Abstract
Objective
The mitochondrial pyruvate carrier (MPC) occupies a critical node in intermediary metabolism, prompting interest in its utility as a therapeutic target for the treatment of obesity and cardiometabolic disease. Dysregulated nutrient metabolism in adipose tissue is a prominent feature of obesity pathophysiology, yet the functional role of adipose MPC has not been explored. We investigated whether the MPC shapes the adaptation of adipose tissue to dietary stress in female and male mice.
Methods
The impact of pharmacological and genetic disruption of the MPC on mitochondrial pathways of triglyceride assembly (lipogenesis and glyceroneogenesis) was assessed in 3T3L1 adipocytes and murine adipose explants, combined with analyses of adipose MPC expression in metabolically compromised humans. Whole-body and adipose-specific glucose metabolism were subsequently investigated in male and female mice lacking adipocyte MPC1 (Mpc1AD−/−) and fed either standard chow, high-fat western style, or high-sucrose lipid restricted diets for 24 weeks, using a combination of radiolabeled tracers and GC/MS metabolomics.
Results
Treatment with UK5099 or siMPC1 impaired the synthesis of lipids and glycerol-3-phosphate from pyruvate and blunted triglyceride accumulation in 3T3L1 adipocytes, whilst MPC expression in human adipose tissue was negatively correlated with indices of whole-body and adipose tissue metabolic dysfunction. Mature adipose explants from Mpc1AD−/− mice were intrinsically incapable of incorporating pyruvate into triglycerides. In vivo, MPC deletion restricted the incorporation of circulating glucose into adipose triglycerides, but only in female mice fed a zero fat diet, and this associated with sex-specific reductions in tricarboxylic acid cycle pool sizes and compensatory transcriptional changes in lipogenic and glycerol metabolism pathways. However, whole-body adiposity and metabolic health were preserved in Mpc1AD−/− mice regardless of sex, even under conditions of zero dietary fat.
Conclusions
These findings highlight the greater capacity for mitochondrially driven triglyceride assembly in adipose from female versus male mice and expose a reliance upon MPC-gated metabolism for glucose partitioning in female adipose under conditions of dietary lipid restriction.
Keywords: Adipose, Mitochondria, Sexual dimorphism, Lipogenesis, Glyceroneogenesis
Highlights
-
•
The MPC gates pyruvate-driven triglyceride synthesis in white adipose tissue (WAT).
-
•
Both de novo lipogenesis and glyceroneogenesis are blunted by WAT MPC inhibition.
-
•
Adipocyte MPC facilitates glucose storage in female WAT during dietary lipid restriction.
-
•
Sex-specific depletion of TCA cycle intermediates in female MPC-null WAT.
-
•
Adiposity and metabolic health are preserved in female and male Mpc1AD−/− mice.
1. Introduction
Adipose tissue contributes to the intricate regulation of whole-body energy metabolism through the cyclic synthesis and breakdown of triglyceride stores. Nutrient fluxes into healthy adipocytes serve to buffer postprandial increases in circulating lipids and glucose whilst, in the postabsorptive state, lipolysis and free fatty acid (FFA) re-esterification balance systemic FFA availability with peripheral energy demands [1,2]. Under conditions of sustained nutrient excess, the progressive failure of these systems leads to chronic elevations in plasma FFA, ectopic lipid deposition (i.e. in skeletal muscle or liver) and the gradual worsening of peripheral insulin resistance [3,4]. Therefore, dysregulated nutrient handling in adipose tissue is an important hallmark of obesity that is directly implicated in the pathogenesis of common cardiometabolic diseases like type 2 diabetes and metabolic dysfunction-associated liver disease.
Adipose mitochondria are increasingly recognized as important modifiers of obesity-related pathophysiology. Unlike their counterparts in highly oxidative tissues (e.g. skeletal muscle and brown adipose tissue), mitochondria in white adipose tissue (WAT) are specialized for nutrient storage [5,6]. Mitochondrial fluxes in WAT support triglyceride assembly by fueling the de novo synthesis of both fatty acids (lipogenesis; DNL) and glycerol-3-phosphate (glyceroneogenesis). Under normal dietary conditions, the contribution of DNL to adipose triglyceride storage in humans is believed to be less than 10% [7]. However, studies in rodents [8] and humans [9] have implicated an important role for adipose DNL in diverting dietary carbohydrates towards lipid storage, under conditions of excess carbohydrate availability. Moreover, impairments in adipose DNL and triglyceride storage have been associated with the development of insulin resistance in humans [10]. Glyceroneogenesis is an important source of glycerol-3-phosphate generation in adipose [11] and facilitates FFA esterification to sustain the healthy deposition of triglycerides [12]. The regulation of these integrated mitochondrial pathways thus underpins the adaptive plasticity of WAT following dietary perturbations [13].
The mitochondrial pyruvate carrier (MPC) regulates cellular nutrient partitioning by gating pyruvate entry into mitochondria [14,15] where, in WAT, it is used both as a precursor for DNL and as the obligate substrate for glyceroneogenesis. Despite emergent tissue-specific functions for the MPC in numerous metabolic organs [[16], [17], [18], [19], [20], [21], [22], [23], [24], [25]], the relevance of MPC activity in WAT has received surprisingly little attention. In vitro data demonstrate that mitochondrial pyruvate transport may be dispensable for the differentiation of 3T3L1 pre-adipocytes [26], but this does not preclude a role for the MPC in mature WAT in vivo. On the contrary, prior studies suggest that reductions in WAT MPC expression, either by heterogenous knockdown [27] or in response to a high fat diet [26], might be associated with alterations in systemic metabolism. However, the effects of MPC manipulation in WAT have not been directly investigated and thus its functional relevance in this tissue remains unclear.
Our understanding of the mechanisms through which dietary perturbations trigger adipose dysfunction has been convoluted by (often overlooked) sex-specific differences in adipose nutrient handling. For example, female adipose tissue displays greater intrinsic capacity for triglyceride synthesis and lipolysis than males, especially in subcutaneous depots [8,28]. Moreover, sex differences in adipose tissue influence the phenotypic responses to different diets [29] and likely contribute to the increased incidence of obesity-related complications in males versus females [30]. Despite their fundamental importance in the development of precision therapeutics against obesity-related disease, the molecular events underpinning sex-specific responses to dietary stress remain poorly characterized.
We reasoned that mitochondrial substrate partitioning might shape the sex-specific impact of dietary nutrients on metabolic health and, specifically, that the MPC would play a central role in this process. To address these questions, we explored the relationship between MPC-gated intermediary metabolism and adipocyte function, assessed the requirement for MPC of metabolic adaptations to dietary lipid restriction and excess, and compared the role of WAT MPC between male and female mice.
2. Materials and methods
2.1. Experimental models and animal details
2.1.1. Cell culture
3T3L1 adipocytes were maintained in DMEM supplemented with GlutaMAX™ and were differentiated in absence of thiazolidinediones as previously described [31]. For siRNA transfection experiments, 3T3L1 adipocytes were trypsin detached five days post-differentiation and reverse transfected with 50 pmols of Dharmacon SmartPool siSCR (#D-001810-10-05) or siMPC1 (#L-040908-01-0005) using Lipofectamine® RNAiMAX reagent (#13778; ThermoFisher, MA, USA) following published protocols [32]. Based upon initial optimization experiments of MPC1 depletion (Supplemental Fig. 1A), subsequent assays were performed 5 days post transfection.
2.1.2. Human studies
All studies involving human subjects were approved by the University of Texas Health Science Center at San Antonio Institutional Review Board. Fasting abdominal subcutaneous adipose tissue specimens were obtained from subjects with and without adipose tissue insulin resistance (AdipoIR), as previously described [33]. All subjects underwent a standard 2-h oral GTT to confirm normal glucose tolerance (NGT) or impaired glucose tolerance (IGT). Fasting AdipoIR was calculated from free fatty acid and insulin data [34].
2.1.3. Animal studies
For experiments in genetically obese mice, eight-week old wild type (C57BL/6J; JAX #000664) and Ob/Ob (B6 ob; JAX #000632) male mice were purchased from The Jackson Laboratory, housed in environmentally-controlled conditions (23 °C, 12 h light/dark cycles) and provided ad-libitum access to water and a standard chow diet (70% kcal carbohydrate, 10% kcal fat, 20% kcal protein; D12450J; Research Diets Inc.). Experiments were conducted in 12–14-week aged mice following four weeks of diet treatments. For Mpc1 knockout mice, mice with LoxP sites flanking the Mpc1 allele, backcrossed through C57Bl/6J mice, were a gift from Eric Taylor and have been described previously [17]. Adiponectin-Cre (Adipoq-Cre) mice were purchased from The Jackson Laboratory (stock # 010803) (Bar Harbor, ME). LoxP/LoxP littermate controls were used in all experiments. Mice were housed in environmentally controlled conditions (23 °C, 12-hour light/dark cycles), provided ad-libitum access to water and a fed a standard chow or, starting at 6 weeks of age, a zero fat diet (D04112303) or a western-style high fat diet (D09100310) from Research Diets Inc. (NJ, USA). Experiments were conducted in 32-week aged mice following 24–26 weeks of diet treatments. All procedures were approved by the Institutional Animal Care and Use Committee at University of Texas Health Science Center at San Antonio.
2.2. Method details
2.2.1. Western blotting and quantitative real-time polymerase chain reaction (qRT-PCR)
Immunoblot analysis was carried out on cell or adipose lysates using mouse or human primary antibodies against MPC1 (Sigma #HPA045119), MPC2 (Cell Signaling Technologies #46141), GAPDH (Sigma #G8795) and β-Tubulin (Abcam #179513) and developed by chemiluminescence (ECL). QRT-PCR was performed using pre-designed TaqMan probes (Life Technologies, CA, USA) or SYBR green primer assays (Integrated DNA Technologies, Iowa, USA). Data were normalized to the geometric mean of the reference genes B2m, Hmbs, Gapdh, Rplpo, and β-actin.
