Review Highlights
-
•
Summarize the most efficient method for extracting Volatile Organic Compounds from soil and plants.
-
•
Evaluate the significance of TFME-MS assay sampling in enhancing the production of volatile compounds associated with it compared to various methods.
-
•
Thin film solid-phase microextraction is an innovative technique that has not yet been utilized to examine soil samples.
Keywords: LC–MS/MS, GC–MS, HS-SPME, SPME fiber, Plant tissue, Soil, Thin-film microextraction, Sample preparation
Method name: A techique for determining volatile organic compound from plant tissue and soil samples
Abstract
This review critically assesses the determination of low molecular weight volatiles by different methods, providing context for the development of suitable techniques to determine volatile content in plant tissue and soil samples as well as the associated analytical challenges. Although sensitive analytical methods have been reported in recent decades, studies on their application in modern investigative techniques are lacking. Herein, the latest sampling methods in volatile biochemistry, current advancements in the understanding of these analytes, and the significance of these findings for other types of volatiles are summarized. Gas chromatography, high-performance liquid chromatography, ion chromatography, thin-film microextraction, and real-time monitoring techniques are discussed and critically determined. This review concerns the methods most suitable for future research in this area.
Graphical abstract
Specifications table
| Subject area: | Chemistry |
| More specific subject area: | Metabolite and volatile organic compound analysis |
| Name of the reviewed methodology: | A technique for determining volatile organic compounds from plant tissue and soil samples |
| Keywords: | LC–MS/MS; GC–MS; HS-SPME; SPME fiber; Plant tissue; Soil; Thin-film microextraction, sample preparation |
| Resource availability: | N/A |
| Review question: | What is the significance of SPME in plants and soil? How does TFME-MS assay detect plant tissue? What advantages does TF-MS offer over conventional laboratory-based plant and soil detection methods? |
Introduction
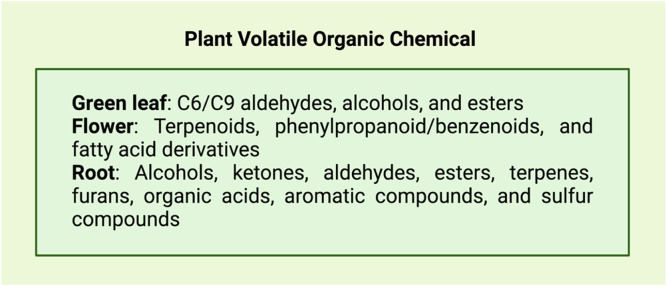
Plant volatile organic compounds (PVOCs) include multiple volatile plant secondary metabolites. Plant volatiles can be divided into green leaf, floral, and fruit volatiles according to the different organs of the plant. C6 straight-chain alcohols, aldehydes, and their ester derivatives are the primary sources of green leaf odors, while terpenes, such as monoterpenes, sesquiterpenes, and diterpenes, and aromatic compounds are the main constituents of floral plant odors (Fig. 1). The metabolic pathways of different plants, such as the cystine and cysteine metabolic pathways of the lily family and the methionine metabolic pathway of the cruciferous family, can produce specific odors [1,2]. The ability of a plant to produce PVOCs, including their diversity and abundance, is strongly influenced by biotic and abiotic factors [3,4]. These PVOCs have essential functions in plant–plant, plant–insect, and plant–soil microbial interactions. The study of PVOCs is limited by the number of compounds involved and the complexity of their interactions. For example, multiple PVOCs with different functions often originate from the same plant tissue or organ, which then interact with various insect species [5].
Fig. 1.
PVOCs from different plant organs [6].
VOCs in soils are mainly produced by plants [7,8] and microorganisms [9]. Recent research has uncovered and suggested the roles of volatile organic compounds (VOCs) released by plant roots, their symbiotic mycorrhizal fungi, and young plants [8,10]. VOC-mediated plant–microbe interactions as well as the production and measurement of PVOCs have been discussed in several reviews [[11], [12], [13]] and are far beyond the scope of this review. The investigation of soil VOCs production is complex, as the soil is a highly variable parameter that influences the production of microbial volatile compounds (MVOCs). Besides differences in soil-specific community composition, MVOCs production in soils strongly depends on nutrient and oxygen availability in addition to the physiological state of the microorganisms, which are affected by environmental factors, such as soil moisture, soil texture, and microbial activity [14].
Research objective
The purpose of this paper is to review the current state of volatile sampling and the analysis of VOCs in plant tissue and soil emissions. This review highlights the challenges of sampling, analytical determination, and speciation before presenting an approach for the development of rapid, sensitive, and reproducible tools for the characterization of bioaerosols by analyzing samples from a variety of environmental sources.
