Abstract
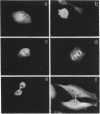
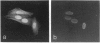
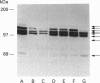
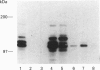
The mechanisms that regulate ubiquitin-mediated degradation of proteins such as the mitotic cyclins at defined stages of the cell cycle are poorly understood. The initial step in the conjugation of ubiquitin to substrate proteins involves the activation of ubiquitin by the ubiquitin-activating enzyme, E1. Previously we have described the subcellular localization of this enzyme to both nuclear and cytoplasmic compartments. In the present study, we have used the 1C5 anti-E1 monoclonal antibody in immunofluorescent-microscopy and subcellular-fractionation techniques to examine the distribution of E1 during the HeLa cell cycle. E1 is both cytoskeletal and nuclear during the G1-phase. As the cells progress into S-phase, E1 is exclusively cytoskeletal and has a perinuclear distribution. During G2-phase, E1 reappears in the nucleus before breakdown of the nuclear envelope. In mitotic cells, E1 localizes to both the mitotic spindle and the cytosol, but is absent from the chromosomes. Immunoblot analysis reveals multiple forms of E1 in HeLa whole cell extract. This heterogeneity is not a result of polyubiquitination and may represent inactive pools of E1. Only the characteristic E1 doublet is able to activate ubiquitin. Cell-fractionation studies reveal a differential distribution of specific E1 isoforms throughout the cell cycle. Therefore we propose that the subcellular localization of E1 may play a role in regulating cell-cycle-dependent conjugation of ubiquitin to target proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agell N., Mezquita C. Cellular content of ubiquitin and formation of ubiquitin conjugates during chicken spermatogenesis. Biochem J. 1988 Mar 15;250(3):883–889. doi: 10.1042/bj2500883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applegate M. L., Moore M. M., Broder C. B., Burrell A., Juhn G., Kasweck K. L., Lin P. F., Wadhams A., Hozier J. C. Molecular dissection of mutations at the heterozygous thymidine kinase locus in mouse lymphoma cells. Proc Natl Acad Sci U S A. 1990 Jan;87(1):51–55. doi: 10.1073/pnas.87.