2.2.2. [2,3–14C] pyruvate incorporation assays
Approximately 5 × 105 adherent 3T3L1 adipocytes, eight days post-differentiation, were washed twice with PBS and incubated with complete growth media supplemented with 0.1 μCi/ml [2,3–14C] pyruvate or [U–14C] acetate with or without UK5099 (5uM) or insulin (300 pM) for 6 h. Cells were subsequently washed with PBS and quenched with ice cold methanol. For explant studies, 20–30 mg of freshly excised WAT was incubated in media as above, then washed with PBS and snap frozen in liquid nitrogen. Total lipids were extracted from cell lysates or frozen extracts by the Folch method [35] and dried under oxygen-free nitrogen. Lipid residues were saponified in methanolic KOH (1.5M) for 1 h at 70°, neutralized with methanolic HCl (3M) and re-extracted for separation of glycerol-glyceride and fatty acyl fractions. Dried fractions were resuspended and mixed with scintillation fluid prior to scintillation counting.
2.2.3. Triglyceride content
Triglyceride content was determined in 3T3L1 lysates (5% NP-40) using a calorimetric kit (Ab65336, Abcam) following the manufacturer's instructions.
2.2.4. Ex vivo lipolysis assays
Glycerol and free fatty acid release from freshly excised inguinal and epididymal fat explants (20–30 mg) were assessed under basal, insulin-treated, and maximal (10 μM forskolin/5 μM Triacsin C) conditions using published protocols [36]. Media glycerol (F6428, Sigma) and NEFA (Wako) concentrations were determined by calorimetric assay.
2.2.5. Mouse physiology studies
2.2.5.1. Body composition
Lean mass, fat mass and total body water were determined in 24-week-old WD and ZFD mice by qMRI at the San Antonio Nathan Shock Centre Aging Animal Models and Longevity Assessment Core.
2.2.5.2. Oral glucose tolerance test
Overnight fasted (16 h) mice were administered with dextrose (2 g/kg bodyweight) by oral gavage. Glucose concentration was determined in whole-blood sampled at baseline and at 15-, 30-, 60- and 120-minute intervals from a tail incision using a glucometer (Contour next EZ).
2.2.5.3. [U–14C] glucose incorporation assays
Overnight fasted mice were administered 2 nCi [U–14C] glucose in 500 μl saline by intraperitoneal injection and were euthanized by isoflurane (inhaled) 5 h later. Tissues were excised, snap frozen in liquid nitrogen and stored at −80°, with ∼200 mg portions used for lipid extraction and scintillation counting as described above.
2.2.6. Plasma analytes
Plasma insulin was determined by ELISA (#90080; Crystal Chem, IL, USA). Plasma free fatty acids (999-34691; FUJIFILM Wako Chemicals; VA, USA), glycerol (F6428; Sigma, MO, USA) and triglycerides (TR22421; ThermoFisher, MA, USA) were determined by enzymatic calorimetric assay.
2.2.7. GC/MS polar metabolomics
Approximately 50 mg of tissue plus 30 pmol/mg tissue of internal standard (MSK-A2-1.2, Cambridge Isotope Laboratories, Inc) were extracted in chloroform:methanol:saline (2:1:1) by bead homogenization. Extracts were centrifuged at 10,000g for 5 min at 4 °C and the upper layer (polar fraction) was evaporated to dryness under oxygen-free nitrogen. Residues were reconstituted in 20 μl methoxyamine HCl (20 mg/ml in pyridine) and incubated at 37 °C for 1 h 20 μl MBSTFA was added prior to a further 30-minute incubation at 37 °C. Polar derivatives were analysed by GC–MS using a DB-35MS column (30 m × 0.25 mm internal diameter × 0.25 μm, Agilent J&W Scientific) installed in an Agilent 7890 A gas chromatograph interfaced with an Agilent 5975 C mass spectrometer as previously described [37]. Spectra were quantified in MatLab and natural isotope abundance was corrected using custom-built scripts [38]. Metabolite abundances were normalized to their respective internal standard (in pmol/mg tissue weight) or, for metabolites without a respective internal standard, normalized to 13C valine (relative abundance).
2.3. Quantification and statistical analyses
Differences between genotypes, sexes and/or diets were assessed using two-way analysis of variance with multiple comparisons controlled post-hoc, as appropriate. Further details for individual comparisons are provided in figure legends. Significance testing was performed using GraphPad Prism 10. Data are presented as mean ± standard error, and data were considered significantly different at P < 0.05.
3. Results
3.1. Mitochondrial pyruvate transport maintains triglyceride storage in 3T3L1 adipocytes
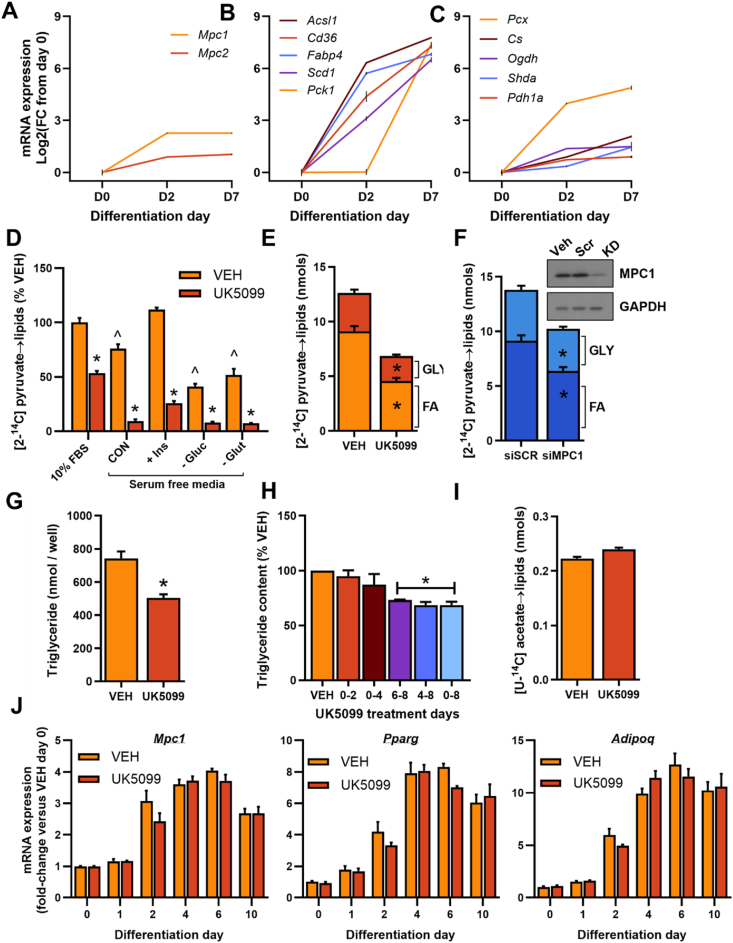
To initially explore the role of mitochondrial pyruvate transport in adipocyte biology, we extracted the transcriptional profile of MPC RNA expression from a publicly available dataset (GSE20696) of 3T3L1 adipocyte differentiation [39]. Both Mpc1 and Mpc2 were rapidly induced during differentiation of pre-adipocytes to adipocytes (Figure 1A). The increase in MPC components was modest compared to several PPARγ targets (Figure 1B), the major transcriptional programme in adipocyte differentiation, but closely followed the induction of downstream enzymatic machinery for mitochondrial pyruvate metabolism (Figure 1C). Interestingly, a recent proteomic screen identified MPC1 and MPC2 as two of the most strongly induced features of 3T3L1 differentiation at the protein level [40]. To directly probe the functional significance of mitochondrial pyruvate transport in adipocytes, we monitored [2–14C] pyruvate incorporation into cellular lipids. Treatment of differentiated 3T3L1 adipocytes cultured in complete growth media with the MPC inhibitor UK5099 (5 μM) led to a ∼50% reduction in pyruvate incorporation into the total lipid pool (Figure 1D). However, near-complete suppression of pyruvate-driven lipid synthesis was observed when adipocytes were treated with UK5099 in serum-free media (Figure 1D). Pyruvate flux into lipids was stimulated by insulin and was partially dependent upon both glucose and glutamine availability but was abrogated by UK5099 treatment regardless of substrate conditions (Figure 1D).
Figure 1.
Mitochondrial pyruvate transport maintains triglyceride storage in 3T3L1 adipocytes. (A–C) Analysis of selected genes from GSE20696 showing mRNA expression during the differentiation of 3T3L1 pre-adipocytes (day 0) to adipocytes (days 2 and day 7), including MPC components (A), the most significantly induced genes (B), and genes involved in mitochondrial pyruvate metabolism (C). Data are expressed as Log base 2 of the fold change from day 0. (D–F) Incorporation of [2–14C] pyruvate into total lipids (D), or the fatty acyl or glycerol-glyceride fraction of lipids (E – F) following treatment of differentiated 3T3L1 adipocytes with 5 μM UK5099 (D–E) or siRNA against MPC1 or scramble control (F). (G–H) Total triglyceride accumulation in adipocytes treated with 5 μM UK5099 either throughout (G), or at various time points during (H), differentiation. (I) Incorporation of [U–14C] acetate into total lipids in differentiated 3T3L1 adipocytes treated with or without 5 μM UK5099. (J) mRNA expression of selected genes in 3T3L1 adipocytes treated with DMSO vehicle (VEH) or 5 μM UK5099 throughout differentiation, normalized to 18S control and presented relative to VEH day 0. Data are mean ± SE for at least three experimental replicates (different cell passages). ∗P < 0.05 vs control, ^P < 0.05 vs 10% FBS condition in (D) by paired t-test (E - F and I), one-way ANOVA (H) or 2-way ANOVA (D).
