Abstract
This study aims to elucidate the anti-hypoxia mechanism of sesamoside, an active component of Phlomis younghusbandii Mukerjee, through a network pharmacology approach. Sesamoside has demonstrated potential anti-oxidant and antiglycation activities. The hypoxia-related disease targets were collected from databases like GeneCards and OMIM. Protein-protein interaction (PPI) networks were constructed using the STRING database. GO/KEGG enrichment analysis was performed using the Metascape database to identify biological processes and signaling pathways. Our results indicate that sesamoside interacts with multiple targets related to glucose and lipid metabolism, nucleotide metabolism, and inflammatory, and we find that AKR1B1 (AR) plays a crucial role in sesamoside responses to hypoxia. Molecular docking studies were performed using Autodock software, revealing good binding activity between sesamoside and AR. We then use CCK-8 assay, qPCR, WB, and ELISA analysis to validate the role of sesamoside in regulating AR and participating in anti-hypoxia through cell experiments. The results show that compared with the hypoxia group, sesamoside treatment significantly improves the expression of AR and inflammation cytokines. In summary, this study sheds light on the anti-hypoxia mechanism of sesamoside using a network pharmacology approach, providing a theoretical basis and experimental foundation for its application in the prevention and treatment of hypoxic diseases.
Keywords: sesamoside, hypoxia, network pharmacology, AKR1B1
Graphical Abstract.
Introduction
With the advancement of modern transportation and tourism, an increasing number of individuals have the opportunity to visit high-altitude regions and experience their majestic scenery. However, high altitudes’ unique low-pressure and hypoxic environments challenge human health. Among these, high-altitude pulmonary edema (HAPE) stands as a severe altitude-related disease that has garnered significant attention from the medical community. HAPE occurs when individuals rapidly ascend to high altitudes, exposing themselves to low-pressure, hypoxic conditions, which lead to abnormal accumulation of fluid in the lungs, manifesting as symptoms such as dyspnea, cough, and the production of frothy sputum.1,2 In severe cases, it can even be life-threatening, with a mortality rate as high as 50%.3,4 The treatment and prevention strategies for HAPE are continuously being explored and improved. Existing treatment methods primarily include symptomatic treatment, causal treatment, and pharmacological therapy, aiming to alleviate patient symptoms, reduce the extent of pulmonary edema, and improve patients’ quality of life.5-7 However, these treatment methods still have limitations and shortcomings in practical applications, such as inconsistent treatment and significant side effects.
Environmental factors such as high-altitude hypoxia and low pressure lead to pathological and physiological changes such as excessive elevation of pulmonary arterial pressure, increased pulmonary capillary permeability, and dysregulation of fluid transport, which trigger HAPE. Firstly, hypoxia activates transcriptional factors such as Hypoxia-inducible factor (HIF), thereby regulating the transcription and translation of numerous genes. 8 These changes in gene expression can affect energy metabolism, homeostasis, and neuroendocrine systems and disrupt fluid balance and oxidative stress responses.9-11 These molecular alterations collectively contribute to the pathogenesis of HAPE in the lung tissue. Secondly, the increased permeability of pulmonary capillaries plays a pivotal role in the development of HAPE. Bronchoalveolar lavage fluid from HAPE patients shows high protein levels, inflammatory cells, increased red blood cell count, and C-reactive protein, indicating that HAPE is a form of exudative edema. 12 Animal studies suggest that vascular endothelial growth factor levels rise significantly under hypoxic conditions, increasing blood flow and damaging the vascular endothelium. NO is a vasodilator. In hypoxic conditions, individuals susceptible to HAPE experience a significant decrease in NO levels regulated by NO synthase (NOS). 13 Schemer and colleagues discovered that long-term residents of high-altitude areas have significantly higher NO levels in their lung tissues than newcomers. 2 Endothelin-1 (ET-1) is a potent vasoconstrictor synthesized in pulmonary endothelial cells, where it regulates the contraction of vascular smooth muscle cells and maintains vascular tension. Studies found that individuals susceptible to HAPE in high-altitude hypoxic environments have increased levels of ET-1 in their lungs. 14 The hypoxic high-altitude environment induces pulmonary vascular endothelial cells to secrete ET-1 and other vasoactive substances and adhesion molecules. These endothelial cells mediate the adhesion and aggregation of inflammatory cells through ET-1, contributing to the remodeling of pulmonary vessels and the onset of pulmonary hypertension. 15