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold J. E., Gevers W. Auto-ubiquitination of ubiquitin-activating enzymes from chicken breast muscle. Biochem J. 1990 May 1;267(3):751–757. doi: 10.1042/bj2670751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball E., Karlik C. C., Beall C. J., Saville D. L., Sparrow J. C., Bullard B., Fyrberg E. A. Arthrin, a myofibrillar protein of insect flight muscle, is an actin-ubiquitin conjugate. Cell. 1987 Oct 23;51(2):221–228. doi: 10.1016/0092-8674(87)90149-8. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., Lampert M. A., Lipsick J. S., Baluda M. A. Avian myeloblastosis virus and E26 virus oncogene products are nuclear proteins. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4265–4269. doi: 10.1073/pnas.81.14.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne P. S., Levy E. R., McLaren A. Spermatogenic failure in male mice lacking H-Y antigen. Nature. 1986 Mar 13;320(6058):170–172. doi: 10.1038/320170a0. [DOI] [PubMed] [Google Scholar]
- Carlson N., Rechsteiner M. Microinjection of ubiquitin: intracellular distribution and metabolism in HeLa cells maintained under normal physiological conditions. J Cell Biol. 1987 Mar;104(3):537–546. doi: 10.1083/jcb.104.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson N., Rogers S., Rechsteiner M. Microinjection of ubiquitin: changes in protein degradation in HeLa cells subjected to heat-shock. J Cell Biol. 1987 Mar;104(3):547–555. doi: 10.1083/jcb.104.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A., DiGiuseppe J. A., Bercovich B., Orian A., Richter J. D., Schwartz A. L., Brodeur G. M. Degradation of nuclear oncoproteins by the ubiquitin system in vitro. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):139–143. doi: 10.1073/pnas.88.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A., Elias S., Heller H., Hershko A. "Covalent affinity" purification of ubiquitin-activating enzyme. J Biol Chem. 1982 Mar 10;257(5):2537–2542. [PubMed] [Google Scholar]
- Ciechanover A., Finley D., Varshavsky A. Ubiquitin dependence of selective protein degradation demonstrated in the mammalian cell cycle mutant ts85. Cell. 1984 May;37(1):57–66. doi: 10.1016/0092-8674(84)90300-3. [DOI] [PubMed] [Google Scholar]
- Ciechanover A., Schwartz A. L. How are substrates recognized by the ubiquitin-mediated proteolytic system? Trends Biochem Sci. 1989 Dec;14(12):483–488. doi: 10.1016/0968-0004(89)90180-1. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Nuclear targeting sequences--a consensus? Trends Biochem Sci. 1991 Dec;16(12):478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- Ellison M. J., Hochstrasser M. Epitope-tagged ubiquitin. A new probe for analyzing ubiquitin function. J Biol Chem. 1991 Nov 5;266(31):21150–21157. [PubMed] [Google Scholar]
