertebra (C7) spinous process and the tip of the thumb while the participants placed their thumb along the spinal column [28]. A shorter distance reflects greater HBB ROM. The final score of each angle was counted by the average from three measurements.
Heart rate variability Heart rate variability (HRV) was used as a measure of cardiac autonomic modulation [29]. The participants were instructed to avoid alcohol and caffeinated beverages in the 3 h preceding HRV assessment. The participants sat for 10 min, and then their cardiac waveforms were continuously recorded over 5 min using a validated wearable device (Polar H10 heart rate sensor) [30] that was firmly strapped immediately below the chest muscle. The time-domain measures included the mean normal-to-normal (NN) interval (RRi), the standard deviation of NN intervals (SDNN), and the root mean square of the successive difference of NN intervals (RMSSD). The frequency-domain measures included the low-frequency (LF), the high-frequency (HF), and the LF/HF ratio [31]. Data analysis was performed using the Kubios software (Kubios HRV Standard ver. 3.5.0) [32].
Mood The Chinese (Cantonese) Hospital Anxiety and Depression Scale (HADS-C) was used to measure levels of anxiety and depression [33]. This instrument consists of anxiety and depression subscores, and a higher score represents high levels of anxiety and depression symptoms (subscore score ranges: 0–21). The Chinese version of HADS demonstrated good internal consistency with Cronbach's α of 0.87 for both subscales in cancer patients [34].
Fatigue The Chinese/Cantonese version of the Fatigue Assessment Scale (C-FAS) was a validated and reliable instrument to determine levels of fatigue [35]. It includes 10 items across two subscales (physical and mental fatigue). A higher score indicates higher level of fatigue (total score range: 10–50).
Health-related quality of life Health-related quality of life was assessed using the traditional Chinese version of the Functional Assessment of Cancer Therapy – Lymphedema questionnaire (FACT-B + 4) [36], which consists of 41 items across six subscales (physical, social, emotional, functional well-being, breast cancer, and arm symptoms). A higher score represents a better quality of life (total score range: 0–148). The FACT-B + 4 demonstrated good internal consistency (Cronbach's α = 0.81) in Chinese women with breast cancer [37].
2.8. Statistical analyses
A Kolmogorov–Smirnov test was performed to check the normality of the data. A last-observation-carried-forward intention-to-treat analysis was adopted to manage missing data and dropouts. The effectiveness of the intervention was tested by using a two-way mixed-design repeated-measures analysis of covariance to measure between-group (intervention versus control) changes in outcome measures across time. Sociodemographic and baseline variables were treated as covariates if there were any significant between-group differences. Partial eta-square (ηp2) of 0.01 (small), 0.06 (medium) and 0.14 (large) effect sizes were applied. Post-hoc test would be done if a variable exhibited significant time-by-group interaction effects. Paired t-tests (Bonferroni adjustment) and independent t-tests were performed for within-group and between-group comparisons, respectively. Alpha (α) value was at <0.05 as the significance level, and the adjusted α of <0.008 (0.05/6) was adopted to prevent occurrence of Type 1 error in view of multiple comparisons. All statistical analyses were conducted by SPSS 26.0 software (IBM, Armonk, New York).
3. Results
3.1. Participants’ characteristics
Thirty-five women with breast cancer were recruited as participants: 17 were assigned to the YG, and 18 were assigned to the CG. There were no significant between-group differences in terms of sociodemographic or clinical characteristics (Table 1).
Table 1.
Participants’ demographics and clinical characteristics.
| YG (n = 16), n (%) | CG (n = 18) n (%) | t or χ2 | p | |
|---|---|---|---|---|
| Age, mean (SD) | 48.63 (8.77) [range:37–63] | 45.78 (9.25) [range:36–63] | t = −0.918 | 0.366 |
| Education level | χ2 = 2.242 | 0.131 | ||
| Primary or below | 0 | 0 | ||
| Secondary | 4 (25) | 9 (50) | ||
| Tertiary | 12 (75) | 9 (50) | ||
| Living status | χ2 = 3.702 | 0.094 | ||
| alone | 3 (18.8) | 0 | ||
| family | 13 (81.3) | 18 (100) | ||
| Employment status | χ2 = 0.410 | 0.980 | ||
| Full-time | 10 (62.5) | 11 (61.1) | ||
| Part-time | 2 (12.5) | 2 (11.1) | ||
| Others (i.e., housewife) | 4 (25) | 5 (27.8) | ||
| Marital status | χ2 = 0.283 | 0.868 | ||
| Single | 4 (25) | 6 (33.3) | ||
| Married | 11 (68.8) | 11 (61.1) | ||
| Divorced | 1 (6.3) | 3 (5.6) | ||
| Widowed | 0 | 0 | ||
| Hand Dominance | χ2 = 1.889 | 0.487 | ||
| Right | 16 (100) | 16 (88.9) | ||
| Left | 0 | 2 (11.1) | ||
| Cancer stage | χ2 = 0.360 | 0.835 | ||
| I | 4 (25) | 5 (27.8) | ||
| II | 8 (50) | 10 (55.6) | ||
| III | 4 (25) | 3 (16.7) | ||
| Duration of cancer diagnosis (months), mean (SD) | 18.22 (9.07) [Range: 3.5–44] | 22.58 (9.51) [Range: 5–38] | t = 1.365 | 0.182 |
| Post-surgery duration (months), mean (SD) | 16.75 (9.44) [Range: 3–43] | 22.08 (9.55) [Range: 4.5–37.5] | t = 1.634 | 0.112 |
| Post-chemotherapy/radiotherapy duration (months), mean (SD) | 12.72 (9.49) [Range: 2.5–39] | 16.75 (10.75) [Range: 2.5–37] | t = 1.153 | 0.258 |
| Surgery-mastectomy | χ2 = 0.472 | 0.732 | ||
| Mastectomy | 9 (56.3) | 8 (44.4) | ||
| Partial mastectomy | 7 (43.8) | 10 (55.6) | ||
| Surgery-lymph node | χ2 = 4.386 | 0.112 | ||
| none | 1 (6.3) | 5 (27.8) | ||
| Sentinel node dissection | 7 (43.8) | 3 (16.7) | ||
| Lymph node dissection | 8 (50) | 10 (55.6) | ||
| Adjuvant treatments | ||||
| Chemotherapy | 11 (68.8) | 17 (81.3) | χ2 = 3.848 | 0.078 |
| Radiotherapy | 14 (87.5) | 13 (72.2) | χ2 = 1.209 | 0.405 |
| Hormonal therapy | 6 (37.5) | 6 (33.5) | χ2 = 0.064 | 1.000 |
| Affected side | χ2 = 2.902 | 0.234 | ||
| Right | 5 (43.8) | 9 (50) | ||
| Left | 7 (31.3) | 8 (44.4) | ||
| Both | 4 (25) | 1 (5.6) |
t, independent-sample t-test; χ2, chi-squared test; YG, yoga intervention group; CG, control group.
One participant in the YG withdrew from the study before the start of the intervention because of a schedule clash. Thus, there were 16 participants (mean age: 48.63 ± 8.77 years) in the YG, and they completed all of the assessments. There were 18 participants (mean age: 45.78 ± 9.25 years) in the CG, but only 16 completed all of the assessments; two participants did not complete the 1-month follow-up assessment due to a busy schedule (one participant) and a leg injury (one participant). The 16 participants in the CG who completed all of the assessment would receive the yoga program after they had completed the final assessment. No adverse events were reported during the yoga sessions, and the average attendance rate was 92 % for the intervention group.