Incorporation of pyruvate into the total lipid pool reflects the de novo synthesis (and subsequent esterification) of either fatty acids or glycerol-3-phosphate (gly-3P). Tracing pyruvate into the fatty acyl or gly-3P moieties of total lipids demonstrated that both these pathways were sensitive to inhibition by UK5099 (Figure 1E). The reliance of pyruvate-driven lipogenesis on MPC-linked metabolism was further validated in 3T3-L1 adipocytes transfected with Mpc1 siRNA to deplete MPC abundance by ∼50% (Figure 1F and Supplementary Fig. 1). Since precursors other than pyruvate (e.g. glutamine) could support anabolic pathways in adipocytes [41], we next assessed the overall dependence of triglyceride accumulation on mitochondrial pyruvate transport. Treatment with UK5099 during adipocyte differentiation, despite the presence of 2 mM glutamine, resulted in a 35% reduction in total triglyceride content (Figure 1G). Notably, delaying UK5099 treatment until the end of differentiation (days 6–8) suppressed triglyceride levels to a similar extent as to when UK5099 treatment was maintained throughout differentiation (Figure 1H), whereas treatment on days 0–4 had a negligible impact. Importantly, lipogenic pathways downstream of the MPC were unaffected by UK5099 treatment, as shown by the preservation of [U–14C] acetate incorporation into the triglyceride pool (Figure 1I). Moreover, UK5099 treatment had no impact on Mpc1 mRNA expression or on the induction of adipogenic genes during 3T3L1 differentiation (Figure 1J and Supplemental Fig. 1C). These data support previous observations that the MPC is dispensable for the early events of adipogenesis [26], but also now illustrate an obligate role for pyruvate-driven metabolism in maintaining triglyceride accumulation in differentiated adipocytes.
3.2. Adipose tissue MPC expression is altered in metabolically compromised mice and humans
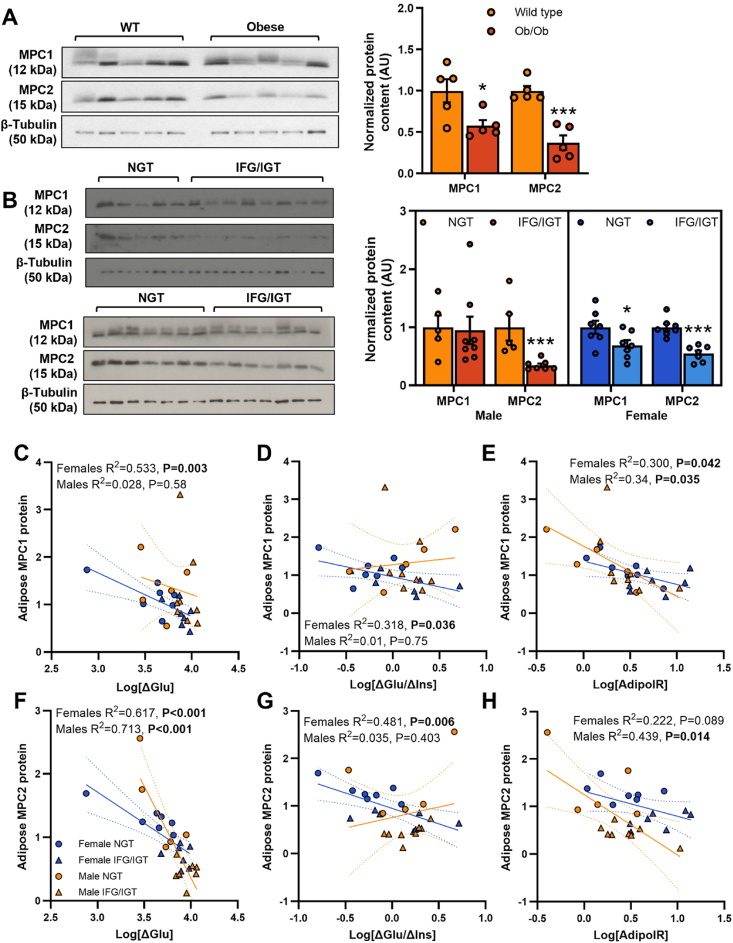
The ability to accumulate triglycerides is an important function of healthy adipose tissue and this becomes dysregulated under metabolic stressors such as obesity and type 2 diabetes [42,43]. To establish the pathological relevance of mitochondrial pyruvate transport in mature adipose tissue, we quantified adipose MPC expression in tissue from metabolically compromised mice and humans. Both MPC1 and MPC2 proteins were significantly lower in the subcutaneous inguinal WAT (iWAT) from obese versus lean mice (Figure 2A). Similarly, MPC1 protein expression was reduced in subcutaneous WAT from female (but not male) prediabetic human subjects displaying impaired fasting glucose, impaired glucose tolerance, and impaired adipose function compared to control subjects with normal glucose tolerance (Figure 2B and Supplementary Fig. 2). MPC2 protein expression was reduced in both prediabetic females and prediabetic males. Moreover, adipose tissue MPC expression was negatively correlated with oral glucose tolerance (Figure 2C–D and F-G) and adipose tissue insulin resistance (Figure 2E,H) across all subjects. Together with our in vitro experiments, these findings highlight a potential link between reduced MPC expression in WAT, adipose tissue function, and the progression of metabolic disease.
Figure 2.
Adipose tissue MPC expression is altered in metabolically compromised mice and humans. (A–B) Normalized, quantified protein expression and representative western blot of mitochondrial pyruvate carrier proteins in subcutaneous adipose tissue from age-matched, chow fed wild type vs genetically obese (ob/ob) male mice (A) and from female and male human subjects with normal glucose tolerance vs impaired fasting glucose plus impaired glucose tolerance (B). (C–H) Pearson correlations between the protein expression of MPC1 (A, D, E) or MPC2 (F, G, H) in subcutaneous adipose tissue and indices of glucose tolerance (C and F), insulin sensitivity (D and G) and adipose insulin resistance (E and H) in human subjects separated by sex. Data are n = 5–8 in each group. ∗P < 0.05, ∗∗∗P < 0.001 vs control group (A and B).
3.3. MPC1 knockdown blunts capacity for triglyceride synthesis in mature adipose tissue
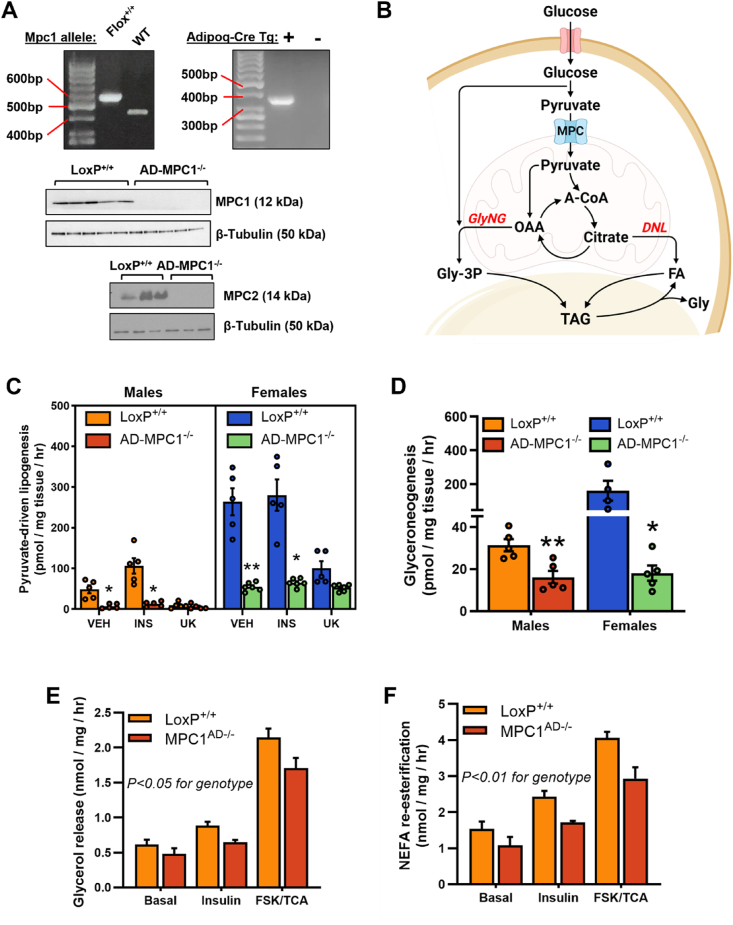
To test the hypothesis that a reduction in adipose tissue MPC expression could exacerbate metabolic dysfunction by restricting the healthy expansion of triglyceride stores, adipose specific Mpc1 knockout mice were generated using the Cre-lox system with an Adiponectin-Cre promoter. Cre recombination in homozygous floxed mice silenced MPC1 protein expression in subcutaneous adipose tissue from Mpc1AD−/− mice and, consistent with previous studies [17], was associated with concomitant loss of MPC2 protein (Figure 3A). To examine the functional significance of adipose MPC ablation, we first compared ex vivo rates of pyruvate-driven lipid synthesis in adipose tissue explants from Mpc1AD−/− mice with LoxP+/+ controls (Figure 3B). Basal and insulin-stimulated rates of lipogenesis were markedly suppressed in visceral epididymal (eWAT, Figure 3C) and iWAT (Supplemental Fig. 3A) explants from Mpc1AD−/− mice. Moreover, residual rates of lipogenesis in Mpc1AD−/− explants were insensitive to further inhibition by UK5099 (Figure 3C), confirming the complete loss of MPC gated lipid synthesis in Mpc1-silenced adipose tissue. In agreement with our in vitro findings in 3T3-L1 cells, pyruvate-driven gly-3P synthesis was also compromised, by 50–90%, in Mpc1AD−/− explants (Figure 3D). Notably, rates of both lipogenesis and gly-3P synthesis were substantially higher in explants from female mice compared to males (Figure 3C,D and Supplemental Fig. 3A), which is consistent with previous reports on sexual dimorphism in the lipogenic capacity of adipose tissue [8,44].
Figure 3.
