Abstract
Antibiotics are currently recognized as environmental pollutants. In this work, the methods involved in the degradation of a β-lactam antibiotic (i.e., DXC) by treatments based on inorganic peroxides and UVC (e.g., UVC alone, UV-C/H2O2, UVC/peroxymonosulfate, and UVC/peroxydisulfate) are presented. The methodology of computational calculations to obtain frontier orbitals and Fukui indices for DXC, and elucidate the reactive moieties on the target substance is also shown. Finally, the direct oxidation by peroxides and UV-C/H2O2 action to treat DXC in simulated pharmaceutical wastewater are depicted. The chromatographic and theoretical analyses allowed for determining the degrading performance of inorganic peroxides and UVC-based treatments toward the target pollutant in aqueous samples.
-
•
Treatments based on inorganic peroxides and UVC as useful methods for degrading the β-lactam antibiotic dicloxacillin.
-
•
Persulfates and UV-C/H2O2 showed high degrading action on the target pharmaceutical.
-
•
Methodologies based on theoretical calculations for the identification of reactive moieties on the DXC susceptible to radical attacks are presented.
Keywords: Advanced oxidation, Pharmaceuticals elimination, Photochemistry, Water decontamination
Method name: Oxidative and photochemical methods to degrade the antibiotic dicloxacillin in water
Graphical abstract

Specifications table
| Subject area: | Environmental Science |
| More specific subject area: | Pollutants degradation |
| Name of your method: | Oxidative and photochemical methods to degrade the antibiotic dicloxacillin in water |
| Name and reference of original method: | Not applicable |
| Resource availability: | All resources are detailed in this article. |
Background
Wastewater from the pharmaceutical industry (PWW) is recognized as a source of the input of pharmaceuticals into the municipal sewage systems, which then are usually treated in urban wastewater treatment plants, or directly discharged into the environment [1]. Pharmaceuticals such as antibiotics are highly consumed and they are very common in municipal wastewater. For instance, DXC (a β-lactam antibiotic) is widely consumed and up to 65 % of this pharmaceutical is excreted after consumption [1], thus leading to a high discharge into the sewage systems and environmental water.
The entrance of β-lactam antibiotics into environmental water is worrying due to the development of antimicrobial resistance [2]. Thus, the input of compounds such as DXC into the environment must be avoided. Therefore, to diminish the entrance of pharmaceuticals from PWW into the aquatic environment, effective treatments are required. Oxidative processes based on the use of inorganic peroxides and photochemical systems (involving the combination of UVC with inorganic peroxides are useful methods to eliminate organic pollutants recalcitrant to biological systems [1]. Oxidative processes that use hydrogen peroxide (H2O2), peroxymonosulfate (HSO5-, PMS), or peroxydisulfate (S2O82-, PDS) are capable of directly inducing some transformations on the organic compounds. Meanwhile, the photochemical treatments that involve UVC irradiation combined with inorganic peroxides can promote the production of radical species generated by the homolytic cleavage of the —O—O— bond (Eqs. (1)–(3)) [3,4]. The formed radicals can attack and degrade organic contaminants such as β-lactam antibiotics (e.g., DXC) in aqueous samples [[3], [4], [5]].
| H2O2 + UVC → 2 HO• | (1) |
| HSO5- + UVC → HO• + SO4•- | (2) |
| S2O82- + UVC → 2 SO4•- | (3) |
Considering the concern generated by β-lactam antibiotics and the degradation capability of inorganic peroxides acting alone or in combination with UVC light, this study presents the methods involved in oxidative and photochemical systems (focused on DXC degradation), demonstrating the efficacy of UV/PMS and UV/PDS processes in comparison to more commonly used methods such as UV-C and UV/H2O2. Moreover, as another novel aspect, this work presents the methodological parts related to the theoretical analyses (Fukui indexes) to identify the regions on DXC susceptible to attacks by degrading species coming from the combination of UVC light with inorganic peroxides. The experimental and theoretical methods and procedures are detailed below.