3.2. Effects of yoga training
3.2.1. Primary outcomes
Upper limb function
There was a statistically significant group-by-time interaction effect (F (3, 96) = 9.246, p < 0.001 with a large effect size (ηp2) = 0.224) on QuickDASH scores (Table 2). However, post-hoc analysis revealed that there was no significant between-group difference at all time-points (mid-intervention: mean difference (MD) = −2.384, t = −0.438, 95 % CI: −13.464 to 8.696, p = 0.664; post-intervention: MD = −10.448, t = −1.810, 95 % CI: −22.275 to 1.380, p = 0.081; 1-month follow-up: MD = −5.193, t = −1.027, 95 % CI: −15.529 to 5.143, p = 0.313).
Table 2.
Between-group and within-group comparison on upper limb function and sleep.
| YG (n = 16) Mean (SD) |
CG (n = 18) Mean (SD) |
Group-by-time Interaction |
Group Effect |
Time Effect |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T1 | T2 | T3 | T4 | F-value | p | Effect size, ηp2 | F-value | p | Effect size, ηp2 | F-value | p | Effect size, ηp2 | |
| Upper limb function | |||||||||||||||||
| QuickDASH | 26.56 (16.47) | 18.32 (14.43) | 15.06 (12.08)a | 18.04 (11.45)a | 22.85 (19.53) | 20.71 (16.97) | 25.50 (20.87) | 23.23 (17.68) | 9.246 | 0.000* | 0.224 | 0.429 | 0.517 | 0.013 | 5.770 | 0.003* | 0.153 |
| Sub-scores of QuickDASH | |||||||||||||||||
| -QuickDASH (work) | 23.05 (14.74) | 17.58 (12.75) | 14.45 (13.25) | 14.84 (15.45) | 22.22 (18.72) | 17.71 (15.20) | 19.79 (16.64) | 20.49 (17.26) | 2.464 | 0.079 | 0.071 | 0.258 | 0.615 | 0.008 | 5.740 | 0.002* | 0.152 |
| -QuickDASH (sports) | 31.25 (17.53) | 28.52 (20.02) | 21.88 (20.16) | 22.66 (15.12) | 28.82 (20.91) | 23.94 (21.03) | 27.78 (19.67) | 18.75 (16.88) | 1.494 | 0.221 | 0.045 | 0.050 | 0.824 | 0.002 | 3.775 | 0.013* | 0.106 |
| Sleep | |||||||||||||||||
| PSQI (global) | 7.75 (2.96) | 6.50 (2.56) | 5.81 (1.91) | 5.06 (2.62)a | 6.89 (5.03) | 6.78 (4.05) | 6.89 (4.54) | 6.67 (3.45) | 3.975 | 0.010* | 0.110 | 0.211 | 0.649 | 0.007 | 5.111 | 0.003* | 0.138 |
| Sub-scores of PSQI | |||||||||||||||||
| -Duration | 0.75 (0.68) | 0.88 (0.50) | 0.94 (0.58) | 0.63 (0.72) | 0.83 (0.71) | 0.72 (0.58) | 0.83 (0.71) | 0.78 (0.65) | 1.131 | 0.340 | 0.034 | 0.001 | 0.978 | 0.000 | 1.192 | 0.317 | 0.036 |
| -Disturbance | 1.50 (0.52) | 1.19 (0.54) | 1.13 (0.50) | 1.13 (0.50)# | 1.28 (0.67) | 1.39 (0.50) | 1.39 (0.50) | 1.56 (0.51) | 4.116 | 0.017* | 0.114 | 1.443 | 0.238 | 0.043 | 0.721 | 0.504 | 0.022 |
| -Latency | 1.56 (0.81) | 1.25 (0.78) | 0.69 (0.60)a,b,# | 0.63 (0.89)a,b | 1.50 (1.04) | 1.28 (0.90) | 1.33 (1.09) | 1.28 (1.13) | 4.995 | 0.006* | 0.135 | 1.266 | 0.269 | 0.038 | 9.403 | 0.000* | 0.227 |
| -Day dysfunction | 1.00 (0.89) | 1.00 (0.73) | 1.19 (0.83) | 1.06 (0.85) | 0.94 (1.00) | 1.17 (0.92) | 1.22 (1.00) | 1.00 (0.84) | 0.497 | 0.632 | 0.015 | 0.006 | 0.941 | 0.000 | 1.706 | 0.185 | 0.051 |
| -Quality | 1.75 (0.68) | 1.44 (0.63) | 1.25 (0.68) | 1.19 (0.54) | 1.22 (1.00) | 1.22 (0.88) | 1.28 (0.83) | 1.17 (0.62) | 2.423 | 0.084 | 0.070 | 0.715 | 0.404 | 0.022 | 2.599 | 0.069 | 0.075 |
| -Efficiency | 0.94 (0.77) | 0.50 (0.52) | 0.56 (0.63) | 0.31 (0.60) | 0.72 (0.96) | 0.56 (0.71) | 0.56 (0.71) | 0.44 (0.51) | 0.737 | 0.491 | 0.023 | 0.002 | 0.963 | 0.000 | 4.694 | 0.011* | 0.128 |
| -Medication | 0.25 (0.58) | 0.25 (0.68) | 0.06 (0.25) | 0.13 (0.50) | 0.39 (0.98) | 0.44 (1.04) | 0.28 (0.83) | 0.44 (1.04) | 0.299 | 0.719 | 0.009 | 0.774 | 0.386 | 0.024 | 1.271 | 0.286 | 0.038 |
T1, baseline; T2, mid-intervention; T3, post-intervention, T4, 1-month follow-up; YG, yoga intervention group; CG, control group.
QuickDASH, shortened version of the Disabilities of the Arm, Shoulder and Hand; PSQI, Pittsburgh Sleep Quality Index.
*p < 0.05, group-by-time interaction, group and time effect; #p < 0.05, between-group comparison at each time-point.
ap < 0.008, within-group comparison relative to T1; bp < 0.008, within-group comparison relative to T2.
Sleep
There were statistically significant group-by-time interaction effects on PSQI global scores (F = 3.975, p = 0.010, ηp2 = 0.110), and on sleep disturbance (F = 4.116, p = 0.017, ηp2 = 0.114) and sleep latency (F = 4.995, p = 0.006, ηp2 = 0.135) sub-scores (Table 2). Despite post-hoc analysis revealed that there was no significant between-group difference in PSQI scores at all time-points (mid-intervention: MD = −0.278, t = −0.236, 95 % CI: −2.680 to 2.125, p = 0.815, post-intervention: MD = −1.076, t = −0.920, 95 % CI: −3.495to 1.343, p = 0.367; 1-month follow-up: MD = −1.604, t = −1.512, 95 % CI: −3.765 to 0.556, p = 0.140), the YG had significantly lower sleep latency sub-scores (MD = −0.646, t = −2.177, 95 % CI: −1.254 to −0.037, p = 0.038) at post-intervention and significantly less sleep disturbance (MD = −0.431, t = −2.476, 95 % CI: −0.785 to −0.076, p = 0.019) at 1-month follow-up than the CG.