MPC1 knockdown blunts capacity for triglyceride synthesis in mature adipose tissue. (A) Mpc1 floxed allele and Adipoq-Cre recombination was visualized by semiquantitative RT-PCR and MPC complex proteins knockdown efficacy was verified by western blot of adipose lysates. (B) Schematic illustrating major pathways of pyruvate incorporation into adipose triglycerides. (C - D) Ex vivo incorporation of [2–14C] pyruvate into the fatty acyl (lipogenesis C) or glycerol (glyceroneogenesis D) moieties of total lipids in epididymal adipose explants from male and female Mpc1AD−/− mice or LoxP+/+ controls. (E–F) Rates of glycerol release (E) and non-esterified fatty acid re-esterification (F) from epididymal adipose explants from male Mpc1AD−/− mice or LoxP+/+ controls under basal (unstimulated) conditions or during treatment with insulin or forskolin plus triacsin C. ∗P < 0.05, ∗∗P < 0.01 for Mpc1AD−/− vs LoxP+/+; ^^P < 0.01 for female vs male. Data are mean ± SE for at least five mice per group.
A considerable fraction of the FFA liberated from adipose TAG during lipolysis is re-esterified, requiring a readily available pool of gly-3P [11,12]. We therefore investigated whether the impairment in pyruvate-driven gly-3P synthesis due to MPC deletion also impacted upon the capacity for lipolysis or FFA recycling in WAT. Free glycerol release from iWAT explants was slightly lower in Mpc1AD−/− mice compared to LoxP+/+ controls (Figure 3E), with the greatest effect observed when lipolysis was stimulated with forskolin and triacsin C. A similar trend was observed in eWAT (Supplemental Fig. 3B), indicating a blunted lipolytic capacity in Mpc1AD−/− WAT. Moreover, this reduction in lipolysis was paralleled by lower rates of FFA re-esterification in Mpc1AD−/− explants (Figure 3F and Supplemental Fig. 3C). As a result of the similar reduction in both lipolysis and FFA re-esterification, absolute rates of explant NEFA release were comparable between Mpc1AD−/− and LoxP+/+ mice (Supplemental Figs. 3D–E). Thus, MPC1 knockout compromises the intrinsic capacity of mature adipose tissue for both anabolic (glyceroneogenesis, lipogenesis and FFA recycling) and catabolic (lipolysis) processes relevant to metabolic function.
3.4. Sex-specific dependencies on the MPC for adipose DNL in vivo
Circulating glucose is a major precursor of the intracellular pyruvate pool and an important physiological substrate for the subsequent synthesis of glycerol-3-phosphate and fatty acids in adipose. Accordingly, we next determined whether loss of MPC-gated lipid synthesis impacted upon the incorporation of an oral bolus of [U–14C] glucose into adipose lipids in Mpc1AD−/− and LoxP+/+ mice. Since reliance on adipose DNL may be augmented under certain dietary conditions [8], mice were challenged with either a high-fat western-style diet (WD) or a zero-fat sucrose-enriched diet (ZFD) for 24 weeks.
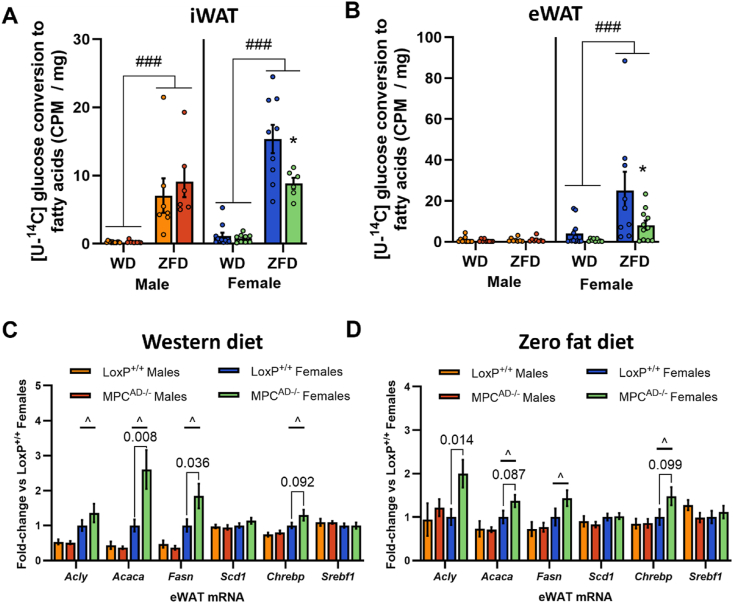
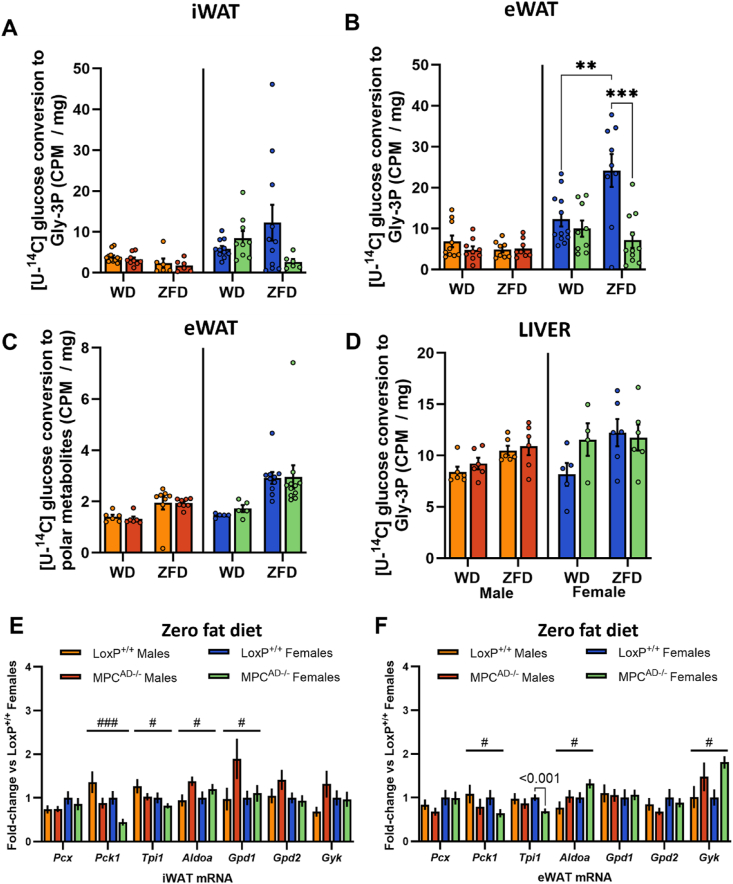
In iWAT, the conversion of glucose into fatty acids (i.e. DNL from glucose) was significantly higher in LoxP+/+ females compared to males and was robustly increased by ZFD versus WD in both sexes (Figure 4A). Whereas the induction of DNL with ZFD was preserved in iWAT from Mpc1AD−/− males, it was blunted by 43% (Figure 4A) in Mpc1AD−/− females. In eWAT, DNL from glucose was unaffected by diet or genotype for male mice but was again markedly reduced in Mpc1AD−/− females fed ZFD (Figure 4B). These data show that reliance on MPC-gated metabolism for glucose flux into fatty acids is greater in female than male adipose.
Figure 4.
Sex-specific dependencies on the MPC for adipose DNL in vivo. (A - B) In vivo incorporation of intraperitoneally administered [U–14C] glucose into the fatty acyl moieties of total lipids (lipogenesis) in inguinal (A) and epididymal (B) adipose tissue depots and (C–D) mRNA expression of lipogenic genes in epididymal adipose tissue (eWAT) for male and female Mpc1AD−/− mice or LoxP+/+ controls fed either a high fat western-style diet (WD, C) or a zero fat, sucrose enriched diet (ZFD, D) for 24 weeks. ∗P < 0.05 for MPCAD−/− vs LoxP+/+; ###P < 0.001 for ZFD vs WD; ^P < 0.05 for female vs male. Data are mean ± SE for 8-10 mice per group.
To gain further insight into the rewiring of adipose DNL in Mpc1AD−/− females, we also measured the mRNA expression of lipogenic enzymes and transcription factors. Consistent with our ex vivo and in vivo tracer data, lipogenic gene expression was typically higher in adipose depots from female mice compared to males, especially in eWAT (Figure 4C–D and Supplemental Figs. 4A and B). Moreover, certain lipogenic genes, including Acly, Acaca and Fasn, were upregulated in adipose depots from female, but not male, Mpc1AD−/− mice (Figure 4C,D and Supplemental Figs. 4A and B). These data suggest that transcriptional upregulation of rate-limiting lipogenic enzymes downstream of the MPC might compensate for the loss of glucose dependent DNL flux in MPC-deficient adipose tissue, especially in female mice.
Blockade of adipose DNL has previously been shown to promote a compensatory increase in liver DNL under ZFD conditions [8]. As with adipose tissue, incorporation of [U–14C] glucose into hepatic fatty acids was greater following ZFD than WD, especially in female mice, but no differences were detected between Mpc1AD−/− and LoxP+/+ mice regardless of sex (Supplemental Fig. 4C). Similarly, lipogenic gene expression, although increased in female livers versus males, was unchanged by the loss of adipose MPC (Supplemental Fig. 4D). Finally, MPC deletion in brown adipocytes is reportedly associated with a compensatory increase in fat oxidation [45]. However, lipid oxidation genes were not impacted by MPC knockdown in Mpc1AD−/− mice, regardless of sex (Supplemental Fig. 4E).
3.5. Sex-specific dependencies on the MPC for adipose glycerol-3-phosphate synthesis in vivo
We next monitored the incorporation of [U–14C] glucose into the Gly-3P moiety of adipose lipids, which captures MPC-dependent glyceroneogenesis following the conversion of glucose to pyruvate, as well as the MPC-independent direct synthesis of Gly-3P from glucose via glycolysis. In iWAT, Gly-3P synthesis was higher in females than males, but largely unaffected by diet or genotype in either sex (Figure 5A). In contrast, whereas eWAT Gly-3P synthesis was again comparable between diets and genotypes in male mice, it was markedly upregulated by ZFD in LoxP+/+ females but entirely blunted in Mpc1AD−/− females (Figure 5B).