Method details
Sample preparation is the most important step of analysis. A sample needs to undergo a series of procedures, including sampling, sample treatment (e.g., hydrolysis or extraction) to isolate the analyte from sample matrices, concentration, and derivatization, to make it suitable for separation and detection. For example, any compounds analyzed by gas chromatography (GC) must be volatile, as the presence of nonvolatile contaminants results in poor analytical response and potential unwanted peaks in the chromatogram. Thus, sample preparation often requires the removal of these nonvolatile components. Solvent extraction and solid-phase extraction (SPE) are common sample preparation techniques. However, these methods are tedious and time-consuming, require large volumes of samples and solvents, have multi-step procedures that create analyte loss, and pose a serious threat to laboratory personnel and the environment due to the use of hazardous solvents [15,16].
Literature search strategy
The PRISMA (Preferred Reporting Items for Systematic Reviews and MetaAnalyses) methodology should serve as the foundation for a thorough investigation. Using PRISMA ensures accurate and accessible execution and reporting of the systematic review. Gaining a thorough grasp of regulatory recommendations and current practices in the topic of study will require completing this initial step. The inclusion and exclusion criteria can also be described using PRISMA principles, and the reporting should be done in compliance with the rules provided by Equator Network (https://www.equator-network.org/). Following that, a thorough and methodical search should be conducted using the keywords in main, secondary, and guideline literature databases. To ensure objectivity and lower the possibility of bias, this search should be carried out by at least two researchers, and the inter-reviewer reliability of selections should be recorded. This process will provide an exact record devoid of repetitions. Following this preliminary stage, papers that may be relevant should be chosen by looking over their titles and abstracts. The entire texts of the selected articles should be carefully examined in order to assess a publication's eligibility. We had comprehensive literature search was performed using databases, such as Google Scholar, Springer, PubMed, Scopus, Web of Science, IEEE Xplore, ScienceDirect, and ResearchGate to identify studies that report the use of extraction of volatile organic compounds from plant tissue and soil samples also strategies detected strategies analysis untargeted metabolomic of main active compound. Articles related to detection of volatile compounds by using CC-MS were identified from the databases ScienceDirect, ResearchGate and Elsevier using the search terms word (secondary metabolites extraction and volatile sampling). Only English language articles were chosen. Moreover, we go deep search to high quality of journal to look for a new method for volatile extraction such as trend in plant science, metabolite and new phytologist etc.
We systematically searched IEEE Xplore, Web of Science, ResearchGate, and Elsevier for English articles (May 1976 - May 2042), by employing the PRISMA diagram to visualize the systematic search process. A total of 892 articles were identified from three major databases (242 from Web of Science, 200 from Elsevier, and 450 from ResearchGate and google scholar). Subsequently, 40 articles were selected, with an additional four from different sources.
Methods for volatile organic compound (VOC) extraction from plant tissue
To extract volatile organic compounds from a wide variety of plant materials, including herbs, fruits, and vegetables, researchers use multiple techniques and methods. This section presents the updated outlines for the extraction techniques used to identify volatile organic compounds in plant materials using various methods (Table. 1).
Table 1.
Comparison of different extraction method of volatile from plant tissue.
| Extraction method | Advantages | Disadvantages |
|---|---|---|
| Drying Extraction | Simple; well established and widely used; can be easily applied on an industrial scale. | Long extraction time, The need for optimal extraction temperature. |
| Direct Infusion Mass Spectrometry (DI-MS) for real time techniques | High detection of a volatile compound | soft ionization conditions that limit the fragmentation of chemical compounds, used in real-time measurements. |
| Film Solid-phase Micro-Extraction (TF-SPME) | Require small sample volumes and decrease operation costs, TF-SPME devices are available with several different extraction phases to cover a wide range of polarity. | The need for optimal extraction temperature and accurate procedure setting. |
| Headspace Solid Phase Microextraction (HS-SPME) | Short analysis time, Simple analysis conducting andmaintenance, Low analysis cost | Sensitivity of PDMS-coated fiber to presence of suspended matter. |
| Solid Phase Microextraction Fiber (SPME-Fiber) | Short analysis time, Simple apply to plant volatile and soil, High detection of a volatile compound | Sorption processes of analytes on fiber compete with analytes'sorption on suspended matter surface. |
Herb drying processes for the extraction of volatiles
Herb drying is a common stabilization technique used to preserve the vital qualities of essential oils and bioactive compounds [17]. This process is highly valued due to the consumer expectation of product quality and its use in high-yield chemical extraction [18]. Drying is also an important step in preserving the essential oils contained in the trichomes of epidermal plant cells [19]. The selection of an appropriate drying method is also important to minimize the damage to these glands in the trichomes during the heating process [20].