3.2.2. Secondary outcomes
Shoulder muscle strength and mobility
Regarding shoulder muscle strength, there were significant group-by-time interaction effects on bilateral shoulder flexion (both p < 0.001) and affected-side shoulder abduction (p = 0.031), but not on unaffected-side shoulder abduction (p = 0.068) (Table 3). For the affected-side shoulder, when compared with the CG, the YG demonstrated significantly greater body-weight-normalized muscle strength in flexion angle across mid-intervention to 1-month follow-up (mid-intervention: MD = 0.042, t = 3.326, 95 % CI: 0.017 to 0.071, p = 0.002, post-intervention:, MD = 0.038, t = 3.134, 95 % CI: 0.013 to 0.063, p = 0.004; 1-month follow-up: MD = 0.042, t = 2.169, 95 % CI: 0.003 to 0.081, p = 0.038), and abduction angle at mid-intervention and post-intervention (mid-intervention: MD = 0.027, t = 2.278, 95 % CI: 0.003 to 0.051, p = 0.030, post-intervention: MD = 0.038, t = 2.800, 95 % CI: 0.010 to 0.065, p = 0.009; 1-month follow-up: MD = 0.033, t = 1.718, 95 % CI: −0.006 to 0.072, p = 0.095). For the unaffected-side shoulder, when compared with the CG, the YG demonstrated significantly greater body-weight-normalized muscle strength in flexion angle at post-intervention and 1-month follow-up (mid-intervention: MD = 0.023, t = 1.734, 95 % CI: −0.004 to 0.051, p = 0.093, post-intervention: MD = 0.044, t = 3.117, 95 % CI: 0.015 to 0.073, p = 0.004; 1-month follow-up: MD = 0.038, t = 2.339, 95 % CI: 0.005 to 0.071, p = 0.026), and abduction angle at post-intervention (mid-intervention: MD = 0.022, t = 1.682, 95 % CI: −0.005 to 0.049, p = 0.102, post-intervention: MD = 0.040, t = 2.459, 95 % CI: 0.007 to 0.073, p = 0.020; 1-month follow-up: MD = 0.033, t = 1.815, 95 % CI: −0.004 to 0.071, p = 0.079).
Table 3.
Comparison of effects of yoga intervention on upper limb strength and shoulder mobility between groups over 4 time-points measures.
| YG (n = 16) Mean (SD) |
CG (n = 18) Mean (SD) |
Group-by-time Interaction |
Group Effect |
Time Effect |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T1 | T2 | T3 | T4 | F-value | p | Effect size, ηp2 | F-value | p | Effect size, ηp2 | F-value | p | Effect size, ηp2 | |
| Muscle strength-Affected side (normalized to body weight) [muscle strength, kg] | |||||||||||||||||
| Shoulder flexion | 0.11 (0.03) [6.32 ± 1.71] | 0.17 (0.04)a,# [9.89 ± 2.32] | 0.16 (0.04)a,# [9.36 ± 2.23] | 0.16 (0.07)a,# [9.19 ± 2.72] | 0.12 (0.05) [6.85 ± 2.37] | 0.13 (0.04) [7.14 ± 1.77] | 0.13 (0.03) [7.04 ± 1.69] | 0.12 (0.05) [6.83 ± 2.22] | 8.490 | 0.000* | 0.210 | 4.838 | 0.035* | 0.131 | 10.294 | 0.000* | 0.243 |
| Shoulder abduction | 0.11 (0.03) [6.18 ± 1.61] | 0.15 (0.03)a,# [8.69 ± 2.26] | 0.16 (0.04)a,# [8.86 ± 2.37] | 0.15 (0.07)a,# [8.31 ± 3.10] | 0.11 (0.04) [6.32 ± 2.20] | 0.12 (0.03) [6.92 ± 1.58] | 0.12 (0.04) [6.66 ± 1.91] | 0.12 (0.04) [6.50 ± 2.08] | 3.606 | 0.031* | 0.101 | 3.901 | 0.057 | 0.109 | 6.226 | 0.003* | 0.163 |
| Muscle strength-Unaffected side (normalized to body weight) [muscle strength, kg] | |||||||||||||||||
| Shoulder flexion | 0.12 (0.03) [7.13 ± 1.32] | 0.16 (0.04)a [9.32 ± 2.07] | 0.18 (0.05)a,# [10.25 ± 2.32] | 0.17 (0.05)a,# [9.79 ± 2.58] | 0.14 (0.05) [7.71 ± 2.09] | 0.14 (0.04) [7.82 ± 1.74] | 0.14 (0.04) [7.76 ± 1.88] | 0.13 (0.04) [7.56 ± 2.08] | 9.351 | 0.000* | 0.226 | 3.522 | 0.070 | 0.099 | 8.145 | 0.000* | 0.203 |
| Shoulder abduction | 0.12 0.02) [7.02 ± 1.34] | 0.14 (0.04) [8.34 ± 2.46] | 0.16 (0.05)# [9.16 ± 2.87] | 0.16 (0.06) [8.81 ± 3.29] | 0.12 (0.05) [6.99 ± 2.65] | 0.12 (0.04) [6.90 ± 1.75] | 0.12 (0.05) [6.82 ± 2.35] | 0.12 (0.05) [6.84 ± 2.43] | 2.553 | 0.068 | 0.0740 | 3.990 | 0.054 | 0.111 | 2.015 | 0.117 | 0.059 |
| Shoulder ROM-Affected side | |||||||||||||||||
| Flexion (°) | 161.90 (10.45) | 168.38 (9.33) | 167.63(8.95) | 167.00 (8.50) | 160.00 (8.05) | 160.39(9.66) | 162.82 (9.15) | 162.95 (9.26) | 2.077 | 0.108 | 0.061 | 2.864 | 0.100 | 0.082 | 5.132 | 0.002* | 0.138 |
| Abduction(°) | 158.19 (15.75) | 165.92(13.79)a | 165.15(11.32)a | 166.31 (12.51)a | 159.04 (10.92) | 158.9(10.48) | 158.76 (10.49) | 159.41 (9.38) | 4.447 | 0.011* | 0.122 | 1.650 | 0.208 | 0.049 | 4.604 | 0.009* | 0.126 |
| HBB-length (cm) | 19.39 (6.58) | 15.99 (5.45)a | 15.21 (4.78)a | 14.94 (4.97)a | 17.97 (5.77) | 17.44 (6.48) | 17.18 (6.05) | 17.38 (6.70) | 6.287 | 0.004* | 0.164 | 0.324 | 0.573 | 0.010 | 11.817 | 0.000* | 0.270 |
| Shoulder ROM-Unaffected side | |||||||||||||||||
| Flexion (°) | 165.81 (10.56) | 168.71 (8.47) | 171.32(9.72) | 170.14 (6.46) | 164.26 (7.45) | 166.81(6.55) | 163.23 97.33) | 166.76 (6.88)c | 2.314 | 0.092 | 0.067 | 3.023 | 0.092 | 0.086 | 2.228 | 0.101 | 0.065 |
| Abduction(°) | 160.27 (11.79) | 165.52(11.87) | 167.61 (9.68)a | 167.81 (8.11)a | 162.83 (10.71) | 163.02(9.54) | 163.24 (9.21) | 162.42 (8.97) | 3.522 | 0.018* | 0.099 | 0.639 | 0.430 | 0.020 | 3.510 | 0.018* | 0.099 |
| HBB-length (cm) | 17.78 (5.22) | 16.09 (4.32) | 14.53 (4.83)a | 15.22 (4.45)a | 16.18 (5.29) | 15.74 (6.16) | 15.71 (5.69) | 16.55 (6.03) | 4.196 | 0.008* | 0.116 | 0.006 | 0.937 | 0.000 | 5.084 | 0.003* | 0.137 |
T1, baseline; T2, mid-intervention; T3, post-intervention, T4, 1-month follow-up; YG, yoga intervention group; CG, control group.
*p < 0.05, group-by-time interaction, group and time effect; #p < 0.05, between-group comparison at each time-point.
ap < 0.008, within-group comparison relative to T1.
Regarding shoulder mobility, there were significant group-by-time interaction effects on bilateral limb HBB motion and abduction ROM (p = 0.004–0.018), however, further analysis showed that there was no significant between-group difference at all time-points.