Figure 5.
Sex-specific dependencies on the MPC for adipose glycerol-3-phosphate synthesis in vivo. (A - D) In vivo incorporation of intraperitoneally administered [U–14C] glucose into the glycerol moieties of total lipids (glyceroneogenesis A, B, D) or polar metabolites (C) in inguinal adipose (A), epididymal adipose (B - C), or liver (D) for male and female Mpc1AD−/− mice or LoxP+/+ controls fed either a high fat western-style diet (WD) or a zero fat, sucrose enriched diet (ZFD) for 24 weeks. (E–F) mRNA expression of genes involved in glycerol metabolism in inguinal (E) or epididymal (F) adipose tissue for male and female Mpc1AD−/− mice or LoxP+/+ controls fed a zero fat, sucrose enriched diet (ZFD). ∗∗P < 0.01, ∗∗∗P < 0.001 for Mpc1AD−/− vs LoxP+/+; #P < 0.05, ###P < 0.001 main effect of genotype. Data are mean ± SE for 8-10 mice per group.
To confirm that the observed changes in gly-3P synthesis were not related to differences in adipose tissue glucose uptake, we also measured tracer enrichment in the polar fraction. Although flux from [U–14C] glucose into polar metabolites was increased by ZFD, and to a greater extent in female mice, it was unaffected by MPC knockdown (Figure 5C). Similarly, glucose incorporation into hepatic gly-3P was comparable between LoxP+/+ and Mpc1AD−/− mice, regardless of sex or diet (Figure 5D). Thus, glucose trafficking towards gly-3P in visceral adipose is increased under conditions of dietary lipid restriction, but only in female mice and, moreover, this adaptation is entirely reliant upon MPC-mediated glyceroneogenesis.
We also investigated whether the transcriptional regulation of glyceroneogenesis was altered by MPC knockdown. Notably, Pck1, which encodes for the rate-limiting enzyme glyceroneogenic enzyme phosphoenolpyruvate carboxykinase 1, was consistently reduced in adipose depots from ZFD fed Mpc1AD−/− mice, regardless of sex (Figure 5E–F). We also observed changes in Aldoa (increased) and Tpi1 (decreased), both terminal enzymes in the glycolytic generation of glyceraldehyde-3-phosphate, as well as increased Gdp1, responsible for the final step of gly-3P synthesis, in Mpc1AD−/− adipose. Interestingly, glycerol kinase (Gyk) expression was increased in Mpc1AD−/− iWAT (Figure 5F), indicating a potential role for free glycerol as an alternative substrate for Gly-3P synthesis via triglyceride-glycerol recycling [46]. We also measured GYK expression in human adipose tissue, although no differences were observed between female patients with NGT or IFG/IGT (Supplemental Fig. 4F).
3.6. Decreased adipose TCA cycle pool size in female Mpc1AD−/− mice fed a lipid restricted diet
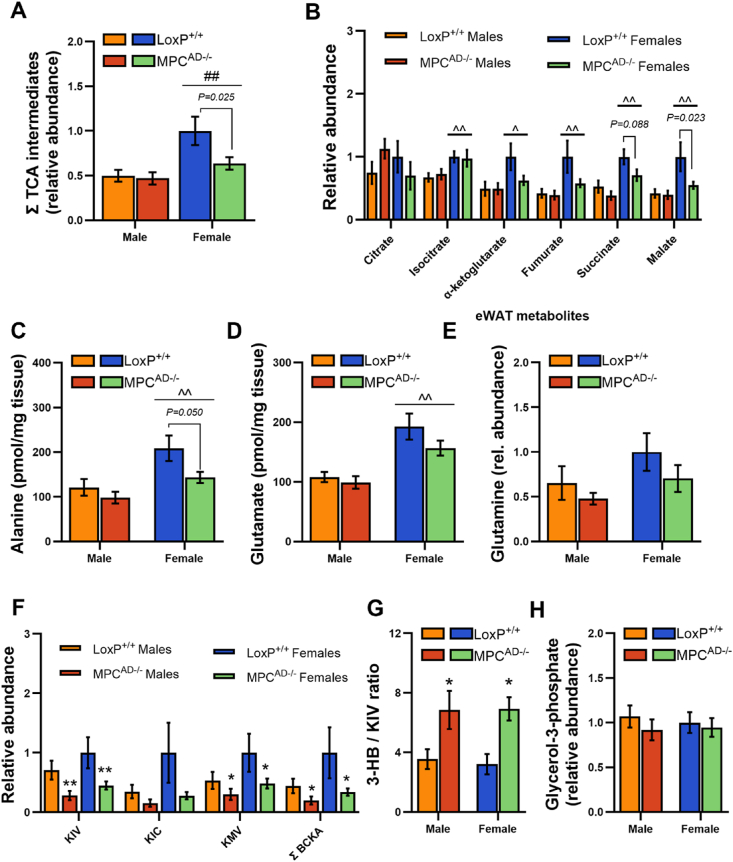
To better understand the metabolic pathways underpinning female-specific reliance on the MPC for DNL and gly-3P synthesis, we performed a targeted analysis of intermediary metabolites in eWAT from ZFD fed mice. We initially focused on intermediates within the TCA cycle, since both DNL and glyceroneogenesis rely on the anaplerotic and cataplerotic pathways of the TCA cycle. The relative pool size of TCA cycle intermediates in female LoxP+/+ adipose was double that of males but was 35% lower in Mpc1AD−/− females (Figure 6A). This was largely driven by reductions in malate and succinate abundance in Mpc1AD−/− females, although other TCA intermediates followed a similar pattern (Figure 6A). Thus, MPC deficiency disrupts the balance between anaplerosis and cataplerosis in adipose from Mpc1AD−/− female, but not male mice, consistent with the sex-specific impairment of adipose DNL and gly-3P synthesis.
Figure 6.
Decreased adipose TCA cycle pool size in female Mpc1AD−/− mice fed a lipid restricted diet Polar metabolite abundances in epididymal adipose tissue from male and female Mpc1AD−/− mice or LoxP+/+ controls fed a zero fat, sucrose enriched diet (ZFD) determined by gas chromatography mass spectrometry. (A) Summed and (B) individual TCA cycle intermediates, (C) alanine, (D) glutamate, (E) glutamine, (F) branched chain alpha keto acids, (G) the ratio of 3-hydroxybutyrate to alpha keto isovalerate and (H) glycerol-3-phosphate. Abundance data represent relative abundances of metabolites (normalized to labeled valine internal standard) except for alanine and glutamate (pmol/mg tissue). ^P < 0.05, ^^P < 0.01 for females vs males; ∗P < 0.05, ∗∗P < 0.01 main effect of genotype. Data are mean ± SE for 7-8 mice per group.
In absence of MPC-gated metabolism, alternate anaplerotic substrates (e.g. amino acids) have been shown to support TCA cycle dependent pathways [17,19]. Alanine levels were lower in adipose tissue from Mpc1AD−/− mice, especially in females (Figure 6C), whilst glutamate (Figure 8D) and glutamine (Figure 6E) levels were not significantly different. Interestingly, branched chain keto acid levels were consistently reduced in Mpc1AD−/− mice, regardless of sex (Figure 6E). Moreover, the ratio of 3-hydroxybutyrate to alpha keto isovalerate, a surrogate for branched chain keto dehydrogenase activity, was robustly increased in Mpc1AD−/− mice (Figure 6F). These changes suggest that flux through branched chain amino acid (BCAA) catabolism is increased in MPC null adipose tissue and are consistent with recent reports following MPC inhibition in hepatocytes [47]. Despite reductions in glucose driven gly-3P synthesis in Mpc1AD−/− females (Figure 5B), levels of adipose gly-3P were comparable between Mpc1AD−/− mice and LoxP+/+ controls (Figure 6H).
Figure 8.
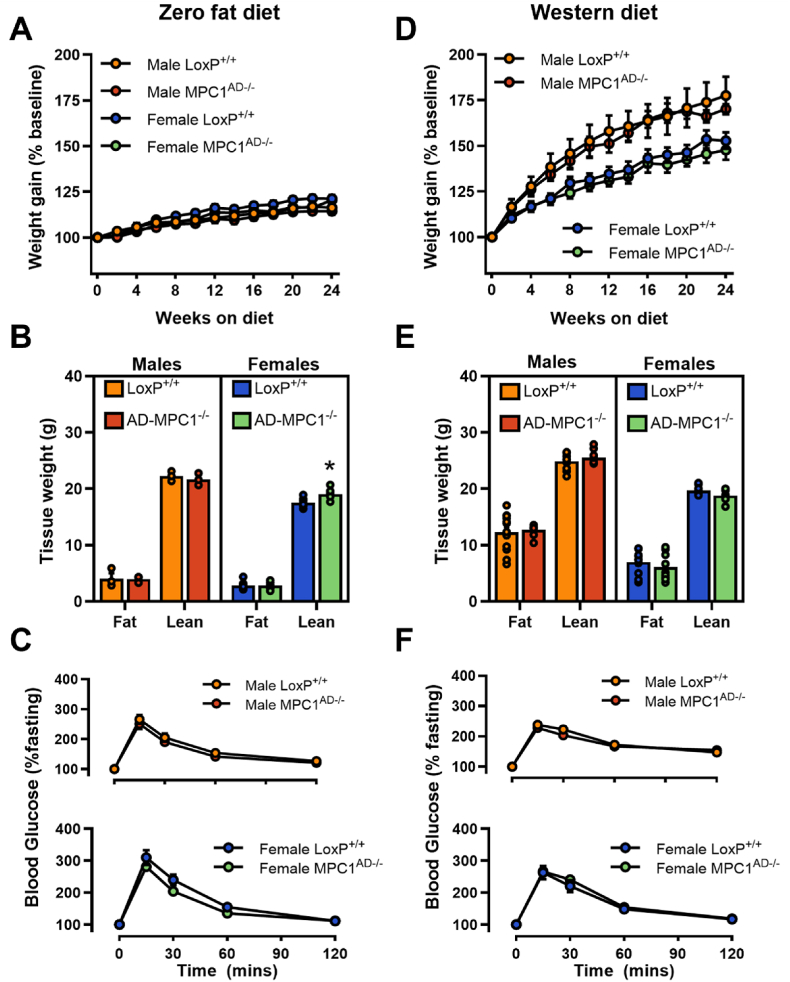
Total adiposity is maintained in Mpc1AD−/− mice fed a lipid restricted diet. (A, D) Percentage body weight gain, (B, E) total fat and lean mass, and (C, F) incremental oral glucose tolerance for male and female MPCAD−/− mice or LoxP+/+ controls fed either a zero fat, sucrose enriched diet (A–C) or a high fat western-style diet (D–F) for 24 weeks. ∗P < 0.05 for MPCAD−/− vs LoxP+/+. Data are mean ± SE for 8-10 mice per group.