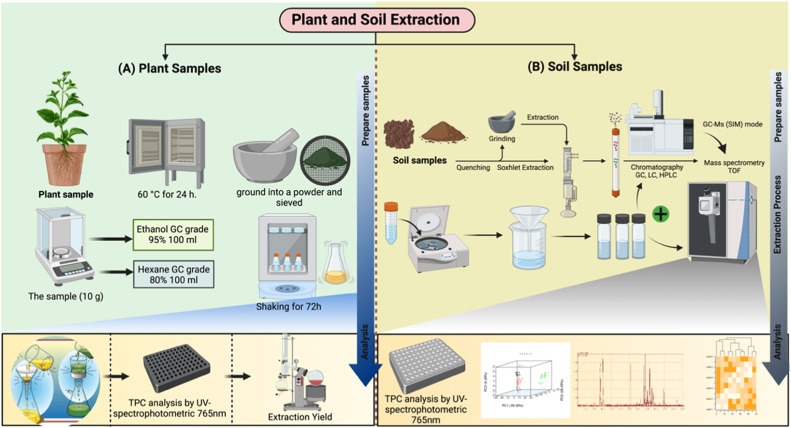
A JEDI dry herb vaporizer (Sino Xing Science and Technology, Shenzhen City, China, sinocig.com) was used to dry the plants. The device consisted of a 14-mm diameter ceramic chamber surrounded by a metallic body with a glass mouthpiece connected to the top of the chamber and a programmable temperature control system that ranges between 150 and 240 °C. The device was heated to the set temperature in 20–30 s and then continuously heated for 5 min at the programmed temperature. The components used in the plant extraction process are shown in Fig. 2A. The septum cap from a 2-mL vial was placed over the mouthpiece to contain the headspace components in the chamber of the vaporizer. Aluminum foil rolled into a cylinder was inserted into the heating chamber below the mouthpiece to contain the sample, allowing for easy cleanup and reduced carryover after each extraction. The estimated volume of the sample chamber including the mouthpiece was about 3 mL with some modifications [21].
Fig. 2.
Volatile extraction from (A) plant and (B) soil samples, modified from [22].
Solid-phase microextraction (SPME) was performed in the headspace of the dry-plant vaporizer. Briefly, different amounts of individual samples (i.e., 500 mg of horseradish, 20 mg of cinnamon, and 400 mg of gasoline-spiked soil) were first loaded in the ceramic heating chamber of the vaporizer, lined with aluminum foil to minimize contamination of the heating source. The mouthpiece was screwed on the top of the chamber and covered with a septum-lined cap from a 2 mL vial. The sample was loaded into the vaporizer and heated to the desired temperature (150, 200, or 240 °C), which constituted the desorption period when the sample was heated prior to extraction. The gas-phase components were then sampled by inserting a manual SPME fiber for 5 min at the selected extraction temperature. This study was designed as a proof of concept for a novel analytical method. As currently configured, the dry-herb vaporizer was not optimized for the times and temperatures used in headspace SPME (HS-SPME), so the development of a quantitative method was not pursued. The adsorbed components were thermally desorbed in the GC injection port for GC–MS analysis using a 100-µm polydimethylsiloxane coated SPME fiber and holder purchased directly from Supelco (Bellefonte, PA, USA). The fiber was preconditioned or cleaned at 250 °C for 30 min before each extraction in the GC injection port. Different samples were taken at different times at the desired temperature based on the sample size and type [21].