HRV
There was a significant group-by-time interaction effect on the RMSSD index (F = 4.264, p = 0.008, ηp2 = 0.118) (Table 4). In addition, at post-intervention, the YG demonstrated significantly higher RMSSD index values than the CG (MD = 8.692, t = 2.182, 95 % CI: 0.454 to 16.929, p = 0.040). Otherwise, there was no significant between-group difference in other HRV indices.
Table 4.
Comparison of effects of yoga intervention on heart rate variability between groups over 4 time-points measures.
| YG (n = 16) Mean (SD) |
CG (n = 18) Mean (SD) |
Group-by-time Interaction |
Group Effect |
Time Effect |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T1 | T2 | T3 | T4 | F-value | p | Effect size, ηp2 | F-value | p | Effect size, ηp2 | F-value | p | Effect size, ηp2 | |
| Heart rate variability | |||||||||||||||||
| Minimum HR (bpm) | 68.06 (8.71) | 63.38 (15.00) | 66.44 (9.19) | 68.75 (10.30) | 69.83 (9.67) | 67.07 (7.24) | 67.72 (8.44) | 66.89 (5.65) | 0.991 | 0.400 | 0.030 | 0.222 | 0.641 | 0.007 | 1.844 | 0.144 | 0.054 |
| Maximum HR (bpm) | 84.50 (13.83) | 89.50 (12.34) | 85.81 (12.34) | 88.00 (12.70) | 89.56 (13.10) | 87.17 (8.67) | 87.06 (9.48) | 87.11 (7.19) | 1.951 | 0.145 | 0.057 | 0.052 | 0.821 | 0.002 | 0.492 | 0.635 | 0.015 |
| Time-Domain | |||||||||||||||||
| -RRi (ms) | 823.00(104.66) | 814.88(104.01) | 837.75(99.86) | 773.75 (79.70) | 781.56 (90.49) | 753.50(95.19) | 752.56 (89.98) | 748.06 (76.00) | 1.175 | 0.323 | 0.035 | 4.817 | 0.036* | 0.131 | 2.323 | 0.080 | 0.068 |
| -SDNN (ms) | 24.88 (11.08) | 30.24 (13.03) | 34.93 (13.30) | 28.44 (10.02) | 24.70 (9.78) | 23.45 (9.54) | 27.54 (11.29) | 24.91 (9.99) | 2.594 | 0.072 | 0.075 | 1.791 | 0.190 | 0.053 | 6.974 | 0.001* | 0.179 |
| -RMSSD (ms) | 28.00 (11.78) | 28.26 (12.07) | 34.33 (14.05)# | 28.88 (10.39) | 28.44 (11.55) | 30.12 (13.25) | 25.63 (7.96) | 27.49 (9.77) | 4.264 | 0.008* | 0.118 | 0.325 | 0.573 | 0.010 | 0.575 | 0.633 | 0.018 |
| Frequency-Domain | |||||||||||||||||
| -LF (nu) | 38.13 (13.61) | 45.89 (21.27) | 43.49 (18.09) | 40.89 (21.27) | 37.99 (13.12) | 41.98(12.27) | 38.39 (15.20) | 39.42(15.48) | 0.545 | 0.604 | 0.017 | 0.281 | 0.600 | 0.009 | 2.541 | 0.079 | 0.074 |
| -HF (nu) | 65.15 (13.66) | 60.29 (14.82) | 58.27 (18.92) | 58.90 (21.40) | 62.40 (13.96) | 60.61(11.91) | 61.18 (15.16) | 61.72(16.95) | 0.579 | 0.630 | 0.018 | 0.049 | 0.827 | 0.002 | 1.172 | 0.325 | 0.035 |
| -LF/HF ratio | 2.01 (1.06) | 1.77 (1.15) | 1.96 (1.75) | 2.27 (1.91) | 1.99 (1.20) | 1.62 (0.67) | 2.05 (1.38) | 2.01 (1.32) | 0.278 | 0.841 | 0.009 | 0.047 | 0.830 | 0.001 | 1.664 | 0.180 | 0.049 |
T1, baseline; T2, mid-intervention; T3, post-intervention, T4, 1-month follow-up; YG, yoga intervention group; CG, control group.
HR, heart rate; bpm, beat per minute, RRi, mean normal to normal (NN) interval; SDNN, standard deviation of NN intervals; RMSSD, root mean square of the successive difference of NN intervals; LF, low-frequency; HF, high-frequency; ms, millisecond; nu, normalized unit.
*p < 0.05, group-by-time interaction, group and time effect; #p < 0.05, between-group comparison at each time-point.
Anxiety, depression, and fatigue
There was a significant group-by-time interaction effect on HADS-C anxiety subscale scores (F = 3.965, p = 0.010, ηp2 = 0.110) (Table 5), however, there was no significant between-group difference at all time-points. In addition, there were no significant interaction effects on HADS-C depression or fatigue subscale scores.
Table 5.
Comparison of effects of yoga intervention on mood and fatigue between groups over 4 time-points measures.
| YG (n = 16) Mean (SD) |
CG (n = 18) Mean (SD) |
Group-by-time Interaction |
Group Effect |
Time Effect |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T1 | T2 | T3 | T4 | F-value | p | Effect size, ηp2 | F-value | p | Effect size, ηp2 | F-value | p | Effect size, ηp2 | |
| Anxiety | |||||||||||||||||
| HADS-Anxiety | 7.56 (2.68) | 6.69 (2.68) | 6.37 (3.10) | 6.19 (2.40)a | 6.89 (2.91) | 7.44 (3.05) | 7.11 (2.91) | 7.28 (3.56) | 3.965 | 0.010* | 0.110 | 0.252 | 0.619 | 0.008 | 1.541 | 0.209 | 0.046 |
| Depression | |||||||||||||||||
| HADS-Depression | 5.75 (3.19) | 5.69 (2.92) | 5.50 (2.83) | 5.63 (2.55) | 5.56 (3.29) | 5.67 (2.72) | 5.61 (2.73) | 5.67 (3.48) | 0.109 | 0.955 | 0.003 | 0.000 | 0.987 | 0.000 | 0.072 | 0.975 | 0.002 |
| Fatigue | |||||||||||||||||
| FAS | 26.38 (6.81) | 25.25 (6.36) | 25.06 (5.62) | 24.94 (5.77) | 25.78 (7.37) | 25.89 (7.78) | 26.17 (7.07) | 26.61 (7.73) | 1.262 | 0.292 | 0.038 | 0.175 | 0.679 | 0.005 | 0.285 | 0.801 | 0.009 |
| Sub-score of FAS | |||||||||||||||||
| -FAS (physical) | 14.12 (3.83) | 14.06 (3.49) | 13.69 (3.01) | 13.94 (3.79) | 14.17 (4.06) | 14.28 (4.31) | 14.39 (4.58) | 14.44 (4.76) | 0.230 | 0.809 | 0.006 | 0.026 | 0.873 | 0.001 | 0.043 | 0.954 | 0.001 |
| -FAS (mental) | 12.13 (3.48) | 11.19 (3.45) | 11.19 (3.27) | 11.00 (2.63) | 11.61 (3.70) | 11.50 (3.78) | 11.89 (3.53) | 12.06 (3.80) | 1.200 | 0.314 | 0.036 | 0.513 | 0.479 | 0.016 | 0.503 | 0.681 | 0.015 |
T1, baseline; T2, mid-intervention; T3, post-intervention, T4, 1-month follow-up; YG, yoga intervention group; CG, control group.
HADS, Hospital Anxiety and Depression Scale; FAS, Fatigue Assessment Scale.
*p < 0.05, group-by-time interaction, group and time effect.
ap < 0.008, within-group comparison relative to T1.