- Finley D., Chau V. Ubiquitination. Annu Rev Cell Biol. 1991;7:25–69. doi: 10.1146/annurev.cb.07.110191.000325. [DOI] [PubMed] [Google Scholar]
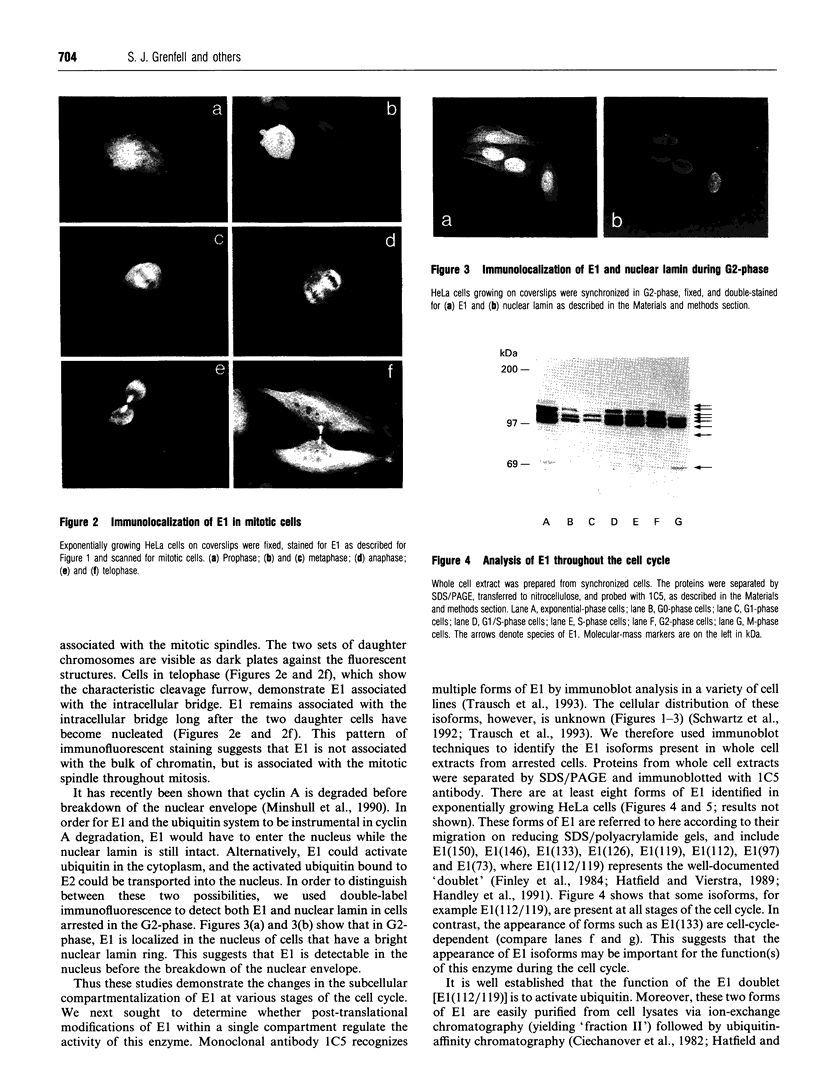
- Finley D., Ciechanover A., Varshavsky A. Thermolability of ubiquitin-activating enzyme from the mammalian cell cycle mutant ts85. Cell. 1984 May;37(1):43–55. doi: 10.1016/0092-8674(84)90299-x. [DOI] [PubMed] [Google Scholar]
- Glotzer M., Murray A. W., Kirschner M. W. Cyclin is degraded by the ubiquitin pathway. Nature. 1991 Jan 10;349(6305):132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Haas A. L., Rose I. A. The mechanism of ubiquitin activating enzyme. A kinetic and equilibrium analysis. J Biol Chem. 1982 Sep 10;257(17):10329–10337. [PubMed] [Google Scholar]
- Handley P. M., Mueckler M., Siegel N. R., Ciechanover A., Schwartz A. L. Molecular cloning, sequence, and tissue distribution of the human ubiquitin-activating enzyme E1. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):258–262. doi: 10.1073/pnas.88.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield P. M., Callis J., Vierstra R. D. Cloning of ubiquitin activating enzyme from wheat and expression of a functional protein in Escherichia coli. J Biol Chem. 1990 Sep 15;265(26):15813–15817. [PubMed] [Google Scholar]
- Hershko A., Ganoth D., Pehrson J., Palazzo R. E., Cohen L. H. Methylated ubiquitin inhibits cyclin degradation in clam embryo extracts. J Biol Chem. 1991 Sep 5;266(25):16376–16379. [PubMed] [Google Scholar]
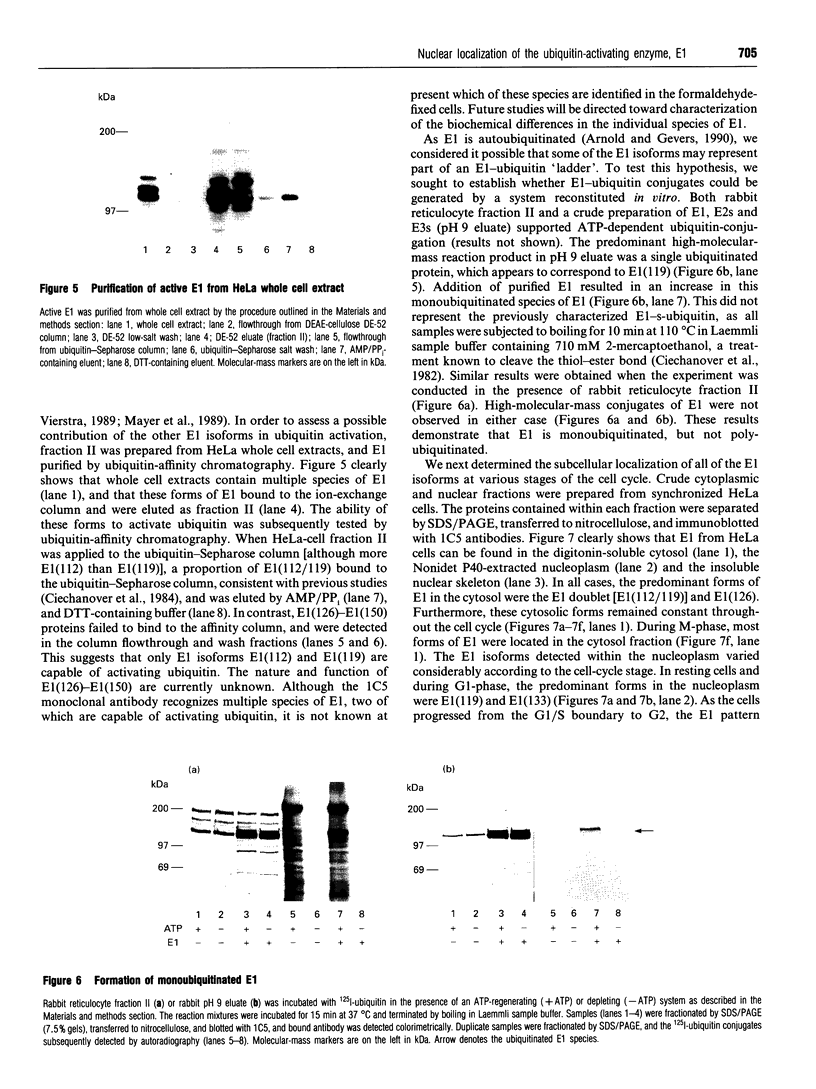
- Hershko A. The ubiquitin pathway for protein degradation. Trends Biochem Sci. 1991 Jul;16(7):265–268. doi: 10.1016/0968-0004(91)90101-z. [DOI] [PubMed] [Google Scholar]
- Jabben M., Shanklin J., Vierstra R. D. Ubiquitin-phytochrome conjugates. Pool dynamics during in vivo phytochrome degradation. J Biol Chem. 1989 Mar 25;264(9):4998–5005. [PubMed] [Google Scholar]
- Jentsch S., Seufert W., Sommer T., Reins H. A. Ubiquitin-conjugating enzymes: novel regulators of eukaryotic cells. Trends Biochem Sci. 1990 May;15(5):195–198. doi: 10.1016/0968-0004(90)90161-4. [DOI] [PubMed] [Google Scholar]
- Kay G. F., Ashworth A., Penny G. D., Dunlop M., Swift S., Brockdorff N., Rastan S. A candidate spermatogenesis gene on the mouse Y chromosome is homologous to ubiquitin-activating enzyme E1. Nature. 1991 Dec 12;354(6353):486–489. doi: 10.1038/354486a0. [DOI] [PubMed] [Google Scholar]