Direct infusion mass spectrometry (DI-MS) for real-time techniques
Accurate mass measurement is one of the best methods for the identification of chemical compounds. The closer the result is to the exact mass of the compound, the higher the likelihood of its correct identification, with the ideal case being the exact determination of the monoisotopic mass of the compound of interest. Conversely, the unambiguous identification of a chemical compound or ion may be challenging without the correct determination of its exact mass. For instance, isoprene (C5H8), a common biogenic volatile compounds (BVOCs) emitted by plants, has a molecular formula of and a nominal mass of 68 Da; however, furan (C4H4O), a compound emitted into the atmosphere through combustion processes, has the same nominal mass despite having different structure and chemical properties [23]. Nevertheless, both isobaric compounds can be discerned based on their exact masses of 68.0626 Da (isoprene) and 68.0262 Da (furan). More challenging still is the discrimination between structural isomers, such as isoprene and piperylene (1,3-pentadiene; C5H8), which have the same exact mass [24]. These isomers must be discerned through other methods, such as retention indices (GC), fragmentation patterns (MS), or observation (i.e., piperylene is a substrate used in the synthesis of polymers and is not a plant BVOC) [25]. While DI-MS is ideal for the analysis of VOCs, specifically BVOCs, their successful characterization requires (i) a versatile and well-researched ionization mechanism, (ii) soft ionization conditions that limit the fragmentation of chemical compounds, (iii) high sensitivity and mass resolution for the trace analysis and determination of the exact mass of the ion, (iv) straightforward sampling, and (v) the ability to conduct the measurement over an extended period. One of the most prominent DI-MS techniques used in real-time measurements is proton transfer reaction mass spectrometry (PTR-MS), which was developed for the analysis of BVOCs. One of its earliest implementations was in the assessment of the emission of volatile metabolites, such as methanol, acetaldehyde, ethanol, acetic acid, isoprene, and methylethylketone, from leaves [26]. Coupling PTR-MS with time-of-flight MS (TOF-MS) drastically improved the monitoring of volatile metabolites, introducing exceptional resolution, detection thresholds in the ppt range, and high acquisition rates (Fig. 3) [27].
Fig. 3.
DI-MS techniques in untargeted plant metabolite profiling. This figure was taken from [28].
Optimization of thin film solid-phase microextraction (TF-SPME) for the determination of volatile compounds
Due to the trace concentrations of target metabolites, adsorptive enrichment is needed before gas chromatography–mass spectrometry (GC–MS) analysis, with SPME best suited for this purpose. Herein, a modification of SPME, thin-film microextraction (TFME), was proposed for the analysis of cellular VOCs, which utilizes a planar mesh coated with a stationary phase to increase the extraction phase volume and active surface area [29].
TF-SPME was first implemented by Bruheim et al. to improve the sensitivity of previous methods such as SPME and stir bar sportive extraction (SBSE) [30,31]. TF-SPME consists of a carbon film (20 × 4.8 mm) covered with an absorbent material, which can be used in HS or DI analysis. The film is placed on a solid or liquid sample in a closed vial for HS techniques, while the film is introduced directly to a liquid sample for DI techniques [32]. TF-SPME is more sensitive than SPME because it uses a larger phase volume, which results in a larger surface area and a higher extraction rate [32,33]. These films incorporate multiple absorbent materials to produce coatings, such as divinylbenzene/polydimethylsiloxane (DVB/PDMS), carboxene/polydimethylsiloxane (CAR/PDMS), and hydrophilic–lipophilic balanced/polydimethylsiloxane (HLB/PDMS), that broaden the polarity range of the compounds that can be extracted [33,34]. TF-SPME can be applied in the extraction of organic compounds in water [31,35]. In this work, the extraction of volatile compounds by TF-SPME provided yields higher than those of SBSE, exhibiting significant advantages over SBSE and SPME in the aforementioned matrices. Thus, it is expected to present the same advantages in other matrices, such as grape must and plant tissue. However, this review aims to provide guidance for the future use of TF-SPME devices in determining the volatile composition of soil samples. In the future, we also intend to assess the compatibility of integrating this extraction methodology with GC–MS, a well-established and effective extraction method.
Headspace SPME (HS-SPME)
In each 20-mL headspace bottle, 20 mg of plant sample and 5 µL of 2-octanol (10 mg/L stock in distilled water) were added as an internal standard. The prep-and-load SPME was employed for sample analysis by GC–MS [36,37]. The incubation period was 30 min at 60 °C, with a 15-min preheating step and a 4-min desorption phase. GC–MS analysis was performed on an Agilent 7890 gas chromatograph connected to a 5977B mass spectrometer using an Agilent DB-Wax GC column in the splitless injection mode. Helium served as the carrier gas, with a front inlet purge flow of 3 mL min−1 and a gas flow rate of 1 mL min−1 through the column. The temperature of the sample was held at 40 °C for 4 min, increased to 245 °C at a rate of 5 °C min−1, and then maintained for an additional 5 min. The ion source, quad, injection, and transfer line temperatures were set at 250, 250, 230, and 150 °C, respectively. In the electron impact mode, the energy was set at –70 eV. MS data was acquired in the scan mode with a solvent delay of 3 min in the m/z range of 20–500 [36,37].