Also, alveolar fluid clearance is impaired, and systemic inflammatory responses may contribute to the pathogenesis of HAPE. In high-altitude environments, the volume of alveolar fluid depends partly on the overflow of fluid from pulmonary vessels, primarily associated with hypoxic pulmonary arterial hypertension, and partly on the reabsorption rate by alveolar epithelium, determined by sodium transport in these cells. Hypoxia disrupts sodium transport in pulmonary epithelial cells, obstructing alveolar fluid clearance. Aldose reductase (AKR1B1, AR) is a member of the aldo-keto reductase superfamily of proteins in various human tissues and organs. 16
AR converts glucose to sorbitol and plays a role in the polyol metabolic pathway.17,18 Sorbitol’s high polarity prevents it from freely crossing the cell membrane, accumulating inside cells, and creating a hyperosmotic state. Hyperglycemia can change the intracellular ratio of reduced nicotinamide adenine dinucleotide phosphate to nicotinamide adenine dinucleotide via AR, resulting in increased production of reactive oxygen species within cells, intensifying severe oxidative stress and leading to apoptosis. 19 Nuclear factor kappa B (NF-κB) binds to promoters in the nucleus that encode inflammatory factors, initiating transcription and playing a role in the development of inflammation. 20 Inhibiting AR significantly reduced the transcriptional activity of NF-kappa-B and eased inflammation. 21 Inflammatory responses play a role in the development of HAPE by increasing pulmonary vascular permeability and pulmonary arterial pressure. 22 In high-altitude hypoxic conditions, NF-κB is activated, initiating various inflammatory factors, such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), initiates systemic inflammatory responses and binds to receptors in lung tissues after a series of inflammatory reactions, causes lysosomal damage and leakage, ultimately leading to lung injury. 23
In terms of treatment, different treatment measures should be taken based on the severity and progression of HAPE. For mild HAPE patients, general treatment measures such as rest and oxygen inhalation can be taken to alleviate symptoms. For severe HAPE patients, more aggressive treatment measures such as mechanical ventilation, diuretics, and pulmonary artery pressure-lowering drugs are needed to reduce the degree of pulmonary edema and improve the patient’s vital signs. 24 In recent years, with the deepening of research on natural drugs, more and more natural compounds have been discovered to have potential anti-hypoxic and anti-altitude disease effects. Among them, Phlomis younghusbandii Mukerjee is a perennial herb of the family Lamiaceae (known as “Lumur” in Tibetan), it has been used in Tibetan medicine for over a thousand years for treating colds, coughs, sores, scabies, rheumatoid arthritis, pneumonia, and bronchitis. 25 To expand the range of medicinal resources, Yongli et al conducted a systematic study on the chemical components of the above-ground parts of this medicinal herb, they identified 8 compounds: 8-acetylshanzhisidemethylester (1), shanzhisidemethylester (2), phlomiol (3), fructose butyrate (4), sesamoside (5), pulchelloside-I (6), luteolin-7-O-β-D-glucopyranoside (7), and daucosterol (8).25-27 These compounds were isolated for the first time from the above-ground parts of the Phlomis younghusbandii Mukerjee. Sesamoside, an active component of Phlomis younghusbandii Mukerjee, has few studies on treating high-altitude pulmonary edema. The preliminary research of our group found that aldose reductase is involved in hypoxic stress and, thus, in the occurrence and development of HAPE. Therefore, we explored the potential targets of sesame glycosides in hypoxic stress through network pharmacology analysis, providing a more theoretical basis for treating HAPE.
Materials and Methods
Reagents
Sesamoside and dexamethasone were purchased from Shanghai Yuanye Bio-Technology Co., Ltd. Cocl2 was purchased from Tianjin Kemiou Chemical Reagent Co., Ltd. BCA protein assay kit, reverse transcription kit, and DEPC-treated water were purchased from Yisheng Biotech Co., Ltd. Cell lysis buffer, PMSF, and SDS-PAGE protein loading buffer were from Beyotime Biotechnology Co., Ltd. SYBR Green qPCR Master Mix was purchased from Sevier Biotechnology Co., Ltd. TNF-α and IL-6 ELISA kit were purchased from Nanjing Jiancheng Bioengineering Institute.