Health-related quality of life
There were significant group-by-time interaction effects on FACT-B + 4 total scores (F = 6.122, p = 0.001, ηp2 = 0.161) and on scores in three domains of the FACT-B + 4 (social well-being, functional well-being, and arm symptoms domains) (Table 6). In addition, the arm symptoms subscores of the YG improved significantly over time compared with those of the CG (mid-intervention: MD = 2.986, t = 2.628, 95 % CI: 0.671 to 5.301, p = 0.013, post-intervention: MD = 3.458, t = 2.882, 95 % CI: 1.014 to 5.902, p = 0.007; 1-month follow-up: MD = 2.556, t = 2.161, 95 % CI: 0.147 to 4.964, p = 0.038). However, there was no significant between-group difference in FACT-B + 4 total score and other subscores.
Table 6.
Comparison of effects of yoga intervention on health-related quality of life between groups over 4 time-points measures.
| YG (n = 16) Mean (SD) |
CG (n = 18) Mean (SD) |
Group-by-time Interaction |
Group Effect |
Time Effect |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T1 | T2 | T3 | T4 | F-value | p | Effect size, ηp2 | F-value | p | Effect size, ηp2 | F-value | p | Effect size, ηp2 | |
| Health-related Quality of Life (Specific to breast cancer) | |||||||||||||||||
| FACT-B (total) | 93.87 (22.33) | 101.25 (21.99)a | 103.60 (23.39)a | 105.15 (20.13)a | 93.94 (21.07) | 93.22(18.60) | 94.18 (17.95) | 93.07 (20.22) | 6.122 | 0.001* | 0.161 | 1.148 | 0.292 | 0.035 | 5.149 | 0.002* | 0.139 |
| Sub-score of FACT-B | |||||||||||||||||
| -Physical | 20.50 (4.34) | 21.81 (3.83) | 22.13 (3.98) | 22.44 (3.56) | 19.72 (5.99) | 20.11 (5.21) | 20.72 (4.62) | 20.17 (5.23) | 1.085 | 0.359 | 0.033 | 1.010 | 0.322 | 0.031 | 3.937 | 0.011* | 0.110 |
| -Social | 18.62 (6.56) | 19.06 (6.68) | 20.54 (6.15)a | 20.65 (4.87) | 19.44 (6.72) | 17.17 (5.92) | 17.73 (6.22) | 16.73 (6.22) | 5.284 | 0.005* | 0.142 | 0.941 | 0.339 | 0.029 | 1.099 | 0.345 | 0.033 |
| -Emotional | 16.94 (3.75) | 18.31 (3.75) | 17.56 (4.32) | 18.75 (3.28) | 17.33 (4.30) | 17.44 (3.43) | 17.22 (3.80) | 17.22 (3.64) | 1.926 | 0.131 | 0.057 | 0.237 | 0.630 | 0.007 | 1.879 | 0.138 | 0.055 |
| -Functional | 17.06 (5.53) | 19.25 (6.42) | 19.25 (5.59)a | 19.06 (5.85) | 17.50 (6.24) | 17.89 (5.84) | 17.17 (6.31) | 16.67 (6.82) | 3.368 | 0.022* | 0.095 | 0.452 | 0.506 | 0.014 | 2.515 | 0.063 | 0.073 |
| -BCS | 20.75 (7.19) | 22.81 (6.74) | 24.13 (7.51) | 24.25 (8.11) | 19.94 (4.80) | 20.61 (4.43) | 21.33 (3.66) | 22.28 (3.92) | 1.400 | 0.248 | 0.042 | 0.998 | 0.325 | 0.030 | 13.294 | 0.000* | 0.294 |
| -Arm symptoms | 14.25 (2.72) | 16.38 (2.55)a,# | 16.63 (2.31)a,# | 16.50 (3.29)a,# | 12.78 (4.19) | 13.39 (2.85) | 13.17 (4.27) | 13.94 (3.57) | 3.194 | 0.027* | 0.091 | 5.521 | 0.025* | 0.147 | 10.243 | 0.000* | 0.242 |
T1, baseline; T2, mid-intervention; T3, post-intervention, T4, 1-month follow-up; YG, yoga intervention group; CG, control group.
FACT-B, Functional Assessment of Cancer Therapy–Breast; BCS, breast cancer subscale.
*p < 0.05, group-by-time interaction, group and time effect; #p < 0.05, between-group comparison at each time-point.
ap < 0.008, within-group comparison relative to T1.
4. Discussion
The preliminary findings showed that the tailor-made yoga program is a well-accepted form of supportive care for women with breast cancer in view of its high compliance rate in this pilot study. Regarding the primary outcomes, there was no significant between-group difference in upper limb function but the YG demonstrated significantly shorter sleep latency and less sleep disturbance than the CG at post-intervention and 1-month follow-up, respectively. Regarding the secondary outcomes, the YG demonstrated significantly greater improvements than the CG in the shoulder muscle strength and arm symptoms subscores of the FACT-B + 4 from mid-intervention until 1-month follow-up, as well as higher HRV (RMSSD) at post-intervention.
4.1. Feasibility of the yoga program
In this pilot study, the dropout rate was low. The reasons for the dropout (schedule clash and leg injury) were unrelated to the yoga intervention. The participant with the leg injury was in the CG and she did not engage the yoga training before withdrawal. Regarding the safety of practicing yoga, there was no recorded adverse events or complaints during and after yoga sessions. Furthermore, a high attendance rate (92 %) was recorded. The possible factors influencing the participation of the intervention may be due to the group-based nature of the intervention, which was treating as a regular social event for the participants. The peer support from the group may have served as motivation for participation. Additionally, an identity-verified QR code for venue entry was texted to the participants individually through mobile phones for each yoga session. Group dynamics and phone reminders may have promoted the intervention adherence. It is believed that our tailor-made yoga program was well-accepted by participants with breast cancer.
4.2. Effects on upper limb performance
Although we found a 43 % reduction in upper limb functional disability (as measured by the QuickDASH) in the YG and an 12 % increase in this disability in the CG, this between-group difference was not statistically significant. There are three possible reasons for this lack of statistical significance. First, our sample size might not have been large enough to detect a significant between-group difference. Second, although the YG overall showed improved upper limb function after the intervention, there were a few outliers in the CG with marked drops in scores that may have confounded the results. Third, although the intervention improved the upper limb function of the YG, the dosage of yoga training might not have been sufficiently intense. Thus, our main study should use a higher dosage of yoga training, such as a higher frequency, a longer duration, and a booster session. Nevertheless, upon inspection of the QuickDASH items, we observed a decreased rating of the items related to the severity of upper limb pain and tingling in the YG, however, there was no ease of difficulties in the items of “doing heavy househlod chores” and “recreational activities requiring some force” after yoga intervention. In line with the positive findings of FACT-B arm symptoms subscores, which evaluated upper limb pain in movement, numbness, stiffness and range of motion, participants in the YG demonstrated improved arm symptoms after yoga training. Therefore, we believe that upper limb function could gradually improve as their arm symptoms improved. However, apart from the arm symptoms, other factors may affect the ability to perform activities. These factors could include fatigue, fear of injury, and time constraints.
As expected, our tailor-made yoga intervention increased the body-weight-adjusted shoulder muscle strength (affected side flexion: 23 %; abduction: 33 %; unaffected side: flexion: 29 %) at post-intervention, when compared with the CG. These findings are consistent with those of a pilot RCT conducted by Loudon et al. [38] that used an 8-week yoga training intervention. They found that this intervention led to 17 % and 20 % increases in affected and unaffected shoulder-abduction muscle strength, respectively, in women with breast cancer-related lymphedema compared with control women. Moreover, our YG demonstrated improvement in affected-shoulder muscle strength at the fourth week of the intervention, and these effects persisted at 1-month follow-up.