Method details
-Reagents and simulated pharmaceutical wastewater (PWW)
Dicloxacillin (DXC) was obtained from Research Pharm LTDA. Sodium persulfate was provided by Fisher Chemical. Acetonitrile, o-phosphoric acid, and H2O2 were purchased from Merk. Oxone (the PMS source) was obtained from Sigma. The PWW was prepared by dissolving 0.1881 g of commercial DXC (MK-Tecnoquímicas) in 10 L of deionized water.
-Equipment and software
For the methods that combine the action of the inorganic peroxides with UVC light, the water sample was placed in a beaker under magnetic stirring inside an aluminum box reactor having five lamps (15 W each and a maximum emission at 254 nm) at the top of the reactor. The degradation experiments were performed at least by duplicate and the average values were reported with their corresponding standard deviation.
The quantification and evolution of the pharmaceutical were performed by using liquid chromatography, injecting 10 µL of sample to be analyzed by a UHPLC (Thermo 3000) equipped with a DAD detector. A Restek Raptor® C18 column (2.7 µm, 150 mm × 3 mm) and a mobile phase with 0.5 % phosphate buffer and acetonitrile were used at a flow rate of 0.4 mL min−1. It was run a gradient elution as specified in Hincapié-Mejía et al., 2022 [5]. The theoretical analyses for the methods involved in the elucidation of the regions on DXC more suitable to interact with oxidizing species were performed using Gaussian 09 (quantum chemistry software).
Method validation
-Methods for degrading DXC in water samples
-Treatment of the target pharmaceutical in distilled water by inorganic peroxides acting alone
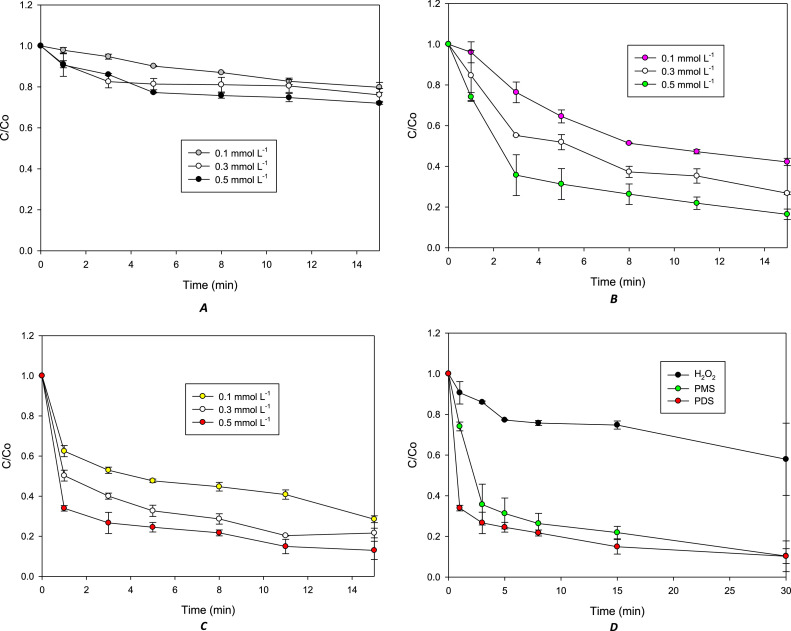
In the direct oxidation method, inorganic peroxides (H2O2, PMS, or PDS) were added individually to a sample of distilled water containing 100 mL of the target pollutant (at 40 µmol L−1). The system was kept for 30 min under magnetic stirring and sampled at 1, 3, 5, 8, 15, and 30 min of treatment. Herein, the influence of the peroxide concentration (0.1, 0.3, and 0.5 mmol L−1) on the degradation method was analyzed (Fig. 1). Figs. 1A, 2B, and C demonstrate that within the evaluated range, the increase of the peroxide concentration enhances DXC degradation. Besides, Fig. 1D compares the data for the treatment of DXC by the oxidative methods at the highest tested concentration of the inorganic peroxides (i.e., H2O2, PMS, or PDS; at 0.5 mmol L−1). It is observed that the direct oxidation of the β-lactam antibiotic was more efficient using PMS or PDS than H2O2 (which can be related to the higher redox potentials of PMS and PDS (E°: 1.82 V and 2.01 V, respectively [6]) than H2O2 (E°: 1.77 V, [7]); indicating that the use persulfates are a good option to directly degrade DXC.