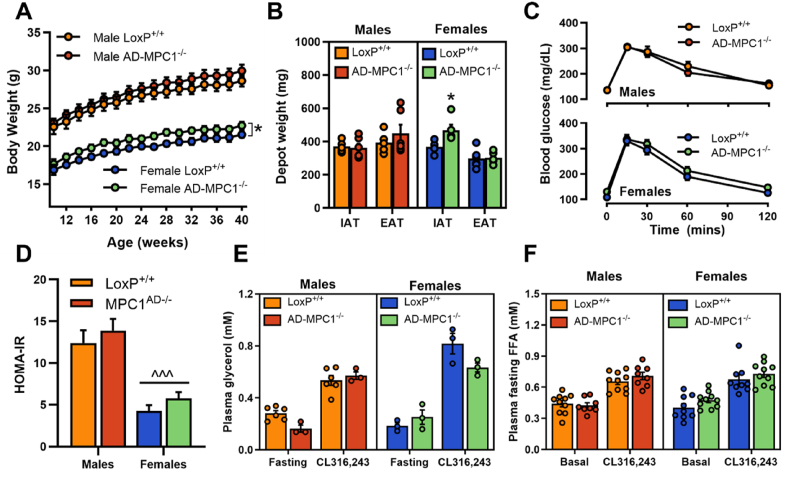
3.7. Whole body lipid and glucose homeostasis are preserved in Mpc1AD−/− mice fed a chow diet
Finally, we determined whether the observed alterations in adipose tissue lipid metabolism impacted weight gain and glucose homeostasis. Despite the observed ex vivo deficit in adipose triglyceride synthesis, weight gain was comparable between Mpc1AD−/− and LoxP+/+ male mice and was marginally (∼5%) increased in Mpc1AD−/− females, when mice were fed a standard chow diet (Figure 7A). Similarly, adipose depot weights were unchanged in Mpc1AD−/− males, whereas iWAT mass was slightly greater in Mpc1AD−/− females compared to LoxP+/+ controls (Figure 7B). No differences were found in glucose tolerance (Figure 7C) or HOMA-IR (fasting glucose x insulin; Figure 7D) between genotypes in either sex. Moreover, circulating free glycerol (Figure 7E) and non-esterified fatty acids (Figure 7F) were unaffected by the loss of adipose MPC1, either in the fasting state or upon simulation with the β3-adrenergic antagonist CL316,243 (10 mg/kg intraperitoneal injection). Together these data demonstrate that, under standard (chow) dietary conditions and regardless of biological sex, mitochondrial pyruvate transport in adipose is not required for the maintenance of whole-body glycemic or lipolytic control in vivo.
Figure 7.
Whole body lipid and glucose homeostasis are preserved in chow fed Mpc1AD−/− mice. (A) Total body weight, (B) inguinal and epididymal adipose depot weights, (C) oral glucose tolerance, (D) HOMA-IR index of insulin resistance, (E - F) fasting and CL316,243-stimulated plasma glycerol (E) and non-esterified fatty acids (F) in male and female Mpc1AD−/− mice or LoxP+/+ controls fed a standard chow diet. ∗P < 0.05 for MPCAD−/− vs LoxP+/+; ^^^P < 0.001 for female vs male. Data are mean ± SE for 8-10 mice per group.
3.8. Total adiposity is maintained in Mpc1AD−/− mice fed a lipid restricted diet
As with chow fed mice, no overt metabolic phenotypes were evident when mice were fed either a WD or ZFD, with weight gain (Figure 8A,D and Supplemental Figs. 5A and C), body composition (Figure 8B,E) and glucose tolerance (Figure 8C,F and Supplemental Figs. 5B and D) comparable between Mpc1AD−/− mice and LoxP+/+ controls. Thus, despite intrinsic impairments in pyruvate driven adipose DNL (Figure 3B and Supplemental Fig. 3A) and sex-specific impairments in glucose-mediated triglyceride assembly under conditions of dietary lipid restriction (Figure 4A–B and Figure 5A–B), total body fat accretion was entirely preserved in Mpc1AD−/− mice regardless of sex or diet.
4. Discussion
Sexual dimorphism in adipose biology is believed to contribute to differential risk profiles for cardiometabolic diseases in males versus females, yet the molecular mechanisms underpinning these differences remain unclear. Here we reveal a sex-dependent role for the mitochondrial pyruvate carrier in adipose tissue glucose metabolism. Adipocyte-specific MPC depletion compromises the synthesis of fatty acids and glycerol-3-phosphate from glucose in female mice, whereas glucose trafficking into adipose triglycerides is entirely preserved in Mpc1AD−/− males. Notably, these alterations in adipose glucose metabolism were only exposed when female mice were fed a carbohydrate rich, lipid restricted diet, consistent with an increased reliance on carbohydrates for endogenous triglyceride synthesis under these conditions [9]. However, loss of adipocyte MPC did not impair body fat accumulation during dietary lipid restriction, regardless of sex. Instead, our findings support a compensatory role for MPC-independent pathways in sustaining triglyceride assembly and storage from non-glucose precursors in Mpc1AD−/− female mice.
Inefficient deposition of dietary nutrients in adipose triglycerides is a purported pathophysiological feature of human obesity and insulin resistance [10]. Whereas research in the obesity field has typically focused on the relevance of mitochondrial content and respiratory capacity, recent findings also highlight the involvement of adipocyte mitochondria in the regulation of nutrient storage [48]. Consistent with the central role of mitochondrial pyruvate transport in intermediary metabolism [54], our in vitro and ex vivo experiments confirmed that pharmacological blockade or genetic depletion of the MPC impairs the de novo synthesis of fatty acids and glycerol-3-phosphate from pyruvate in 3T3L1 adipocytes, whilst these pathways are almost completely abolished in mature adipose tissue explants from Mpc1AD−/− mice. Altogether, these experiments indicate that at least one third of triglyceride accumulation in adipocytes depends upon MPC-gated metabolism, with the remainder presumably supported by alternate substrates which bypass the MPC [17,19]. Despite confirming an obligate role of the MPC for carbohydrate storage in adipose explants, Mpc1AD−/− mice maintained comparable fat mass to control mice, even under conditions of dietary lipid restriction. This discrepancy suggests that in absence of MPC mediated DNL in adipose, whole body lipid synthesis is sustained either through i) MPC-independent DNL within adipocytes or ii) from non-adipose (e.g. hepatic) DNL. As will be discussed, our findings imply that the precise mechanism(s) may depend upon biological sex.
Carbohydrate flux into adipose triglycerides, whether measured ex vivo (from pyruvate) or in vivo (from glucose), was consistently greater in female adipose tissue than males. This was further supported by higher lipogenic gene expression in female adipose and parallels previous findings of higher rates of glucose storage in female adipose compared to males [8,44]. This higher capacity for adipose DNL could reflect a greater mitochondrial capacity in female adipose compared to males [49,50] and, as such, an increased reliance on adipose tissue for whole body lipid synthesis in females, whereas males may obtain a greater contribution from non-adipose (i.e. liver) lipogenesis. This concept was previously illustrated in ZFD-fed mice deficient for adipose ATP citrate lyase (ACLY) [8] and is further supported by the current study. Thus, in the context of a relatively low requirement for adipose DNL, it is perhaps unsurprising that MPC-gated metabolism is dispensable for adipose triglyceride synthesis in male mice. In contrast to males, female Mpc1AD−/− mice displayed intrinsic alterations in adipose metabolism, characterized by impaired nutrient storage pathways, reductions in TCA cycle intermediate pool sizes, and compensatory changes in lipogenic gene expression. Together, these data suggest that mitochondrial pyruvate transport may only be rate limiting for higher rates of DNL or gly-3P synthesis, including those observed in females consuming a ZFD.
A key phenotypic difference between female mice lacking adipose MPC and those lacking adipose ACLY is that, when consuming ZFD, Mpc1AD−/− females did not become lipodystrophic nor develop any overt systemic metabolic abnormalities compared to littermate controls. Thus, whereas adipose ACLY appears conditionally essential for body fat accretion in females [8], reliance on MPC-gated metabolism for adipose accumulation may be obviated by compensatory pathways supporting adipose DNL from non-glucose precursors. Indeed, prior work has demonstrated considerable redundancy in the regulation of metabolic processes by the MPC [17,19,51]. For example, cytosolic conversion of pyruvate to alanine circumvents mitochondrial pyruvate transport to support hepatic gluconeogenesis [19]. Interestingly, ZFD-fed Mpc1AD−/− females displayed reduced adipose alanine concentrations, suggesting increased alanine utilization under these conditions. While studies in brown adipocytes have suggested that fatty acid oxidation may compensate for MPC deficiency [22,45], transcriptional markers of lipid oxidation were unchanged in the current study. Indeed, succinate and malate concentrations were also reduced which, consistent with MPC inhibition in hepatocytes [51], underscores the obligate dependence on the direct import of mitochondrial pyruvate via the MPC to maintain TCA cycle intermediate pool sizes. Notably, lipogenic gene expression was increased in Mpc1AD−/− female adipose, suggesting transcriptional compensation for the loss of glucose-derived lipogenic acetyl-CoA. Further experiments should consider whether cytosolic acetyl-CoA provision (e.g. from acetate) directly sustains adipose DNL in the face of MPC blockade in female adipose.