- Kulka R. G., Raboy B., Schuster R., Parag H. A., Diamond G., Ciechanover A., Marcus M. A Chinese hamster cell cycle mutant arrested at G2 phase has a temperature-sensitive ubiquitin-activating enzyme, E1. J Biol Chem. 1988 Oct 25;263(30):15726–15731. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
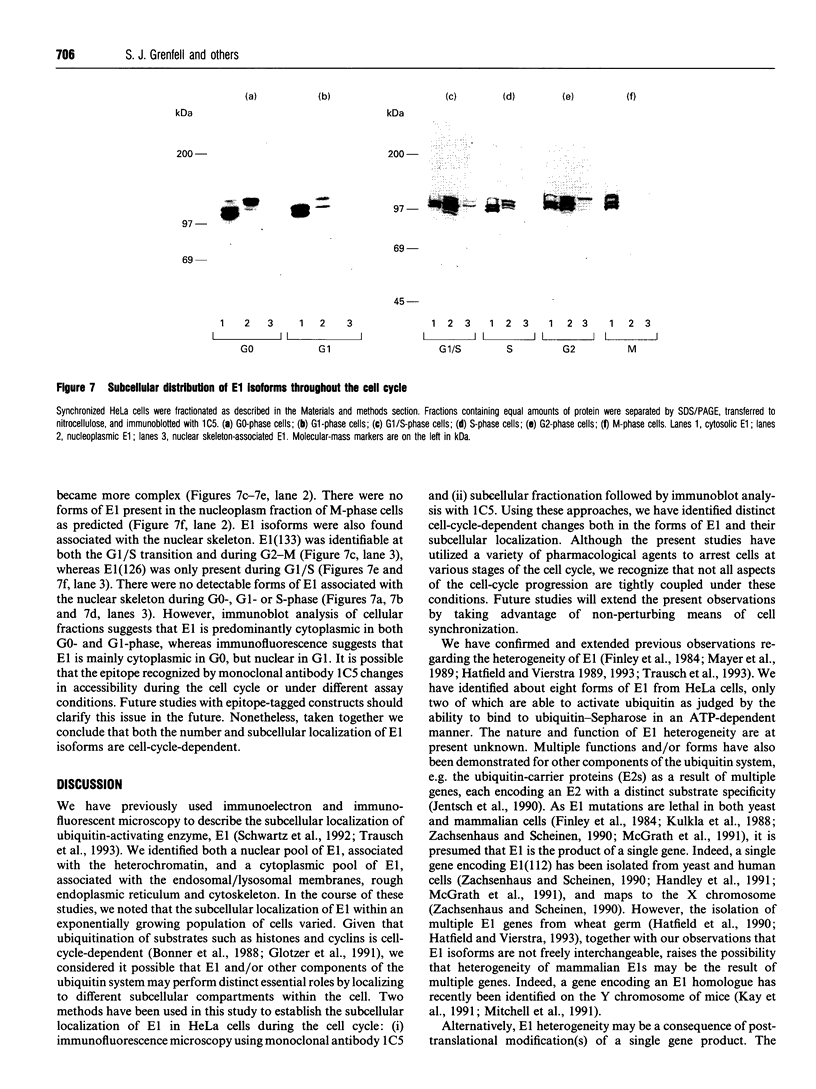
- Leung D. W., Spencer S. A., Cachianes G., Hammonds R. G., Collins C., Henzel W. J., Barnard R., Waters M. J., Wood W. I. Growth hormone receptor and serum binding protein: purification, cloning and expression. Nature. 1987 Dec 10;330(6148):537–543. doi: 10.1038/330537a0. [DOI] [PubMed] [Google Scholar]
- Luca F. C., Shibuya E. K., Dohrmann C. E., Ruderman J. V. Both cyclin A delta 60 and B delta 97 are stable and arrest cells in M-phase, but only cyclin B delta 97 turns on cyclin destruction. EMBO J. 1991 Dec;10(13):4311–4320. doi: 10.1002/j.1460-2075.1991.tb05009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Gropper R., Schwartz A. L., Ciechanover A. Purification, characterization, and rapid inactivation of thermolabile ubiquitin-activating enzyme from the mammalian cell cycle mutant ts85. J Biol Chem. 1989 Feb 5;264(4):2060–2068. [PubMed] [Google Scholar]
- McCurdy D. W., Pratt L. H. Immunogold electron microscopy of phytochrome in Avena: identification of intracellular sites responsible for phytochrome sequestering and enhanced pelletability. J Cell Biol. 1986 Dec;103(6 Pt 1):2541–2550. doi: 10.1083/jcb.103.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. P., Jentsch S., Varshavsky A. UBA 1: an essential yeast gene encoding ubiquitin-activating enzyme. EMBO J. 1991 Jan;10(1):227–236. doi: 10.1002/j.1460-2075.1991.tb07940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshull J., Golsteyn R., Hill C. S., Hunt T. The A- and B-type cyclin associated cdc2 kinases in Xenopus turn on and off at different times in the cell cycle. EMBO J. 1990 Sep;9(9):2865–2875. doi: 10.1002/j.1460-2075.1990.tb07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. J., Woods D. R., Tucker P. K., Opp J. S., Bishop C. E. Homology of a candidate spermatogenic gene from the mouse Y chromosome to the ubiquitin-activating enzyme E1. Nature. 1991 Dec 12;354(6353):483–486. doi: 10.1038/354483a0. [DOI] [PubMed] [Google Scholar]
- Pickart C. M., Rose I. A. Functional heterogeneity of ubiquitin carrier proteins. J Biol Chem. 1985 Feb 10;260(3):1573–1581. [PubMed] [Google Scholar]