Fig. 1.
Treatment of DXC by the direct oxidation methods using H2O2, PMS, or PDS. A. Effect of H2O2 concentration. B. Effect of PMS concentration. C. Effect of PDS concentration. D. comparison of DXC oxidations by the action of peroxides at 0.5 mmol L−1.
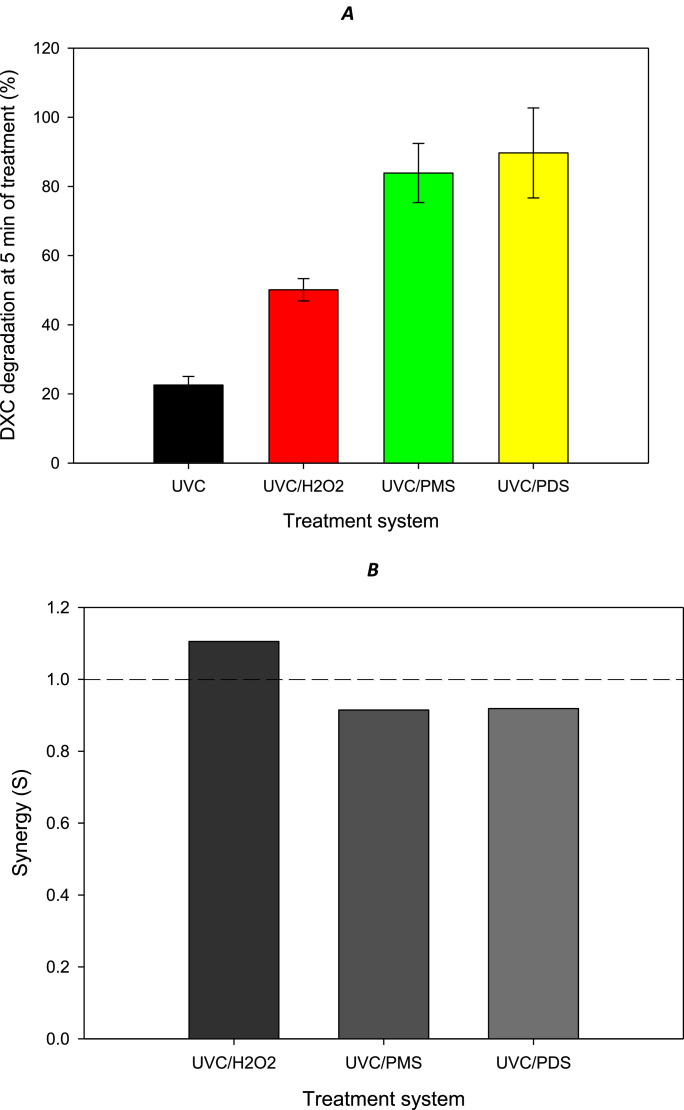
Fig. 2.
Treatment of DXC by the UVC/H2O2, UVC/PMS, and UVC/PDS methods. A. DXC degradation. B. Synergy of the combinations.
-Degradation by UVC combined with the inorganic peroxides
The use of the photochemical systems that combine UVC irradiation with inorganic peroxides to degrade DXC in aqueous samples involves three steps: i) testing the UVC alone performance by the treatment of the target substances under fixed conditions of light power (e.g., 75 W) in distilled water (i.e., a control experiment); ii) perform the treatment of DXC in distilled water by combining UVC with each inorganic peroxide; and iii) evaluate the change in the concentration of the antibiotic, employing the HPLC technique.