ROM is important for activities of daily living, particularly those involving overhead reaching and hand behind back motion. Although there was no significant between-group difference in shouler ROM, the YG exhibited an increase of 14%–23 % in HBB mobility from mid-intervention to 1-month follow-up, compared with baseline. Thus, increasing the yoga training dosage in our future main study may enhance the intervention's effects on shoulder mobility.
Women with breast cancer who undergo surgeries and radiotherapy experience tissue injuries and fibrosis, coupled with post-operative pain and decreased upper limb activity, which results in reduced shoulder mobility and muscle strength. Shamley et al. [39] investigated changes in shoulder muscles following breast cancer treatments, namely mastectomy and radiotherapy; and found that compared with before surgery, after surgery the upper trapezii and rhomboids demonstrated significantly less electromyographic activity. This decreased activity was significantly associated with pain, disability, and time since surgery. Via magnetic resonance imaging, they also determined that the pectoralis major and minor decreased in size on the affected side [39]. Our tailor-made yoga training program (i) engages the upper body to build core stability and strengthen the muscles affected by cancer treatment, and relieves muscle tightness via twist postures to facilitate continual practice in increasing the extent of shoulder movement, and (ii) applies a gradual progression schedule that is adjusted according to the abilities and limitations (for example, arm pain during practice) of women with breast cancer to ensure the safety and effectiveness of practice.
Recent reviews have noted that resistance or weight-lifting exercise is safe for breast cancer patients to perform [40,41]. However, such patients are typically advised to avoid vigorous, repetitive, or excessive upper limb exercise, given the risk of breast cancer-related lymphedema [42]. This has led to a fear that such exercise exacerbates upper limb functional impairment, resulting in a dilemma when recommending exercise for women with breast cancer. In contrast, our tailor-made yoga program based on stabilization of upper-body training and a gradual progression schedule appears to effectively balance the risks and benefits of upper limb exercise in women with breast cancer.
4.3. Effects on sleep quality
Sleep and mood disturbance are bidirectionally influenced. Lengthened sleep latency, represented by difficulty falling asleep, may be caused by hyperarousal of the body due to anxiety and stress. Meanwhile, mood disturbances may be exacerbated by sleep disturbance. Recent HRV-related literature suggests that yoga improves “self-regulation” of the body through regulating the limbic system brain network that is associated with the autonomic nervous system [43]. Yoga practice may enhance parasympathetic tone and is associated with calm mental state [44]. The expected direction of the HRV after yoga intervention would be the enhancement of the vagal tone which was represented by the contribution of parasympathetic nervous system to cardiac regulation and could be reflected by the increase of HRV (RMSSD) index. Consistent with the aforementioned findings, the YG showed significantly better HRV (RMSSD) than the CG (yoga: 34.33 ms; control: 25.63 ms) at post-intervention, although this improvement was not maintained at follow-up. This suggests that the intervention helped with emotional regulation, which is supported by the fact that the YG exhibited an 18 % decrease in anxiety (as measured by the HADS-C anxiety subscale) from baseline to 1-month follow-up. Controlled yogic breathing and meditative practice may help to restore homeostasis through regulatory activation of the autonomic nervous system and modulate the circadian sleep–wake cycle, thereby adjusting sleep architecture and decreasing sleep latency [45,46]. The mechanisms by which yoga training alleviates sleep problems are not fully understood, but our findings demonstrate that yoga training may improve sleep quality. This may be attributable to the components of yoga, namely (i) meditation, which helps an individual to achieve a deep state of psychological rest by harmonizing the body and mind, which could modulate physiological and biomedical functions and thereby mediate sleep [47], and (ii) yoga postures, which help to activate exercise-induced body temperature regulation function and energy consumption [48], and melatonin secretion [49].
4.4. Effects on psychosocial symptoms and health-related quality of life
Inconsistent with previous studies [50,51], our tailor-made yoga training program did not have significant effects on anxiety, depression, and fatigue in the YG. This might have been because the effect size of yoga training was not high (e.g., Cohen's d ≥ 0.80) and was therefore not detectable in our small sample. It might also have been because the YG comprised middle-aged women (the mean age of the YG was 48.63 years) with a low level of fatigue (active members of local self-help groups) and mood disturbance (HADS-C depression domain score <8). Thus, the potential benefits of our tailor-made yoga program on fatigue and mood disturbance were not reflected in the present study. Therefore, a large sample size trial including participants with severe mood disturbance and fatigue was warranted to further investigate the effects of our tailor-made yoga program on these psychosocial symptoms.
The health-related quality of life in the YG was improved in line with the improvement in upper limb motor function as reflected by the improved arm symptoms. It appeared that the above-mentioned improvements in the YG were due to changes in the domains of functional and social well-being. This is consistent with our hypothesis that improvements in upper limb function and sleep quality realized by yoga training lead to improvements in quality of life. Furthermore, the improvements in social well-being in the YG may be attributable to mutual support derived from group participation in yoga practice.
5. Limitations
This study has several limitations. First, the participants were women with breast cancer in primary stage I-III and those had finished cancer treatments more than 1 month. They were recruited from a self-help group and were rather socially active. Thus, our findings may not be generalizable to those women with breast cancer who do not meet our inclusion criteria. Moreover, the effects of yoga intervention might not be detected in the present study due to small sample size. To increase generalizability, large-scale future studies may include women with advanced-stage breast cancer, those with metastasis or recurrence, and those concurrently undergoing cancer-related treatments. Second, we did not record the symptoms of post-menstruation complications (e.g. hot flashes and night sweats) induced by hormonal therapy. This might have confounded the results, especially as psychosocial outcomes are highly associated with vasomotor symptoms. Third, we only included subjectively reported sleep outcomes, objectively assessed sleep parameters (e.g. actigraphy measurement) may reveal different results. Fourth, there may be the potential issue related to therapist effect not being identified as only a single instructor was included in this study. To ensure the intervention fidelity in future trials, yoga instructors will be instructed to adhere the intervention protocol, and video recording of the intervention sessions will be implemented to monitor consistency in intervention delivery among groups. Fifth, we did not investigate the long-term maintenance effects of the intervention. Therefore, we recommend that a large-scale RCT be performed to investigate whether our yoga program has long-lasting positive effects on women with breast cancer. Given the different time-points of measurements in this pilot study, it provided insights into the speed and progress of the intervention on the outcome variables. For instance, we found significantly improved shoulder muscle strength and arm symptoms after 4 weeks of yoga intervention. In contrast, we found significantly improved sleep latency after 8 weeks of yoga intervention. It provided insights for studies to design interventions for improving the upper limb strength, arm symptoms and sleep of women with breast cancer. Finally, the findings of the present study shall be interpreted in cautious. The small sample size of the present study may over- or under-estimated the effects of our tailor-made yoga intervention.
6. Conclusion
This pilot study suggests that our tailor-made yoga program is a feasible and well-accepted supportive therapy for the rehabilitation of women with breast cancer. Despite there was no significant improvement in upper limb function, our participants demonstrated improved sleep latency and upper limb muscle strength after the yoga program. Therefore, a large-scale RCT is warranted to further examine the effects of our tailor-made yoga protocol in women with breast cancer.
Statements & declarations
Ethical approval
The study was conducted in line with the principles of the Declaration of Helsinki. Approval was granted by The Hong Kong Polytechnic University Institutional Review Board (No.: HSEARS20220713002).
Consent to participate
All participants gave their informed written consent prior to the commencement of the study.
Consent to publish
The authors affirmed that the involved research personnel provided informed consent for the publication of the images in the supplementary information.