Network Pharmacology Analysis
Collection of Targets for Sesamoside Extracted From Phlomis younghusbandii Mukerjee
First, the PubChem database was used to identify the chemical structure (https://pubchem.ncbi.nlm.nih.gov/). The compound’s SMILES (Simplified Molecular Input Line Entry System) code was obtained, and the compound’s three-dimensional structure was downloaded. The compounds were then screened using the Swiss Target Prediction and TargetNet (https://targetnet.scbdd.com) (Probability >0) online databases to obtain predicted targets. Finally, gene names of the retrieved targets were converted and standardized using the UniProt database (https://www.uniprot.org/). 28
Drug-Disease Target Prediction
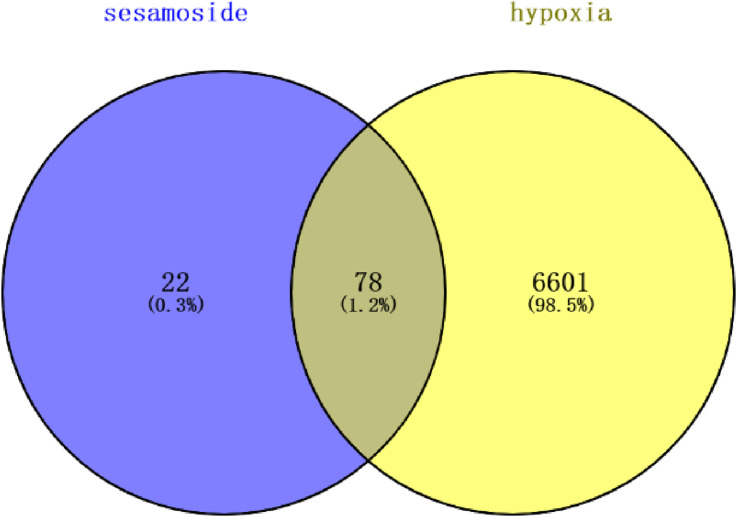
Using the words ‘hypoxia’ and ‘sesamoside’ we searched for disease-related targets in the GeneCards (https://www.genecards.org/), OMIM (https://www.omim.org/), and TTD (https://db.idrblab.net/ttd/). Then, we input the compound targets obtained in the aforementioned method and the disease targets into Venny 2.1.0 to identify the intersecting set. We summarized the intersecting genes to create a Venn diagram for sesamoside-hypoxia.
Construction of Component-Target Networks
The selected sesamoside and hypoxia targets were used to construct an interaction network diagram of targets and compounds through the Cytoscape platform (V3.9.1) (https://www.cytoscape.org/). Core targets were screened, and their degree values were calculated. Degree Centrality: In a network, the greater the degree of a node, the higher its degree of centrality, suggesting that the node is more important within the network.
GO Functional Enrichment Analysis and KEGG Pathway Enrichment Analysis
The selected core targets were analyzed using Metascape (https://metascape.org/), setting the species to ‘Homo sapiens’ for GO function enrichment analysis and KEGG pathway enrichment. 29 The corresponding bubble and bar charts were obtained using the ‘Bioinformatics’ website (https://www.bioinformatics.com.cn/). The results were ordered by P-value from smallest to largest for result analysis. P < .05 indicated statistical significance.
Construction of PPI Network and Screening of Core Targets
The drug-disease targets identified in section ‘2.1.2’ were analyzed using the STRING database (https://string-db.org). 30 The species was set to ‘Homo sapiens’ with a minimum interaction score of ≥0.7. Nodes not connected within the network are not displayed. This process generates a network diagram and outputs the results of the drug-disease target protein interactions.