In addition to supporting adipose DNL, pyruvate is an important substrate for the de novo synthesis of gly-3P, required for acylglycerol assembly and accumulation. Glyceroneogenesis shares its initial enzymatic steps with the gluconeogenic pathway, diverging beyond the metabolism of dihydroxyacetone phosphate. Here we confirmed that genetic or pharmacological blockade of the MPC, like its role in pyruvate mediated gluconeogenesis in the liver [17,19], impairs glyceroneogenesis from pyruvate in adipose tissue. Interestingly, the incorporation of circulating glucose into the glycerol fraction of acylglycerols was also impaired in MPC deficient adipose in vivo, but only in Mpc1AD−/− females fed a ZFD. These findings mirror the partial suppression of glucose synthesis from glycerol via the indirect pathway (i.e. gluconeogenesis) in MPC deficient livers [51]. Our tracer methodology cannot discriminate between the direct and indirect synthesis of glycerol-3-phopshate from glucose. However, based upon the comparable indices of adipose glucose uptake between Mpc1AD−/− and LoxP+/+ mice (Figure 7C), together with the finding that levels of free gly-3P in adipose were similar across genotypes (Figure 8H), it is likely that the direct pathway of gly-3P synthesis from glucose was preserved in Mpc1AD−/− mice. This implies that adipose glyceroneogenesis was specifically compromised in Mpc1AD−/− females fed a ZFD. Nevertheless, the maintenance of fat mass in Mpc1AD−/− females illustrates that, even under lipid restricted conditions, gly-3P generation from glucose was not limiting to triglyceride accumulation. Our transcriptional data hint towards the upregulation of glycerol kinase as a potential compensatory mechanism. Whereas glycerol kinase activity is considered negligible in adipose under normal conditions, it is reportedly induced by the PPARγ agonists thiazolidinediones [46]. Interestingly, these antidiabetic drugs are also purported inhibitors of the MPC [52], highlighting the potential for crosstalk between mitochondrial pyruvate transport and distal metabolic processes. Notably, the increase in glycerol kinase in Mpc1 knockout adipose was not sex-specific, indicating that alternate pathways of gly-3P synthesis also compensate for the reduction in glucose-derived gly-3P in female mice.
It should be acknowledged that tracing pathways of [U–14C] glucose in vivo is complicated by the potential recycling of tracer between tissues (e.g. liver, skeletal muscle). However, based upon prior experiments, the contribution of non-adipose tracer metabolism to circulating labeled metabolites is expected to have a relatively minor influence on the measurement of adipose DNL or glycerol-3-phosphate synthesis over the time course of the studies performed here [53].
In conclusion, we demonstrate that the mitochondrial pyruvate carrier facilitates the increased trafficking of glucose into both fatty acids and gly-3P in female adipose tissue under lipid restricted conditions. Disruption of this process does not alter the whole-body phenotypic adaptation to dietary stress, highlighting the remarkable flexibility for mitochondrial substrate selection towards metabolic homeostasis. Our findings are highly consistent with a recent report that loss of liver MPC constrains hepatic DNL during fasted refeeding in mice without impacting hepatic steatosis or circulating lipid concentrations [51]. However, our findings provide the first evidence of sexual dimorphism in MPC-gated metabolism and thus advance understanding of the molecular mechanisms underlying sex differences in adipose biology and metabolic physiology.
CRediT authorship contribution statement
Christopher E. Shannon: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Terry Bakewell: Writing – review & editing, Project administration, Methodology, Investigation. Marcel J. Fourcaudot: Writing – review & editing, Project administration, Methodology, Investigation. Iriscilla Ayala: Writing – review & editing, Project administration, Methodology, Investigation. Annie A. Smelter: Formal analysis, Investigation. Edgar A. Hinostroza: Formal analysis, Investigation. Giovanna Romero: Writing – review & editing, Methodology, Investigation. Mara Asmis: Writing – review & editing, Methodology, Investigation. Leandro C. Freitas Lima: Writing – review & editing, Methodology, Investigation. Martina Wallace: Writing – review & editing, Resources, Methodology, Investigation, Formal analysis, Conceptualization. Luke Norton: Writing – review & editing, Validation, Supervision, Resources, Project administration, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
C.E.S. is supported by funding from the European Union's Horizon 2020 research and innovation programme under Marie Skłodowska-Curie grant agreement No 945425 and by a Research and Knowledge Exchange Award from The Physiology Society (Grant number TPSRKE02). L.N. is supported by funding from the NIDDK (R01DK128247). The polar metabolomics data was generated with support from Dr Xiaofei Yin and Professor Lorraine Brennan within the UCD Conway Institute of Biomolecular and Biomedical Research Metabolomic Facility.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2024.102005.
Contributor Information
Christopher E. Shannon, Email: christopher.shannon@ucd.ie.
Luke Norton, Email: nortonl@uthscsa.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Frayn K.N. Adipose tissue as a buffer for daily lipid flux. Diabetologia. 2002;45(9):1201–1210. doi: 10.1007/s00125-002-0873-y. [DOI] [PubMed] [Google Scholar]
- 2.Virtanen K.A., Peltoniemi P., Marjamäki P., Asola M., Strindberg L., Parkkola R., et al. Human adipose tissue glucose uptake determined using [(18)F]-fluoro-deoxy-glucose ([(18)F]FDG) and PET in combination with microdialysis. Diabetologia. 2001;44(12):2171–2179. doi: 10.1007/s001250100026. [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo R.A. Dysfunctional fat cells, lipotoxicity and type 2 diabetes. Int J Clin Pract. 2004;Suppl(143):9–21. doi: 10.1111/j.1368-504x.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 4.Norton L., Shannon C., Gastaldelli A., DeFronzo R.A. Insulin: the master regulator of glucose metabolism. Metabolism. 2022;129 doi: 10.1016/j.metabol.2022.155142. [DOI] [PubMed] [Google Scholar]
- 5.Forner F., Kumar C., Luber C.A., Fromme T., Klingenspor M., Mann M. Proteome differences between brown and white fat mitochondria reveal specialized metabolic functions. Cell Metabol. 2009;10(4):324–335. doi: 10.1016/j.cmet.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Rotondo F., Ho-Palma A.C., Remesar X., Fernández-López J.A., Romero M.d.M., Alemany M. Glycerol is synthesized and secreted by adipocytes to dispose of excess glucose, via glycerogenesis and increased acyl-glycerol turnover. Sci Rep. 2017;7(1):8983. doi: 10.1038/s41598-017-09450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strawford A., Antelo F., Christiansen M., Hellerstein M.K. Adipose tissue triglyceride turnover, de novo lipogenesis, and cell proliferation in humans measured with 2H2O. Am J Physiol Endocrinol Metab. 2004;286(4):E577–E588. doi: 10.1152/ajpendo.00093.2003. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez S., Viola J.M., Torres A., Wallace M., Trefely S., Zhao S., et al. Adipocyte ACLY facilitates dietary carbohydrate handling to maintain metabolic homeostasis in females. Cell Rep. 2019;27(9):2772–2784.e2776. doi: 10.1016/j.celrep.2019.04.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aarsland A., Chinkes D., Wolfe R.R. Hepatic and whole-body fat synthesis in humans during carbohydrate overfeeding. Am J Clin Nutr. 1997;65(6):1774–1782. doi: 10.1093/ajcn/65.6.1774. [DOI] [PubMed] [Google Scholar]
- 10.Allister C.A., Liu L.F., Lamendola C.A., Craig C.M., Cushman S.W., Hellerstein M.K., et al. In vivo 2H2O administration reveals impaired triglyceride storage in adipose tissue of insulin-resistant humans. J Lipid Res. 2015;56(2):435–439. doi: 10.1194/jlr.M052860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nye C.K., Hanson R.W., Kalhan S.C. Glyceroneogenesis is the dominant pathway for triglyceride glycerol synthesis in vivo in the rat. J Biol Chem. 2008;283(41):27565–27574. doi: 10.1074/jbc.M804393200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reshef L., Olswang Y., Cassuto H., Blum B., Croniger C.M., Kalhan S.C., et al. Glyceroneogenesis and the triglyceride/fatty acid cycle. J Biol Chem. 2003;278(33):30413–30416. doi: 10.1074/jbc.R300017200. [DOI] [PubMed] [Google Scholar]