Funding
The work was supported by the Hong Kong Metropolitan University, School of Nursing and Health Studies Mini-Grant for Research Projects (code: R5111)
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
CRediT authorship contribution statement
Sarah Suet Shan Wong: Conceptualization, Investigation, Methodology, Project administration, Writing – original draft, Formal analysis. Tai Wa Liu: Conceptualization, Supervision, Writing – review & editing. Shamay Sheung Mei Ng: Project administration, Methodology, Conceptualization, Resources, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
N.A.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e35883.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health Organization Breast Cancer. 2021 https://www.who.int/news-room/fact-sheets/detail/breast-cancer [Google Scholar]
- 2.Miller K.D., Nogueira L., Mariotto A.B., Rowland J.H., Yabroff K.R., Alfano C.M., Jemal A., Kramer J.L., Siegel R.L. Cancer treatment and survivorship statistics, 2019. CA A Cancer J. Clin. 2019;69(5):363–385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 3.Ho S.Y., Rohan K.J., Parent J., Tager F.A., McKinley P.S. A longitudinal study of depression, fatigue, and sleep disturbances as a symptom cluster in women with breast cancer. J. Pain Symptom Manag. 2015;49(4):707–715. doi: 10.1016/j.jpainsymman.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pillai U.S., Kayal S., Cyriac S., Nisha Y., Dharanipragada K., Kamalanathan S.K., Halanaik D., Kumar N., Madasamy P., Muniswamy D.K., Dubashi B. Late effects of breast cancer treatment and outcome after corrective interventions. Asian Pac. J. Cancer Prev. APJCP. 2019;20(9):2673–2679. doi: 10.31557/APJCP.2019.20.9.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayes S.C., Johansson K., Stout N.L., Prosnitz R., Armer J.M., Gabram S., Schmitz K.H. Upper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer. 2012;118(8 Suppl):2237–2249. doi: 10.1002/cncr.27467. [DOI] [PubMed] [Google Scholar]
- 6.Siqueira T.C., Frágoas S.P., Pelegrini A., de Oliveira A.R., da Luz C.M. Factors associated with upper limb dysfunction in breast cancer survivors. Support. Care Cancer. 2021;29(4):1933–1940. doi: 10.1007/s00520-020-05668-7. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez B.D., Eisel S.L., Qin B., Llanos A.A.M., Savard J., Hoogland A.I., Jim H., Lin Y., Demissie K., Hong C.C., Bandera E.V. Prevalence, risk factors, and trajectories of sleep disturbance in a cohort of African-American breast cancer survivors. Support. Care Cancer. 2021;29(5):2761–2770. doi: 10.1007/s00520-020-05786-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leysen L., Lahousse A., Nijs J., Adriaenssens N., Mairesse O., Ivakhnov S., Bilterys T., Van Looveren E., Pas R., Beckwée D. Prevalence and risk factors of sleep disturbances in breast cancersurvivors: systematic review and meta-analyses. Support. Care Cancer. 2019;27(12):4401–4433. doi: 10.1007/s00520-019-04936-5. [DOI] [PubMed] [Google Scholar]
- 9.Strollo S.E., Fallon E.A., Gapstur S.M., Smith T.G. Cancer-related problems, sleep quality, and sleep disturbance among long-term cancer survivors at 9-years post diagnosis. Sleep Med. 2020;65:177–185. doi: 10.1016/j.sleep.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Otte J.L., Carpenter J.S., Russell K.M., Bigatti S., Champion V.L. Prevalence, severity, and correlates of sleep-wake disturbances in long-term breast cancer survivors. J. Pain Symptom Manag. 2010;39(3):535–547. doi: 10.1016/j.jpainsymman.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altice C.K., Banegas M.P., Tucker-Seeley R.D., Yabroff K.R. Financial hardships experienced by cancer survivors: a systematic review. J. Natl. Cancer Inst. 2016;109(2) doi: 10.1093/jnci/djw205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mokhtari-Hessari P., Montazeri A. Health-related quality of life in breast cancer patients: review of reviews from 2008 to 2018. Health Qual. Life Outcome. 2020;18(1):338. doi: 10.1186/s12955-020-01591-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feuerstein G. Hohm Press; Prescott: 1998. The Yoga Tradition: its History, Literature, Philosophy, and Practice. [Google Scholar]
- 14.Wanchai A., Armer J.M. The effects of yoga on breast-cancer-related lymphedema: a systematic review. J Health Res. 2020;34(5):409–418. doi: 10.1108/jhr-09-2019-0210. [DOI] [Google Scholar]
- 15.Saraswathi V., Latha S., Niraimathi K., Vidhubala E. Managing lymphedema, increasing range of motion, and quality of life through yoga therapy among breast cancer survivors: a systematic review. Int. J. Yoga. 2021;14(1):3–17. doi: 10.4103/ijoy.IJOY_73_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W.L., Chen K.H., Pan Y.C., Yang S.N., Chan Y.Y. The effect of yoga on sleep quality and insomnia in women with sleep problems: a systematic review and meta-analysis. BMC Psychiatr. 2020;20(1):195. doi: 10.1186/s12888-020-02566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cramer H., Lauche R., Klose P., Lange S., Langhorst J., Dobos G.J. Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Cochrane Database Syst. Rev. 2017;1(1) doi: 10.1002/14651858.CD010802.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi L.J., Tian X., Jin Y.F., Luo M.J., Jiménez-Herrera M.F. Effects of yoga on health-related quality, physical health and psychological health in women with breast cancer receiving chemotherapy: a systematic review and meta-analysis. Ann. Palliat. Med. 2021;10(2):1961–1975. doi: 10.21037/apm-20-1484. [DOI] [PubMed] [Google Scholar]
- 19.Barbosa K.P., da Silva L.G.T., Garcia P.A., Freitas C.A., da Silva E.C.F., Pereira T.V., Alves A.T., Matheus L.B.G. Effectiveness of Pilates and circuit-based exercise in reducing arthralgia in women during hormone therapy for breast cancer: a randomized, controlled trial. Supportive Care. 2021;29(10):6051–6059. doi: 10.1007/s00520-021-06180-2. [DOI] [PubMed] [Google Scholar]
- 20.Riley K.O., Park C.L. How does yoga reduce stress? A systematic review of mechanisms of change and guide to future inquiry. Health Psychol. Rev. 2015;9(3):379–396. doi: 10.1080/17437199.2014.981778. [DOI] [PubMed] [Google Scholar]
- 21.Mustian K.M., Janelsins M., Peppone L.J., Kamen C. Yoga for the treatment of insomnia among cancer patients: evidence, mechanisms of action, and clinical recommendations. Oncol Hematol Rev. 2014;10(2):164–168. doi: 10.17925/ohr.2014.10.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee E.W., Lau J.S., Chung M.M., Li A.P., Lo S. Evaluation of the Chinese version of the Disability of the Arm, Shoulder and Hand (DASH-HKPWH): cross-cultural adaptation process, internal consistency and reliability study. J. Hand Ther. 2004;17(4):417–423. doi: 10.1197/j.jht.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Cao S., Zhou R., Zhou H., Chen Y., Cui H., Lu Z., Qian Q., Ding Y. Reliability and validity of simplified Chinese version of quick Disabilities of the arm, shoulder, and hand (QuickDASH) questionnaire: cross-cultural adaptation and validation. Clin. Rheumatol. 2019;38(11):3281–3287. doi: 10.1007/s10067-019-04661-8. [DOI] [PubMed] [Google Scholar]