Molecular Docking of Sesamoside With AR
The SMILES code of the compound was obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/), and the three-dimensional structure of the compound was downloaded and saved in SDF format. The three-dimensional structure and protein structure of AR were downloaded from PDB (https://www.rcsb.org/). ‘Homo sapiens,’ ‘X-RAY,’ and ‘Protein’ were selected as the criteria. Proteins with ‘bond to’ or ‘complex with’ were preferred. The docking site was confirmed by clicking on ‘small molecules’ on the detail page, and the file was saved in PDB format. Openbabel converted the downloaded compound’s SDF file into mol2 format, then Autodock vina into pdbqt format. Pymol was used to remove water molecules and other small molecules outside the ligand, extract the ligand, determine the docking box size, and save it in PDB format. The protein’s PDB file was opened with Autodock vina to add hydrogens and charges and saved in pdbqt format. Docking was used to obtain the docking energy. The affinity value was displayed upon successful docking, showing 9 mode values. The best binding site was selected based on the value of mode1. Using Pymol, the protein and compound are combined and saved in PDB format. The file was then uploaded to the website for analysis (https://projects.biotec.tu-dresden.de/plip-web/plip). The pse file was downloaded to create illustrations, and the RST file was used to analyze the specific interactions between the compound and the protein. Finally, Autodock Vina was employed for molecular docking of the small molecule compound with AR, and the results were visualized using Pymol.
CCK-8 Colorimetric Assay
CCK-8 analyzed the cell viability of cocl2 on BEAS-2B cells; cells were treated with 0-, 10-, 300-, 500-, 700-, or 900 μM cocl2 for 24 h, respectively. After cell treatment, CCK-8 was added and incubated at 37°C for 1-4 h, the absorbance was measured at 450 nm.
Cell Culture
Human bronchial epithelial cells (BEAS-2B) were cultured in a MEM complete medium with 10% fetal bovine serum and 1% antibiotics (100 UI/ml penicillin and 100 μg/mL streptomycin). Cells were divided into 4 groups: (1) NC group (blank control), (2) hypoxia group (cells treated with 800 μM cocl2 for 6 h), (3) DEX + hypoxia group (cells pre-treated with 1 mM dexamethasone for 24 h, followed by 800 μM cocl2 for 6 h), (4) sesamoside + hypoxia group (cells pre-treated with 200 μM sesamoside for 6 h, followed by 800 μM cocl2 for 6 h).
Quantitative Analyses of mRNA Expression by qPCR
Total RNA was isolated from cells using Trizol Reagent (Invitrogen) according to the manufacturer’s protocol and 1 μg total RNA was reverse-transcribed. qPCR was performed using the CFX96 connect instrument and a reaction mixture that consisted of SYBR Green PCR Master Mix, cDNA template, and primers listed below.
| Target Gene | Primer Sequences |
|---|---|
| AR | F: ACGCATTGCTGAGAACTTTAAG |
| R: TTCCTGTTGTAGCTGAGTAAGG | |
| 18S | F: GACGACCCATTCGAACGTCT |
| R: CTCTCCGGAATCGAACCCTGA | |
| VEGF-α | F: AGAAGGAGGAGGGCAGAATCATCAC |
| R: GGGCACACAGGATGGCTTGAAG | |
| ERK | F: TCGCCGAAGCACCATTCAAGTTC |
| R: TCCTGGCTGGAATCTAGCAGTCTC | |
| TNF-α | F: CACTTTGGAGTGATCGGCCC |
| R: AGCTTGAGGGTTTGCTACAAC | |
| HIF-1α | F: CCATTAGAAAGCAGTTCCGCAAGC |
| R: GTGGTAGTGGTGGCATTAGCAGTAG | |
| NF-κB | F: CCTGGACAGTGTGGAGTGTTACG |
| R: AGTTCTGCTGGTCAATCTGCTTCC | |
| IL-6 | F: GACAGCCACTCACCTCTTCAGAAC |
| R: GCCTCTTTGCTGCTTTCACACATG |
Western Blots Analysis
Protein concentrations for cell lysates were determined using a BCA protein assay kit. Proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membranes, and blocked with 5% BSA for 1 h at room temperature. Proteins were then detected using primary antibodies incubated overnight at 4°C, followed by secondary antibodies for 1 h at room temperature.
ELISA
Cell supernatant was collected. According to the manufacturer’s instructions, the concentrations of TNF-α and IL-6 were measured with ELISA kits.