SPME fiber method
Each plant sample was placed in a glass headspace bottle container equipped with a wide opening for easy plant removal. A fiber coated with adsorbent was affixed to an SPME fiber holder resembling an adapted syringe for material injection through the septum of the sample container. To expose the fiber to volatiles, it was extended from the needle by pressing the plunger of the SPME fiber holder [13] with some modifications (Fig. 4). The VOCs were meticulously extracted from the volatile emissions of plant samples during concurrent 3 h incubation, which were detected by extracting biochemicals from the headspace emissions of plant samples using the SPME fiber. CAR/PDMS three-phase fibers (StableFlex catalog number 57334-U; 85 µm, Sigma-Aldrich, Australia) with a 24-gauge needle for a manual holder were chosen to absorb volatiles released from three different species of invasive weeds. Each fiber was desorbed in the splitless injector at 250 °C in the GC–MS system. The initial column temperature was set at 60 °C, held for 3 min, increased to 180 °C at a rate of 5 °C min−1, and then held at 250 °C for 5 min. Helium was used as the carrier gas (BOC Gas, Sydney, Australia) at a flow rate of 1:1 mL min−1, while the splitless flow was set to 20 mL/min at 1.5 min. The total GC–MS run time was 45 min. Following the collection of VOCs from the headspace samples, each SPME fiber was retracted into the needle, and the SPME device was removed from the container for the GC analysis of compounds through thermal desorption. The quantity of compound adsorbed by the SPME fiber is influenced not only by the thickness of the fiber coating but also by the distribution constant of the analyte, typically increasing with its molecular weight and boiling point [13]. For all experiments, the following SPME fibers are suggested for the extraction of volatile compounds:
-
•
75/85 µm CAR/PDMS or 100 µm PDMS for bipolar gases and low molecular weight compounds
-
•
100 µm PDMS for non-polar volatiles
-
•
65 µm DVB/PDMS for bipolar volatiles, amines, and nitro-aromatic compounds
-
•
50/30 µm DVB/PDMS/CAR on a StableFlex fiber for bipolar flavor compounds (i.e., volatiles and semi-volatiles)
Fig. 4.
Schematic diagram of important factors in VOC collection and sampling via (A) HS-SPME and (B) SPME-FIBER. GC–MS analysis of concentrated volatile and semi-volatile compounds in headspace samples from plants.
The fibers selected for the absorption of volatiles were conditioned prior to analysis according to the manufacturer's instructions via insertion into the GC injector port [38].
Extraction of VOCs from soil
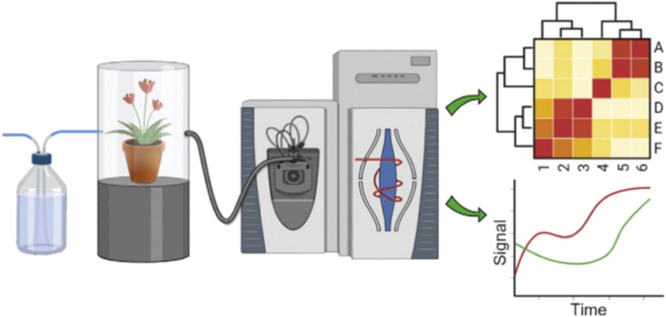
In push–pull systems (Fig. 5), air is pushed into the headspace sampling container at a rate regulated by a flowmeter. Before entering the chamber, the air was cleaned by flowing through a purifying filter material such as charcoal, which adsorbs impurities and can be humidified at a desired rate by mixing with a second air stream of saturated humidity. Inside the container, a uniform airflow over the plant is created. A portion of the air exits the chamber through an adsorbent trap connected to a vacuum pump. The flow rate of the outgoing air stream is regulated by a second flow meter, allowing for the collection of a defined percentage of emitted plant volatiles. The remaining air flow escapes the collection chamber through a vent to prevent overpressure. Compared with pull collection devices, this system offers more flexibility in regulating incoming and outgoing airflow dependent on the emission rates of VOCs in the investigated soil (Fig. 6). This system was employed to collect volatiles from aphid-infested plants over several days [39] and trap VOCs from plant/soil samples over 8 h [40].
Fig. 5.
Dynamic headspace collection systems in soil.
Fig. 6.
Method used to study VOCs produced by the microbiota of soils. Supelco vial technique [41] adapted to soil samples. This figure was created and adapted from app.biorender.com [42].