- 24.Tsai P.S., Wang S.Y., Wang M.Y., Su C.T., Yang T.T., Huang C.J., Fang S.C. Psychometric evaluation of the Chinese version of the Pittsburgh Sleep Quality Index (CPSQI) in primary insomnia and control subjects. Qual. Life Res. 2005;14(8):1943–1952. doi: 10.1007/s11136-005-4346-x. [DOI] [PubMed] [Google Scholar]
- 25.Chong A.M.L., Cheung C. Factor structure of a Cantonese-version Pittsburgh sleep quality index. Sleep Biol. Rhythm. 2012;10(2):118–125. doi: 10.1111/j.1479-8425.2011.00532.x. [DOI] [Google Scholar]
- 26.Harrington S., Padua D., Battaglini C., Michener L.A., Giuliani C., Myers J., Groff D. Comparison of shoulder flexibility, strength, and function between breast cancer survivors and healthy participants. J Cancer Surviv. 2011;5(2):167–174. doi: 10.1007/s11764-010-0168-0. [DOI] [PubMed] [Google Scholar]
- 27.Clarkson H. third ed. Wolters Kluwer/Lippincott Williams & Wilkins Health; Philadelphia: 2013. Musculoskeletal Assessment: Joint Motion and Muscle Testing. [Google Scholar]
- 28.Shirai T., Ijiri T., Suzuki T. Relationship between hand-behind-back motion and internal rotation with the shoulder in extension. J. Phys. Ther. Sci. 2022;34(11):732–736. doi: 10.1589/jpts.34.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas B.L., Claassen N., Becker P., Viljoen M. Validity of commonly used heart rate variability markers of autonomic nervous system function. Neuropsychobiology. 2019;78(1):14–26. doi: 10.1159/000495519. [DOI] [PubMed] [Google Scholar]
- 30.Schaffarczyk M., Rogers B., Reer R., Gronwald T. Validity of the polar H10 sensor for heart rate variability analysis during resting state and incremental exercise in recreational men and women. Sensors. 2022;22(17):6536. doi: 10.3390/s22176536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar P., Das A.K., Prachita, Halder S. Time-domain HRV analysis of ECG signal under different body postures. Procedia Comput. Sci. 2020;167:1705–1710. doi: 10.1016/j.procs.2020.03.435. [DOI] [Google Scholar]
- 32.Tarvainen M.P., Niskanen J.P., Lipponen J.A., Ranta-Aho P.O., Karjalainen P.A. Kubios HRV--heart rate variability analysis software. Comput. Methods Progr. Biomed. 2014;113(1):210–220. doi: 10.1016/j.cmpb.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 33.Lam C.L., Pan P.C., Chan A.W., Chan S.Y., Munro C. Can the Hospital Anxiety and Depression (HAD) Scale be used on Chinese elderly in general practice? Fam. Pract. 1995;12(2):149–154. doi: 10.1093/fampra/12.2.149. [DOI] [PubMed] [Google Scholar]
- 34.Li Q., Lin Y., Hu C., Xu Y., Zhou H., Yang L., Xu Y. The Chinese version of hospital anxiety and depression scale: psychometric properties in Chinese cancer patients and their family caregivers. Eur. J. Oncol. Nurs. 2016;25:16–23. doi: 10.1016/j.ejon.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Ho L.Y.W., Lai C.K.Y., Ng S.S.M. Measuring fatigue following stroke: the Chinese version of the Fatigue Assessment Scale. Disabil. Rehabil. 2021;43(22):3234–3241. doi: 10.1080/09638288.2020.1730455. [DOI] [PubMed] [Google Scholar]
- 36.Coster S., Poole K., Fallowfield L.J. The validation of a quality of life scale to assess the impact of arm morbidity in breast cancer patients post-operatively. Breast Cancer Res. Treat. 2001;68(3):273–282. doi: 10.1023/a:1012278023233. [DOI] [PubMed] [Google Scholar]
- 37.Yeo W., Mo F.K., Pang E., Suen J.J., Koh J., Yip C.H., Yip C.C., Li L., Loong H.H., Liem G.S. Quality of life of young Chinese breast cancer patients after adjuvant chemotherapy. Cancer Manag. Res. 2018;10:383–389. doi: 10.2147/CMAR.S149983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loudon A., Barnett T., Williams A. Yoga, breast cancer-related lymphoedema and well-being: a descriptive report of women's participation in a clinical trial. J. Clin. Nurs. 2017;26(23–24):4685–4695. doi: 10.1111/jocn.13819. [DOI] [PubMed] [Google Scholar]
- 39.Shamley D.R., Srinanaganathan R., Weatherall R., Oskrochi R., Watson M., Ostlere S., Sugden E. Changes in shoulder muscle size and activity following treatment for breast cancer. Breast Cancer Res. Treat. 2007;106(1):19–27. doi: 10.1007/s10549-006-9466-7. [DOI] [PubMed] [Google Scholar]
- 40.Hasenoehrl T., Palma S., Ramazanova D., Kölbl H., Dorner T.E., Keilani M., Crevenna R. Resistance exercise and breast cancer-related lymphedema-a systematic review update and meta-analysis. Support. Care Cancer. 2020;28(8):3593–3603. doi: 10.1007/s00520-020-05521-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wanchai A., Armer J.M. Effects of weight-lifting or resistance exercise on breast cancer-related lymphedema: a systematic review. Int. J. Nurs. Sci. 2018;6(1):92–98. doi: 10.1016/j.ijnss.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harris S.R., Niesen-Vertommen S.L. Challenging the myth of exercise-induced lymphedema following breast cancer: a series of case reports. J. Surg. Oncol. 2000;74(2):95–99. doi: 10.1002/1096-9098(200006)74:2<95::AID-JSO3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 43.Gard T., Noggle J.J., Park C.L., Vago D.R., Wilson A. Potential self-regulatory mechanisms of yoga for psychological health. Front. Hum. Neurosci. 2014;8:770. doi: 10.3389/fnhum.2014.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Streeter C.C., Gerbarg P.L., Saper R.B., Ciraulo D.A., Brown R.P. Effects of yoga on the autonomic nervous system, gamma-aminobutyric-acid, and allostasis in epilepsy, depression, and post-traumatic stress disorder. Med. Hypotheses. 2012;78(5):571–579. doi: 10.1016/j.mehy.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 45.Gong H., Ni C.X., Liu Y.Z., Zhang Y., Su W.J., Lian Y.J., Peng W., Jiang C.L. Mindfulness meditation for insomnia: a meta-analysis of randomized controlled trials. J. Psychosom. Res. 2016;89:1–6. doi: 10.1016/j.jpsychores.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 46.Panjwani U., Dudan S., Wadhwa M. Sleep, cognition, and yoga. Int. J. Yoga. 2021;14(2):100–108. doi: 10.4103/ijoy.IJOY_110_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagendra R.P., Maruthai N., Kutty B.M. Meditation and its regulatory role on sleep. Front. Neurol. 2012;3:54. doi: 10.3389/fneur.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Youngstedt S.D. Effects of exercise on sleep. Clin. Sports Med. 2005;24(2):355–xi. doi: 10.1016/j.csm.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Harinath K., Malhotra A.S., Pal K., Prasad R., Kumar R., Kain T.C., Rai L., Sawhney R.C. Effects of Hatha yoga and Omkar meditation on cardiorespiratory performance, psychologic profile, and melatonin secretion. J. Alternative Compl. Med. 2004;10(2):261–268. doi: 10.1089/107555304323062257. [DOI] [PubMed] [Google Scholar]
- 50.Bower J.E., Garet D., Sternlieb B., Ganz P.A., Irwin M.R., Olmstead R., Greendale G. Yoga for persistent fatigue in breast cancer survivors: a randomized controlled trial. Cancer. 2012;118(15):3766–3775. doi: 10.1002/cncr.26702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zuo X., Li Q., Gao F., Yang L., Meng F. Effects of yoga on negative emotions in patients with breast cancer: a meta-analysis of randomized controlled trials. Int. J. Nurs. Sci. 2016;3(3):299–306. doi: 10.1016/j.ijnss.2016.07.009. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.