Statistical Analysis
The data were expressed as mean ± standard deviation. Significant differences were expressed as *P < .05, **P < .01, and ***P < .001.
Results
Intersection of Disease Targets and Drug Targets
Disease-related targets were retrieved using the GeneCards, OMIM, and TTD databases, and standard targets were predicted using the Swiss Target Prediction and TargetNet online databases. The common targets were obtained by intersecting the component targets with disease targets using Venny 2.1.0 (https://bioinfogp.cnb.csic.es/tools/venny/), resulting in 78 targets (Figure 1). These 78 intersecting genes were inputted into the STRING database for PPI analysis. Using degree as the criterion for evaluation, 24 genes with a degree more significant than the average were identified (Table 1).
Figure 1.
Venn diagram of sesamoside anti-hypoxia targets.
Table 1.
Key Targets of Sesamoside in Anti-Hypoxia.
| Target | Degree |
|---|---|
| GAPDH | 42 |
| EGFR | 36 |
| CASP3 | 32 |
| SRC | 26 |
| HSP90AA1 | 25 |
| IL2 | 19 |
| PTGS2 | 19 |
| MCL1 | 17 |
| MMP2 | 17 |
| CDK1 | 15 |
| CASP8 | 15 |
| ABL1 | 14 |
| TERT | 13 |
| PTPN11 | 13 |
| PTPN1 | 13 |
| ADA | 13 |
| CA9 | 12 |
| ADK | 11 |
| PNP | 11 |
| HK2 | 11 |
| LCK | 10 |
| AKR1B1 | 10 |
| MGAM | 10 |
| CASP1 | 10 |
Table 1 24 genes with a degree more significant than the average were identified by PPI.
Network Pharmacology Analysis of the Target Pathway of Sesamoside for Anti-Hypoxia
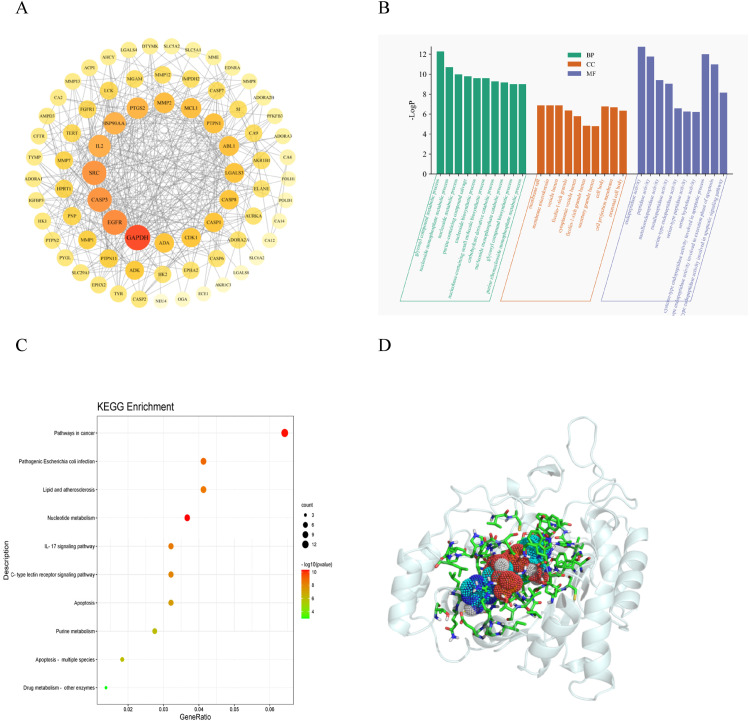
The 78 intersecting genes obtained from the sesamoside anti-hypoxia analysis were imported into the STRING database for PPI analysis. As mentioned in section 2.1.3, the higher the degree value of a node, the more critical it is within the network. Therefore, genes with a degree more significant than the average were identified as genes using degree as the criterion. The interaction network diagram for sesamoside’s anti-hypoxia effect was obtained. Nodes located centrally with more edges, larger sizes, and darker colors will likely be core targets (Figure 2A). The number of adjacent nodes is related to the probability of being a core gene; higher numbers indicate a greater likelihood of being a core target. The results identified 24 genes closely associated with sesamoside’s anti-hypoxia effect, among which AR was found. Subsequently, AR will be used as the gene for molecular docking.