Statistical analysis of volatile compounds
Statistical analysis was performed in which the relative areas of the volatile compounds for each of the described experimental conditions were studied simultaneously to find which conditions maximized the overall extraction of volatile compounds. An exhaustive descriptive analysis was first performed to detect and eliminate any outliers. This descriptive analysis verified the significant degree of correlation between the volatile compounds, justifying the need to analyze all volatile compounds using a multivariate approach. First, principal component analysis was performed to reduce the dimensionality of the data by condensing the volatile compounds into a reduced number of principal components [43]. To interpret the principal components obtained, a varimax rotation was applied. Considering the experimental design described above, a multifactorial analysis of variance (ANOVA) was then used, taking the scores of each principal component as the response variable. Finally, an ANOVA was performed to compare the best conditions of two absorbents (CAR/PDMS and DVB/PDMS). In this case, the volatile compounds were not treated as a set, but the relative areas of each were compared individually.
Conclusion
Volatile organic compounds from soils and plants have been intensively investigated since the 1980s. The excellent review by Stotzky and Schenck (1976) [13,44] summarizes the knowledge of that time, which had not evolved significantly until a few years ago. With newly available tools that even allow online monitoring, soil volatilomics are anticipated to have a bright future. Increasing knowledge on VOCs emission patterns from specific organisms of different habitats and under different conditions, together with modern methods combined with other techniques, may allow for the future monitoring of the community structure, physiological state, and activity of microbial communities without the need for extraction or cultivation procedures. These advancements also would more easily identify the main active compounds for study. To achieve this, further studies of the characterization of plant and soil microorganisms by analyzing their VOCs emissions are necessary. In addition, the investigation of the VOCs output of microbial communities from complex habitats such as soils must be refined to improve VOCs fingerprinting methods.
Overall, various important factors influence the quality of volatiles in various plants and soils. The use of a singular approach is problematic, as the biostructure and volatile chemicals of each type of plant may be beneficial or harmful to the drying method. Exploring multiple drying processes for a specific single soil or plant would thus be beneficial in determining the best method and parameters for extracting the most volatile compounds while maintaining the highest quality of ingredients of a plant or soil sample.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Ethics statements
Not applicable.
CRediT authorship contribution statement
Nipapan kanjana: Conceptualization, Methodology, Investigation, Formal analysis, Validation, Visualization, Data curation, Writing - original draft, Writing – review & editing. Muhammad Afaq Ahmed: Data curation, Writing - original draft, Review-edit graphical abstract figure. Zhongjian Shen: Validation, Investigation, Software, Data curation. Yuyan Li: Writing – review & editing, Data curation. Lisheng Zhang: Supervision, Project administration, Conceptualization, Validation, Writing – review & editing, Funding acquisition.
Data availability
Data will be made available on request.
Acknowledgments
This work was supported by the "National Key R&D Program of China (2023YFE0123000, 2022YFC2601400)" and "the International Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences (CAAS-ZDRW202108).
Data availability
Data will be made available on request.
References
- 1.Laothawornkitkul J., Taylor J.E., Paul N.D., Hewitt C.N. Biogenic volatile organic compounds in the Earth system. New Phytol. 2009;183:27–51. doi: 10.1111/j.1469-8137.2009.02859.x. [DOI] [PubMed] [Google Scholar]
- 2.Dudareva N., Klempien A., Muhlemann K., Kaplan L. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013;198:16–32. doi: 10.1111/nph.12145. [DOI] [PubMed] [Google Scholar]