Following the above-mentioned steps, it was found that the degradation of DXC showed the order: UVC < UVC/H2O2 < UVC/PMS ≈ UVC/PDS (Fig. 2A). The UVC is a highly energetic light able to promote itself a partial degradation of some β-lactam antibiotics such as the target pollutant [3,4]. However, the combination of the ultraviolet light with the peroxides led to DXC degradation percentages higher than 50 %. Although the UVC/inorganic peroxide systems achieved higher removal of DXC than UVC acting solely, it is important to determine the beneficial effect of such a combination. Thus, the synergy must be considered. To calculate the synergy of the combinations (S, at 5 min of treatment), it can be used Eq. (4).
| S = % removal by UVC/peroxide / (% removal by UVC + % removal by peroxide) | (4) |
After the application of Eq. (4) to the combined systems, it can be noted that the UVC/H2O2 was synergistic (S > 1.0; i.e., the combination is better than the system components acting independently, Fig. 2B), whereas the UVC/PMS and UVC/PDS systems were antagonistic (S < 1.0; i.e., the system components acting independently are better than the combination). These observations can be rationalized by considering that in the case of UVC\H2O2, light promotes the production of radicals (Eq. (1)), while in the UVC/PMS and UVC/PDS treatments, there is competition between DXC and light with persulfates. In other words, the DXC molecule undergoes direct oxidation with persulfates (Fig. 1). However, it should be noted that the presence of radiation does not ensure the activation of all persulfate molecules to generate the desired oxidizing species [8].
-Theoretical analysis details on DXC
As mentioned in the previous sections, PMS or PDS acting alone effectively degraded DXC, and also the UVC\H2O2 combination was able to induce a high and synergistic degradation of the pollutant (Fig. 1, Fig. 2). It must be mentioned that theoretical methodologies can be used to elucidate the regions on the organic compounds more suitable to interact with oxidizing species (e.g., PMS, PDS, or HO•) [9,10]. Then, computational calculations of DXC were done. The method comprised a ground state, DFT, B3LYP; and a 6–311 g++ (2d, 2p) basis. Moreover, the neutral molecule of DXC was considered and the dielectric constant for water was considered. Thereby, frontier orbitals and Fukui indices were determined (Table 1, Table 2). Frontier orbitals (i.e., the highest occupied molecular orbital-HOMO and the lowest unoccupied molecular orbital-LUMO, Table 1) can be useful in predicting radical attack to moieties on DXC. Meanwhile, the Fukui indices of DXC (Table 2) aim to identify vulnerable sites toward attacks by radicals (f0), nucleophilic (f+), and electrophilic (f−) species. It can be mentioned that a high value of each Fukui index suggests an increased susceptibility to attacks by degrading species.
Table 1.
HOMO – LUMO energy orbitals.
| Energy of orbitals | |
|---|---|
HOMO Energy: −0,199,192 eV |
LUMO Energy: −0,088,526 eV |
Table 2.
Results of computational analysis (Fukui Function Indices) for DXC.
| Atoms | f + | f − | f ° | |
|---|---|---|---|---|
 |
Cl (1) | 0.040 | 0.013 | 0.027 |
| Cl(2) | 0.041 | 0.014 | 0.028 | |
| S(3) | 0.013 | 0.505 | 0.259 | |
| O (4) | 0.022 | 0.052 | 0.037 | |
| O (5) | 0.022 | 0.010 | 0.015 | |
| O (6) | 0.041 | 0.030 | 0.036 | |
| O (7) | 0.067 | 0.028 | 0.047 | |
| O (8) | 0.079 | 0.007 | 0.043 | |
| N (9) | 0.005 | 0.007 | 0.006 | |
| N (10) | 0.013 | −0.025 | −0.006 | |
| N (11) | 0.017 | 0.009 | 0.063 | |
| C (12) | −0.002 | −0.019 | −0.010 | |
| C (13) | −0.005 | −0.062 | −0.034 | |
| C (14) | −0.005 | 0.004 | −0.001 | |
| C (15) | −0.003 | 0.012 | 0.004 | |
| C (16) | 0.009 | 0.014 | 0.011 | |
| C (17) | −0.003 | −0.014 | −0.008 | |
| C (18) | 0.001 | 0.008 | 0.001 |
*Bold: high value of f+, italic: high-value of f−, and underline: high value of f0.