- Pines J., Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell. 1989 Sep 8;58(5):833–846. doi: 10.1016/0092-8674(89)90936-7. [DOI] [PubMed] [Google Scholar]
- Schwartz A. L., Ciechanover A., Brandt R. A., Geuze H. J. Immunoelectron microscopic localization of ubiquitin in hepatoma cells. EMBO J. 1988 Oct;7(10):2961–2966. doi: 10.1002/j.1460-2075.1988.tb03158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A. L., Ciechanover A., Merritt S., Turkewitz A. Antibody-induced receptor loss. Different fates for asialoglycoproteins and the asialoglycoprotein receptor in HepG2 cells. J Biol Chem. 1986 Nov 15;261(32):15225–15232. [PubMed] [Google Scholar]
- Schwartz A. L., Trausch J. S., Ciechanover A., Slot J. W., Geuze H. Immunoelectron microscopic localization of the ubiquitin-activating enzyme E1 in HepG2 cells. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5542–5546. doi: 10.1073/pnas.89.12.5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinin R. Preliminary characterization of the temperature-sensitive defect in DNA replication in a mutant mouse L cell. Cell. 1976 Jan;7(1):49–57. doi: 10.1016/0092-8674(76)90254-3. [DOI] [PubMed] [Google Scholar]
- Trausch J. S., Grenfell S. J., Handley-Gearhart P. M., Ciechanover A., Schwartz A. L. Immunofluorescent localization of the ubiquitin-activating enzyme, E1, to the nucleus and cytoskeleton. Am J Physiol. 1993 Jan;264(1 Pt 1):C93–102. doi: 10.1152/ajpcell.1993.264.1.C93. [DOI] [PubMed] [Google Scholar]
- Tsuneoka M., Mekada E. Degradation of a nuclear-localized protein in mammalian COS cells, using Escherichia coli beta-galactosidase as a model protein. J Biol Chem. 1992 May 5;267(13):9107–9111. [PubMed] [Google Scholar]
- Turner B. M., Davies S., Whitfield W. G. Characterization of a family of nuclear and chromosomal proteins identified by a monoclonal antibody. Eur J Cell Biol. 1985 Sep;38(2):344–352. [PubMed] [Google Scholar]
- Yarden Y., Escobedo J. A., Kuang W. J., Yang-Feng T. L., Daniel T. O., Tremble P. M., Chen E. Y., Ando M. E., Harkins R. N., Francke U. Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature. 1986 Sep 18;323(6085):226–232. doi: 10.1038/323226a0. [DOI] [PubMed] [Google Scholar]
- Zacksenhaus E., Sheinin R. Molecular cloning, primary structure and expression of the human X linked A1S9 gene cDNA which complements the ts A1S9 mouse L cell defect in DNA replication. EMBO J. 1990 Sep;9(9):2923–2929. doi: 10.1002/j.1460-2075.1990.tb07483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]