- 3.Schiestl F.P., Ayasse M. Post-pollination emission of a repellent compound in a sexually deceptive orchid: a new mechanism for maximising reproductive success? Oecologia. 2001;126:531–534. doi: 10.1007/s004420000552. [DOI] [PubMed] [Google Scholar]
- 4.Majetic C.J., Raguso R.A., Ashman T.-L. Sources of floral scent variation: can environment define floral scent phenotype? Plant Signal Behav. 2009;4:129–131. doi: 10.4161/psb.4.2.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martel C., Romero P.E., Jersakava J., Ayasse M. The evolution of tachinid pollination in Neotinea ustulata is related to floral cuticular composition and the combined high relative production of (Z)-11-C23/C25enes. JSE. 2023;61:487–497. [Google Scholar]
- 6.Qian Q., Cui J., Miao Y., Xu X., Gao H., Xu H., Lu Z., Zhu P. The plant volatile-sensing mechanism of insects and its utilization. Plants. 2024;13:185. doi: 10.3390/plants13020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stotzky G., Schenck S., Papavizas G.C. Volatile organic compounds and microorganisms. Crit. Rev. Microbiol. 1976;4:333–382. doi: 10.3109/10408417609102303. [DOI] [PubMed] [Google Scholar]
- 8.Kesselmeier J., Staudt M. Biogenic volatile organic compounds (VOC): an overview on emission, physiology and ecology. J. Atmos. Chem. 1999;33:23–88. [Google Scholar]
- 9.Stahl P.D., Parkin T.B. Microbial production of volatile organic compounds in soil microcosms. SSSAJ. 1996;60:821–828. [Google Scholar]
- 10.Wenke K., Kai M., Piechulla B. Belowground volatiles facilitate interactions between plant roots and soil organisms. Planta. 2010;231:499–506. doi: 10.1007/s00425-009-1076-2. [DOI] [PubMed] [Google Scholar]
- 11.Penuelas J., Llusià J. The complexity of factors driving volatile organic compound emissions by plants. Biol. Plant. 2001;44:481–487. [Google Scholar]
- 12.Cape J. Effects of airborne volatile organic compounds on plants. Environ. Pollut. 2003;122:145–157. doi: 10.1016/s0269-7491(02)00273-7. [DOI] [PubMed] [Google Scholar]
- 13.Tholl D., Boland W., Hansel A., Loreto F., Rose U., Schnitzler J.P. Practical approaches to plant volatile analysis. Plant J. 2006;45(4):540–560. doi: 10.1111/j.1365-313X.2005.02612.x. [DOI] [PubMed] [Google Scholar]
- 14.McNeal K.S., Herbert B.E. Volatile organic metabolites as indicators of soil microbial activity and community composition shifts. SSSAJ. 2009;73:579–588. [Google Scholar]
- 15.Kataoka H., Lord H.L., Pawliszyn J. Applications of solid-phase microextraction in food analysis. J. Chromatogr. A. 2000;880:35–62. doi: 10.1016/s0021-9673(00)00309-5. [DOI] [PubMed] [Google Scholar]
- 16.de Fatima Alpendurada M. Solid-phase microextraction: a promising technique for sample preparation in environmental analysis. J. Chromatogr. A. 2000;889:3–14. doi: 10.1016/s0021-9673(00)00453-2. [DOI] [PubMed] [Google Scholar]
- 17.Nurhaslina C., Bacho S.A., Mustapa A. Review on drying methods for herbal plants. Mater. Today Proc. 2022;63:S122–S139. [Google Scholar]
- 18.Schaarschmidt S. Public and private standards for dried culinary herbs and spices—Part I: standards defining the physical and chemical product quality and safety. Food Control. 2016;70:339–349. [Google Scholar]
- 19.Hazrati S., Lotfi K., Govahi M., Ebadi M.T. A comparative study: influence of various drying methods on essential oil components and biological properties of Stachys lavandulifolia. Food Sci. Nutr. 2021;9:2612–2619. doi: 10.1002/fsn3.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.C. Turek, F.C. Stintzing, Stability of essential oils: a review, CRFSFS. 12 (2013) 40–53.
- 21.Ahmed A.A., Raynie D.E. Development of dry-herb vaporizer-assisted solid-phase microextraction for the analysis of volatile organic compounds. Adv. Sample Prepr. 2024;10 [Google Scholar]
- 22.Kanjana N., Li Y., Shen Z., Mao J., Zhang L. Effect of phenolics on soil microbe distribution, plant growth, and gall formation. Sci. Total Environ. 2024;924 doi: 10.1016/j.scitotenv.2024.171329. [DOI] [PubMed] [Google Scholar]
- 23.Xu N., Gong J., Huang Z. Review on the production methods and fundamental combustion characteristics of furan derivatives. Renew. Sustain. Energy Rev. 2016;54:1189–1211. [Google Scholar]
- 24.Lacko M., Wang N., Sovova K., Pasztor P., Spanel P. Addition of fast gas chromatography to selected ion flow tube mass spectrometry for analysis of individual monoterpenes in mixtures. Atmos. Meas. Tech. 2019;12:4965–4982. [Google Scholar]
- 25.Behr A., Neubert P. Piperylene-a versatile basic chemical in catalysis. Chem. Cat. Chem. 2014;6:412–428. [Google Scholar]