Figure 2.
(A) Critical targets of sesamoside in the treatment of anti-hypoxia. (B) The GO enrichment analysis of critical targets. (C) The KEGG pathway enrichment analysis of critical targets. (D) Sesamoside-AR molecular docking pattern.
The selected core targets were analyzed using the Metascape online database for GO functional enrichment analysis and KEGG pathway enrichment analysis. GO functional enrichment analysis revealed that sesamoside is mainly involved in sugar and lipid metabolism, membrane vesicles, and peptidase activity in hypoxia-induced HAPE (Figure 2B). KEGG pathway analysis found that it primarily involves lipid metabolism, nucleotide metabolism, and the IL-17 inflammation signaling pathway (Figure 2C).
The structures of the compound and the protein were processed separately, and the size of their docking box was determined using Pymol. The binding energy between them was obtained through docking. Generally, lower binding energy indicates a stronger affinity between the receptor and ligand and a higher likelihood of binding. A binding energy less than 0 is considered significant; values less than −5 typically indicate strong affinity, with larger absolute values of binding energy indicating stronger affinity. The molecular docking results show that sesamoside binds well with the receptor protein (Table 2). The molecular docking binding energy between sesamoside and AR was −5.5 kcal/mol (Figure 2D). Sesamoside and the protein’s active site exhibited a compact binding mode within the active pocket, forming hydrogen bond interactions. In the Autodock environment, an absolute binding energy >.5 indicates the result is meaningful, and an absolute value >5 indicates a good result. We found that sesamoside formed a conformation with low energy and stable binding conformation with the receptor protein AR.
Table 2.
Binding Energy of Sesamoside With AR.
| Mode | Affinity (kcal/mol) | Dist from Best Mode rmsd l. b | rmsd u. b |
|---|---|---|---|
| 1 | −5.5 | 0.000 | 0.000 |
| 2 | −5.4 | 1.271 | 1.334 |
| 3 | −5.1 | 2.687 | 7.621 |
| 4 | −5.0 | 2.528 | 4.741 |
| 5 | −5.0 | 2.440 | 7.929 |
| 6 | −4.9 | 3.307 | 6.319 |
| 7 | −4.8 | 1.820 | 4.646 |
| 8 | −4.7 | 1.749 | 7.434 |
| 9 | −4.6 | 2.835 | 8.139 |