The computational analyses revealed that the S3 atom of DXC has the highest values for f° (Table 2). This is also consistent with the information regarding the HOMO (Table 1), which is mainly located on the β-lactam and dihydrothiazine rings. Those data suggest that such regions on DXC are the most susceptible to transformations by radicals generated in UVC/H2O2 (e.g., HO•), UVC/PMS, or UVC/PDS (e.g., SO4•-). It is relevant to highlight that the Fukui index for electrophilic attacks (f−) also exhibited the highest value on the S3 atom, denoting that this atom of DXC is very prone to be directly oxidized by PMS, PDS, or H2O2. The information presented herein fits well with other previous works that report attacks on the β-lactam ring and thioether group on other antibiotics belonging to the same class of DXC (e.g., ampicillin and cloxacillin [4,10]) as the main primary transformations. This indicates that the theoretical methods are useful tools for predicting the regions/atoms to be attacked by species such as PMS, PDS, hydroxyl radical, or sulfate radical. On the other hand, to verify the theoretical findings about the moieties on DXC more prone to attacks by degrading species (i.e., inorganic peroxides or radicals), it is required the experimental determination of primary degradation products; which should be developed in further works.
-Degradation of DXC in the synthetic pharmaceutical wastewater (PWW)
As a strategy to evaluate the performance of the oxidative systems (i.e., PMS or PDS) and the photochemical process (i.e., UVC/H2O2) in a matrix more complex than distilled water, DXC in a PWW was treated. The PWW was prepared as described previously in the Methods details subsection. PWW (100 mL) was treated by the individual addition of PMS or PDS (at 0.5 mmol L−1) in the dark. Also, 100 mL of the PWW sample experienced the application of the UVC light (75 W) with the addition of hydrogen peroxide (at 0.5 mmol L−1). For the three systems, the change in the concentration of the antibiotic was followed by the HPLC technique. The treatments were applied for 30 min, and Fig. 3 contains the degradation obtained during the treatment.
Fig. 3.
Treatment of DXC in the PWW matrix by PMS, PDS, or UVC/H2O2.
From Fig. 3, it can be noted that the methods of direct oxidation based on PMS or PDS achieved higher degradations (∼85 %) than UVC/H2O2 (∼28 %) in PWW. This can be associated with the higher selectivity of the persulfates acting alone toward DXC; PMS or PDS had low interaction with other matrix components. Meanwhile, in the UVC/H2O2 system (which generates non-selective radical species, HO•), there is a competence among DXC and other components in PWW by HO•, thus leading to a low pollutant degradation. The above findings are well aligned with the Wu et al. report [11], which emphasizes that organic compounds having a thioether moiety in their structure (such as in the case of DXC) are selectively degraded by persulfates (e.g., PMS) even presenting similar degradations in deionized water and complex aqueous matrices. Finally, it is important to mention that the above-presented methods were efficient for the DXC degradation in the PWW at a laboratory and the fundamental aspects were analyzed herein. However, in the future, they could be scaled up to larger levels (e.g., pilot levels) evaluating the best reactor configuration, optimizing the operational parameters (e.g., reagents concentration), and assessing the economic costs to determine the actual viability at large scales.
Concluding remarks about the methods
-
•
The chromatographic methods and the reaction system allowed for determining the degrading performance of inorganic peroxides alone or in combination with UVC light toward DXC in distilled water.
-
•
The increase in the concentration of the inorganic peroxides enhanced the efficacy of the direct oxidation methods on DXC.
-
•
Theoretical methods using quantum chemistry software provided information to elucidate the moieties/atoms on DXC more suitable for attacks by the peroxides and radical species.
-
•
The utilization of the treatment systems in PWW revealed a high suitability of PMS or PDS acting alone to deal with DXC in more complex matrices than distilled water.
Limitations
Not applicable.
Ethics statements
None.
CRediT authorship contribution statement
Fidel Granda-Ramírez: Methodology, Data curation, Investigation, Writing – review & editing. Efraím A. Serna-Galvis: Conceptualization, Data curation, Methodology, Writing – original draft. Yenny Ávila-Torres: Data curation, Software, Writing – review & editing. Ricardo A. Torres-Palma: Conceptualization, Resources, Writing – review & editing. Gina Hincapié-Mejía: Methodology, Investigation, Funding acquisition, Resources, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Institución Universitaria Colegio Mayor de Antioquia (FAI 001DXC) and MINCIENCIAS COLOMBIA, grant number 1115-852-69594, Program to improve the quality of irrigation water through the elimination of emerging contaminants by advanced oxidation processes (PRO-CEC-AGUA).