- 26.Lindinger W., Hansel A., Jordan A. On-line monitoring of volatile organic compounds at pptv levels by means of proton-transfer-reaction mass spectrometry (PTR-MS) medical applications, food control and environmental research. Int. J. Mass Spectrom. Ion Processes. 1998;173:191–241. [Google Scholar]
- 27.Jordan A., Haidacher S., Hanel G., Hartungen E., Mark L., Seehauser H., Schottkowsky R., Sulzer P., Mark T.D. A high resolution and high sensitivity proton-transfer-reaction time-of-flight mass spectrometer (PTR-TOF-MS) Int. J. Mass Spectrom. 2009;286:122–128. [Google Scholar]
- 28.Majchrzak T., Wojnowski W., Rutkowska M., Wasik A. Real-time volatilomics: a novel approach for analyzing biological samples. Trends Plant Sci. 2020;25:302–312. doi: 10.1016/j.tplants.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Filipiak W., Jaroch K., Szeliska P., Zuchowska K., Bojko B. Application of thin-film microextraction to analyze volatile metabolites in A549 cancer cells. Metabolites. 2021;11:704. doi: 10.3390/metabo11100704. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruheim I., Liu X., Pawliszyn J. Thin-film microextraction. Anal. Chem. 2003;75:1002–1010. doi: 10.1021/ac026162q. [DOI] [PubMed] [Google Scholar]
- 31.Tholl D., Boland W., Hansel A., Loreto F., Rose U.S.R., Schnitzler J.-P. Practical approaches to plant volatile analysis. Plant J. 2006;45:540–560. doi: 10.1111/j.1365-313X.2005.02612.x. [DOI] [PubMed] [Google Scholar]
- 32.Niinemets U. Mild versus severe stress and BVOCs: thresholds, priming and consequences. Trends Plant Sci. 2010;15:145–153. doi: 10.1016/j.tplants.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Alonso A., Marsal S., Julià A. Analytical methods in untargeted metabolomics: state of the art in 2015. Front. Bioeng. Biotechnol. 2015;3:23. doi: 10.3389/fbioe.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lubes G., Goodarzi M. Analysis of volatile compounds by advanced analytical techniques and multivariate chemometrics. Chem. Rev. 2017;117:6399–6422. doi: 10.1021/acs.chemrev.6b00698. [DOI] [PubMed] [Google Scholar]
- 35.Majchrzak T., Wojnowski W., Lubinska M., Lubinska-Szczygel M., Rozanska A., Namiesnik J. PTR-MS and GC-MS as complementary techniques for analysis of volatiles: a tutorial review. Anal. Chim. Acta. 2018;1035:1–13. doi: 10.1016/j.aca.2018.06.056. [DOI] [PubMed] [Google Scholar]
- 36.Kind T., Wohlgemuth G., Lee D.Y., Lu Y., Palazoglu M., Shanhbaz S., Fiehn O. FiehnLib: mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal. Chem. 2009;81:10038–10048. doi: 10.1021/ac9019522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joguet N., Jing L., Jamois F., Dumargue P. Characterization of volatile organic compounds (VOCs) from farms effluents: interest of HS-SPME-GC-MS technique for laboratory and field test. Atmosphere. 2023;14:928. [Google Scholar]
- 38.Leroux J., Jing L., Jamois F., Dumargue P. Chapter ten - detection and analysis of novel and known plant volatile apocarotenoids. Methods Enzymol. 2022;670:311–368. doi: 10.1016/bs.mie.2022.03.020. [DOI] [PubMed] [Google Scholar]
- 39.Kunert G., Otto S., Rose U.S.R., Gershenzon J., Weisser W.W. Alarm pheromone mediates production of winged dispersal morphs in aphids. Ecol. Lett. 2005;8:596–603. [Google Scholar]
- 40.Chen F., Tholl D., Auria J.C.D., Farooq A., Pichersky E., Gershenzon J. Biosynthesis and emission of terpenoid volatiles from Arabidopsis flowers. Plant Cell. 2003;15:481–494. doi: 10.1105/tpc.007989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barbosa D.H.S.G., Duarte H., Souza R.M., Viana A.P., Silva C.P. Field estimates of coffee yield losses and damage threshold by Meloidogyne exigua. Nematol. Bras. 2004;28:49–54. [Google Scholar]
- 42.Botelho A.O., Campos V.P., da Silva J.C.P., Freire E.S., de Pinho R.S.C., Barros A.F., Oliveira D.F. Physicochemical and biological properties of the coffee (Coffea arabica) rhizosphere suppress the root-knot nematode Meloidogyne exigua. Biocontrol. Sci. Tech. 2019;29:1181–1196. [Google Scholar]
- 43.G. James, G. James, D. Witten, T. Hastie, R. Tibshirani, An introduction to statistical learning. 112 (2013) 18.
- 44.Stotzky G., Schenck S. Volatile organic compounds and microorganisms. CRC Crit. Rev. Microbiol. 1976;4:333–382. doi: 10.3109/10408417609102303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.
Data will be made available on request.