Table 2 The binding energy between sesamoside with ARwas determined using Pymol.
Sesamoside Reduced AR-Related Gene Expression Induced by Hypoxia
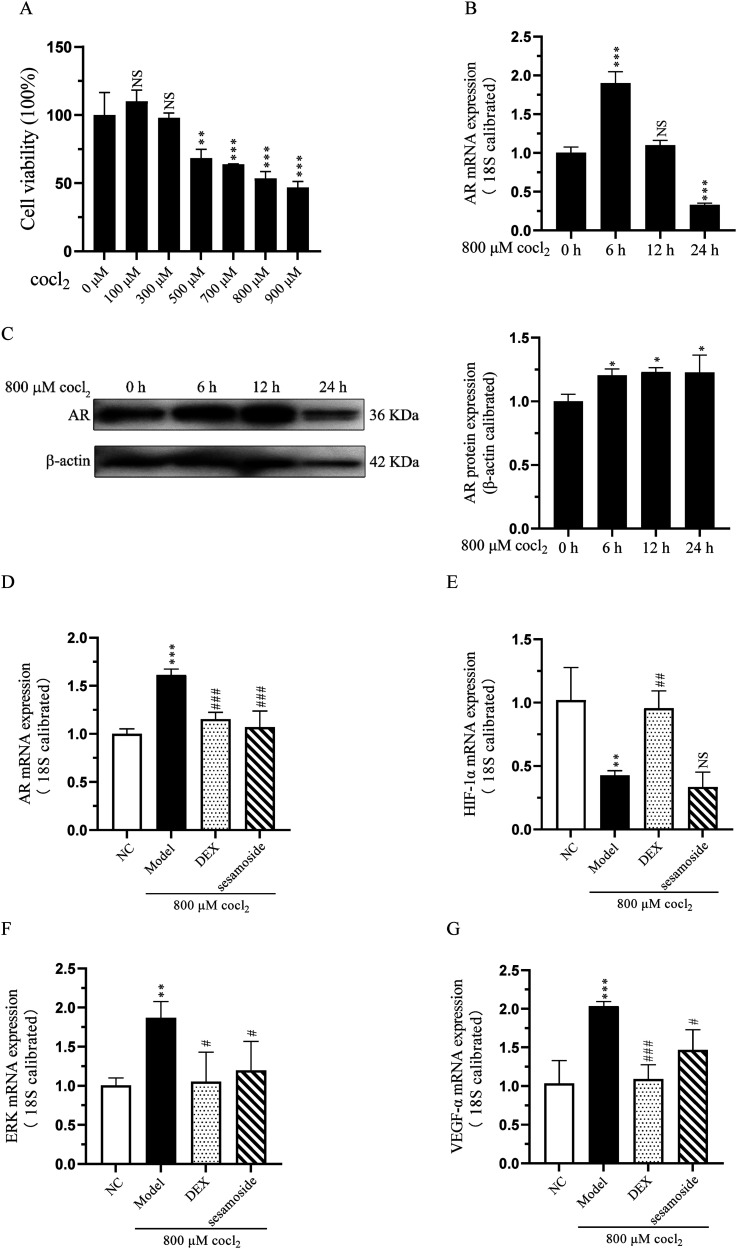
To validate the results of the network pharmacology analysis that sesamoside may exert its anti-hypoxia effect by regulating AR, we used cocl2 to treat BEAS-2B cells and established an in vitro hypoxia model. BEAS-2B cells were treated with a concentration gradient of 0-900 μM cocl2 for 24 h, cell viability was assessed using the CCK-8 assay. As shown in Figure 3A, cocl2 inhibited BEAS-2B cell viability with a 50% inhibitor concentration of 800 μΜ. Then we used 800 μΜ cocl2 stimulated BEAS-2B cell for 0-, 6-, 12-, or 24 h to detect the expression of AR, and found that the up-regulation trend of AR was the most significant at 6 h, therefore, we chose 800 μΜ treatment for 6 h to conduct subsequent experiments (Figure 3B and C).
Figure 3.
(A) The cell viability of cocl2 on BEAS-2B cells. (B) The mRNA expression of AR after cocl2 treatment. (C) The protein expression of AR after cocl2 treatment. (D) The mRNA expression of AR with sesamoside pre-treatment. (E) The mRNA expression of ERK with sesamoside pre-treatment. (F) The mRNA expression of VEGF-α with sesamoside pre-treatment. (G) The mRNA expression of HIF-1α with sesamoside pre-treatment. * P < .05, **P < .01 and *** P < .001 vs control group, NS, not significant. # P < .05, ## P < .01 and ### P < .001 vs model group, NS, not significant.
To determine the effects of sesamosaide on anti-hypoxia, cells were divided into 4 groups: (1) NC group (blank control), (2) hypoxia group, (3) DEX + hypoxia group, (4) sesamoside + hypoxia group. Compared to NC group, the mRNA expression of AR, ERK, and VEGF-α was significantly increased; however, when cells were pre-treated with DEX or sesamoside, the expression of AR, ERK, and VEGF-α was significantly decreased (Figure 3D-F). HIF-1 is a nuclear protein with transcriptional activity, associated with hypoxia adaptation, inflammation development, and tumor growth. In our research, the mRNA expression of HIF-1α was down-regulated compared with the NC group after hypoxia treatment, and sesamoside pre-treatment had no significant impact on its expression (Figure 3G).