Supplementary material and/or additional information
None.
Footnotes
Related research article: None.
For a published article: None.
Data availability
Data will be made available on request.
References
- 1.Patel M., Kumar R., Kishor K., Mlsna T., Pittman C.U., Mohan D. Pharmaceuticals of emerging concern in aquatic systems: chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019;119:3510–3673. doi: 10.1021/acs.chemrev.8b00299. [DOI] [PubMed] [Google Scholar]
- 2.Salam M.A., Al-Amin M.Y., Salam M.T., Pawar J.S., Akhter N., Rabaan A.A., Alqumber M.A.A. Antimicrobial resistance: a growing serious threat for global public health. Healthcare. 2023;11:1946. doi: 10.3390/healthcare11131946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serna-Galvis E.A., Ferraro F., Silva-Agredo J., Torres-Palma R.A. Degradation of highly consumed fluoroquinolones, penicillins and cephalosporins in distilled water and simulated hospital wastewater by UV254 and UV254/persulfate processes. Water Res. 2017;122:128–138. doi: 10.1016/j.watres.2017.05.065. [DOI] [PubMed] [Google Scholar]
- 4.He X., Mezyk S.P., Michael I., Fatta-Kassinos D., Dionysiou D.D. Degradation kinetics and mechanism of β-lactam antibiotics by the activation of H2O2 and Na2S2O8 under UV-254nm irradiation. J. Hazard. Mater. 2014;279:375–383. doi: 10.1016/j.jhazmat.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Hincapié Mejía G., Peñuela G.A., Mueses M.A. Evaluation of a helicoidal flux photoreactor applied in the dicloxacillin degradation by UV-C/H2O2 and UV-A/photo-Fenton including the effect of photon absorption. Results Eng. 2022;15 doi: 10.1016/j.rineng.2022.100519. [DOI] [Google Scholar]
- 6.Kiejza D., Kotowska U., Poli W., Karpi J. Peracids - New oxidants in advanced oxidation processes : the use of peracetic acid, peroxymonosulfate, and persulfate salts in the removal of organic micropollutants of emerging concern − A review. Sci. Total Environ. 2021;790 doi: 10.1016/j.scitotenv.2021.148195. [DOI] [PubMed] [Google Scholar]
- 7.Bacardit J., Oller I., Maldonado M.I., Chamarro E., Malato S., Esplugas S. Simple models for the control of photo-fenton by monitoring H2O2. J. Adv. Oxid. Technol. 2007:10. doi: 10.1515/jaots-2007-0201. [DOI] [Google Scholar]
- 8.Mo C.C., Tian F.X., Xu B., Wang J., Gao Y.Q., Bi D.S., Hu X.J. Efficient trimethoprim removal via cooperation of radical and non-radical pathways in UV/peroxymonosulfate: kinetics, mechanisms and disinfection by-products-associated risks. J. Environ. Chem. Eng. 2024;12(2) doi: 10.1016/j.jece.2024.112368. [DOI] [Google Scholar]
- 9.Zhang Y., Xiao Y., Zhong Y., Lim T.-T. Comparison of amoxicillin photodegradation in the UV/H2O2 and UV/persulfate systems: reaction kinetics, degradation pathways, and antibacterial activity. Chem. Eng. J. 2019;372:420–428. doi: 10.1016/j.cej.2019.04.160. [DOI] [Google Scholar]
- 10.Serna-Galvis E.A., Guateque-Londoño J.F., Silva-Agredo J., Porras J., Ávila-Torres Y., Torres-Palma R.A. Superior selectivity of high-frequency ultrasound toward chorine containing-pharmaceuticals elimination in urine: a comparative study with other oxidation processes through the elucidation of the degradation pathways. Ultrason. Sonochem. 2021;80 doi: 10.1016/j.ultsonch.2021.105814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu S., Zhang R., Fu X., Zhang H., Sun P. Reactivity of unactivated peroxymonosulfate and peroxyacetic acid with thioether micropollutants: mechanisms and rate prediction. Water Res. 2024;256 doi: 10.1016/j.watres.2024.121601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.