- 13.Felix J.B., Cox A.R., Hartig S.M. Acetyl-CoA and metabolite fluxes regulate white adipose tissue expansion. Trends Endocrinol Metabol. 2021;32(5):320–332. doi: 10.1016/j.tem.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bricker D.K., Taylor E.B., Schell J.C., Orsak T., Boutron A., Chen Y.C., et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science. 2012;337(6090):96–100. doi: 10.1126/science.1218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herzig S., Raemy E., Montessuit S., Veuthey J.L., Zamboni N., Westermann B., et al. Identification and functional expression of the mitochondrial pyruvate carrier. Science. 2012;337(6090):93–96. doi: 10.1126/science.1218530. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Caggiano M., Kamynina A., Francois A.A., Prysyazhna O., Eykyn T.R., Krasemann S., et al. Mitochondrial pyruvate carrier abundance mediates pathological cardiac hypertrophy. Nat Metab. 2020;2(11):1223–1231. doi: 10.1038/s42255-020-00276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray L.R., Sultana M.R., Rauckhorst A.J., Oonthonpan L., Tompkins S.C., Sharma A., et al. Hepatic mitochondrial pyruvate carrier 1 is required for efficient regulation of gluconeogenesis and whole-body glucose homeostasis. Cell Metabol. 2015;22(4):669–681. doi: 10.1016/j.cmet.2015.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang S.C., Smith A.M., Everts B., Colonna M., Pearce E.L., Schilling J.D., et al. Metabolic reprogramming mediated by the mTORC2-IRF4 signaling Axis is essential for macrophage alternative activation. Immunity. 2016;45(4):817–830. doi: 10.1016/j.immuni.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCommis K.S., Chen Z., Fu X., McDonald W.G., Colca J.R., Kletzien R.F., et al. Loss of mitochondrial pyruvate carrier 2 in the liver leads to defects in gluconeogenesis and compensation via pyruvate-alanine cycling. Cell Metabol. 2015;22(4):682–694. doi: 10.1016/j.cmet.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCommis K.S., Hodges W.T., Bricker D.K., Wisidagama D.R., Compan V., Remedi M.S., et al. An ancestral role for the mitochondrial pyruvate carrier in glucose-stimulated insulin secretion. Mol Metabol. 2016;5(8):602–614. doi: 10.1016/j.molmet.2016.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCommis K.S., Kovacs A., Weinheimer C.J., Shew T.M., Koves T.R., Ilkayeva O.R., et al. Nutritional modulation of heart failure in mitochondrial pyruvate carrier-deficient mice. Nat Metab. 2020;2(11):1232–1247. doi: 10.1038/s42255-020-00296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panic V., Pearson S., Banks J., Tippetts T.S., Velasco-Silva J.N., Lee S., et al. Mitochondrial pyruvate carrier is required for optimal brown fat thermogenesis. Elife. 2020;9 doi: 10.7554/eLife.52558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shannon C.E., Daniele G., Galindo C., Abdul-Ghani M.A., DeFronzo R.A., Norton L. Pioglitazone inhibits mitochondrial pyruvate metabolism and glucose production in hepatocytes. FEBS J. 2017;284(3):451–465. doi: 10.1111/febs.13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma A., Oonthonpan L., Sheldon R.D., Rauckhorst A.J., Zhu Z., Tompkins S.C., et al. Impaired skeletal muscle mitochondrial pyruvate uptake rewires glucose metabolism to drive whole-body leanness. Elife. 2019;8 doi: 10.7554/eLife.45873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wenes M., Jaccard A., Wyss T., Maldonado-Pérez N., Teoh S.T., Lepez A., et al. The mitochondrial pyruvate carrier regulates memory T cell differentiation and antitumor function. Cell Metabol. 2022;34(5):731–746.e739. doi: 10.1016/j.cmet.2022.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burrell J.A., Richard A.J., King W.T., Stephens J.M. Mitochondrial pyruvate carriers are not required for adipogenesis but are regulated by high-fat feeding in Brown adipose tissue. Obesity. 2020;28(2):293–302. doi: 10.1002/oby.22678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou S., Zhu L., Huang K., Luo H., Xu W., He X. Adipose tissues of MPC1. PeerJ. 2018;6 doi: 10.7717/peerj.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edens N.K., Fried S.K., Kral J.G., Hirsch J., Leibel R.L. In vitro lipid synthesis in human adipose tissue from three abdominal sites. Am J Physiol. 1993;265(3 Pt 1):E374–E379. doi: 10.1152/ajpendo.1993.265.3.E374. [DOI] [PubMed] [Google Scholar]
- 29.Stanhope K.L., Schwarz J.M., Keim N.L., Griffen S.C., Bremer A.A., Graham J.L., et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119(5):1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Després J.P., Couillard C., Gagnon J., Bergeron J., Leon A.S., Rao D.C., et al. Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: the Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) family study. Arterioscler Thromb Vasc Biol. 2000;20(8):1932–1938. doi: 10.1161/01.atv.20.8.1932. [DOI] [PubMed] [Google Scholar]
- 31.Chen X., Ayala I., Shannon C., Fourcaudot M., Acharya N.K., Jenkinson C.P., et al. The diabetes gene and wnt pathway effector TCF7L2 regulates adipocyte development and function. Diabetes. 2018;67(4):554–568. doi: 10.2337/db17-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kilroy G., Burk D.H., Floyd Z.E. High efficiency lipid-based siRNA transfection of adipocytes in suspension. PLoS One. 2009;4(9) doi: 10.1371/journal.pone.0006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winnier D.A., Fourcaudot M., Norton L., Abdul-Ghani M.A., Hu S.L., Farook V.S., et al. Transcriptomic identification of ADH1B as a novel candidate gene for obesity and insulin resistance in human adipose tissue in Mexican Americans from the Veterans Administration Genetic Epidemiology Study (VAGES) PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0119941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gastaldelli A., Gaggini M., DeFronzo R.A. Role of adipose tissue insulin resistance in the natural history of type 2 diabetes: results from the san Antonio metabolism study. Diabetes. 2017;66(4):815–822. doi: 10.2337/db16-1167. [DOI] [PubMed] [Google Scholar]
- 35.Folch J., Lees M., Stanley G.H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 36.Baskaran P., Thyagarajan B. Measurement of basal and forskolin-stimulated lipolysis in inguinal adipose fat pads. J Vis Exp. 2017;125 doi: 10.3791/55625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cordes T., Metallo C.M. Quantifying intermediary metabolism and lipogenesis in cultured mammalian cells using stable isotope tracing and mass spectrometry. Methods Mol Biol. 2019:219–241. doi: 10.1007/978-1-4939-9236-2_14. [DOI] [PubMed] [Google Scholar]
- 38.Fernandez C.A., Des Rosiers C., Previs S.F., David F., Brunengraber H. Correction of 13C mass isotopomer distributions for natural stable isotope abundance. J Mass Spectrom. 1996;31(3):255–262. doi: 10.1002/(SICI)1096-9888(199603)31:3<255::AID-JMS290>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 39.Mikkelsen T.S., Xu Z., Zhang X., Wang L., Gimble J.M., Lander E.S., et al. Comparative epigenomic analysis of murine and human adipogenesis. Cell. 2010;143:156–169. doi: 10.1016/j.cell.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi S., Goswami N., Schmidt F. Comparative proteomic profiling of 3T3-L1 adipocyte differentiation using SILAC quantification. J Proteome Res. 2020;19(12):4884–4900. doi: 10.1021/acs.jproteome.0c00475. [DOI] [PubMed] [Google Scholar]
- 41.Roberts L.D., Virtue S., Vidal-Puig A., Nicholls A.W., Griffin J.L. Metabolic phenotyping of a model of adipocyte differentiation. Physiol Genom. 2009;39(2):109–119. doi: 10.1152/physiolgenomics.90365.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palavicini J.P., Chavez-Velazquez A., Fourcaudot M., Tripathy D., Pan M., Norton L., et al. The insulin-sensitizer pioglitazone remodels adipose tissue phospholipids in humans. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.784391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pietiläinen K.H., Róg T., Seppänen-Laakso T., Virtue S., Gopalacharyulu P., Tang J., et al. Association of lipidome remodeling in the adipocyte membrane with acquired obesity in humans. PLoS Biol. 2011;9(6) doi: 10.1371/journal.pbio.1000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macotela Y., Boucher J., Tran T.T., Kahn C.R. Sex and depot differences in adipocyte insulin sensitivity and glucose metabolism. Diabetes. 2009;58(4):803–812. doi: 10.2337/db08-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veliova M., Ferreira C.M., Benador I.Y., Jones A.E., Mahdaviani K., Brownstein A.J., et al. Blocking mitochondrial pyruvate import in brown adipocytes induces energy wasting via lipid cycling. EMBO Rep. 2020;21 doi: 10.15252/embr.201949634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guan H.P., Li Y., Jensen M.V., Newgard C.B., Steppan C.M., Lazar M.A. A futile metabolic cycle activated in adipocytes by antidiabetic agents. Nat Med. 2002;8(10):1122–1128. doi: 10.1038/nm780. [DOI] [PubMed] [Google Scholar]
- 47.Ferguson D., Eichler S.J., Yiew N.K.H., Colca J.R., Cho K., Patti G.J., et al. Mitochondrial pyruvate carrier inhibition initiates metabolic crosstalk to stimulate branched chain amino acid catabolism. Mol Metabol. 2023;70 doi: 10.1016/j.molmet.2023.101694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu Q., An Y.A., Scherer P.E. Mitochondrial regulation and white adipose tissue homeostasis. Trends Cell Biol. 2022;32(4):351–364. doi: 10.1016/j.tcb.2021.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Norheim F., Hasin-Brumshtein Y., Vergnes L., Chella Krishnan K., Pan C., Seldin M.M., et al. Gene-by-Sex interactions in mitochondrial functions and cardio-metabolic traits. Cell Metabol. 2019;29:932–949.e934. doi: 10.1016/j.cmet.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chella Krishnan K., Vergnes L., Acín-Pérez R., Stiles L., Shum M., Ma L., et al. Sex-specific genetic regulation of adipose mitochondria and metabolic syndrome by Ndufv2. Nat Metab. 2021;3:1552–1568. doi: 10.1038/s42255-021-00481-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yiew N.K.H., Deja S., Ferguson D., Cho K., Jarasvaraparn C., Jacome-Sosa M., et al. Effects of hepatic mitochondrial pyruvate carrier deficiency on. iScience. 2023;26(11) doi: 10.1016/j.isci.2023.108196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Divakaruni A.S., Wiley S.E., Rogers G.W., Andreyev A.Y., Petrosyan S., Loviscach M., et al. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proc Natl Acad Sci U S A. 2013;110:5422–5427. doi: 10.1073/pnas.1303360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Björntorp P., Krotkiewski M., Larsson B., Somlo-Szücs Z. Effects of feeding states on lipid radioactivity in liver, muscle and adipose tissue after injection of labelled glucose in the rat. Acta Physiol Scand. 1970;80(1):29–38. doi: 10.1111/j.1748-1716.1970.tb04766.x. [DOI] [PubMed] [Google Scholar]
- 54.Nathaniel M.V., Ajit S.D., Courtney R.G., Seth J.P., Robert R.H., Theodore P.C., et al. Regulation of substrate utilization by the mitochondrial pyruvate carrier. Mol Cell. 2014;56(3):425–435. doi: 10.1016/j.molcel.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.