Sesamosed Alleviates the Inflammatory Response Caused by Hypoxia
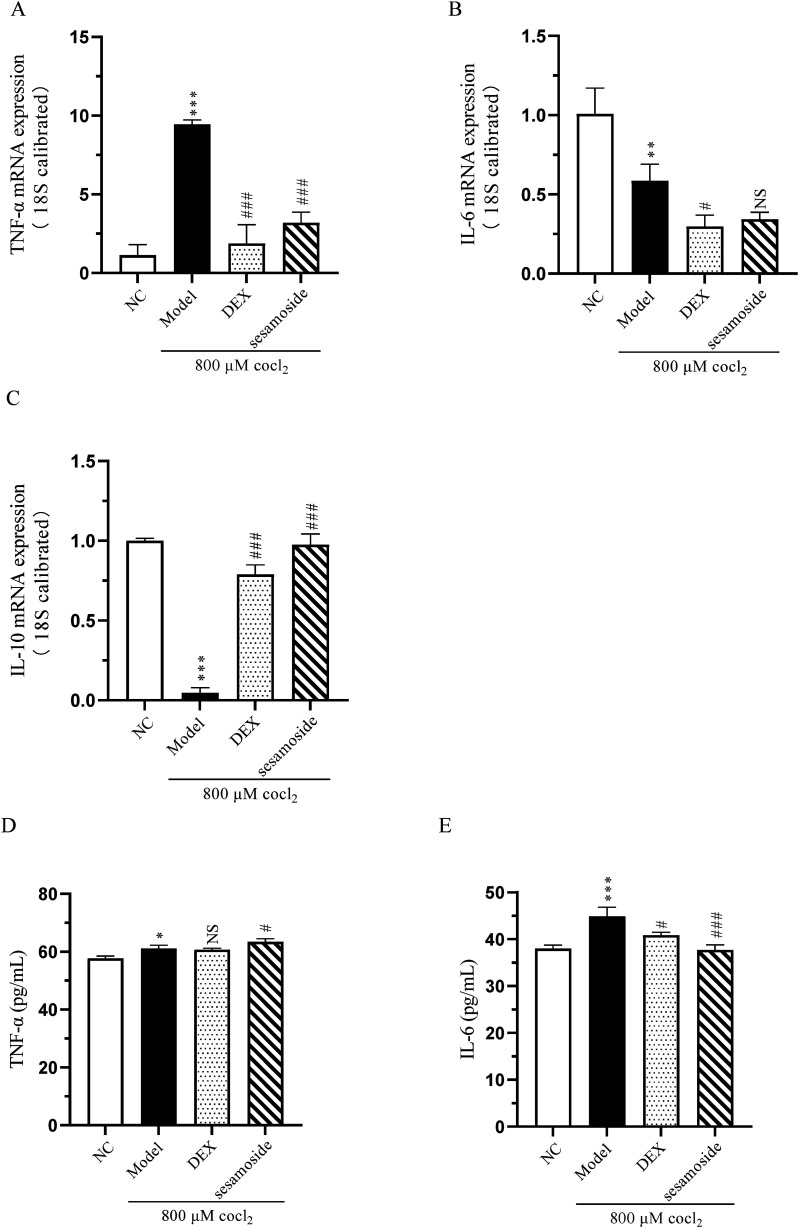
To determine the effect of sesamoside on hypoxia-induced inflammation response, we examined the transcription and secretion of cytokines TNF-α, IL-6 and IL-10. The qPCR and ELISA results revealed that compared with NC group, the expression of TNF-α and IL-6 was up-regulated and IL-10 was down-regulated in hypoxia group. Meahwhile, sesamoside pre-treatment improved this situation somewhat (Figure 4A-E). These findings suggest that sesamoside alleviates the inflammatory response caused by hypoxia.
Figure 4.
(A) The transcription of TNF-α. (B) The transcription of IL-6. (C) The transcription of IL-10. (D) The secretion of TNF-α. (E) The secretion of IL-6. * P < .05, ** P < .01 and *** P < .001 vs control group. # P < 0.05 and ### P < 0.001 vs model group, NS, not significant.
Discussion
With the rapid development of modern pharmacology and bioinformatics, network pharmacology, as an emerging research approach, has shown significant potential in drug discovery and mechanism exploration. The anti-hypoxic mechanism of sesamoside, a natural compound with potential pharmacological value, is essential for developing novel anti-hypoxia drugs. Network pharmacology allows us to construct a complex network model that can capture the interactions between sesamoside and their potential targets and how these targets participate in hypoxia-related biological processes. The unique low-pressure and hypoxic environments at high altitudes pose significant health challenges, particularly in the form of HAPE, a severe altitude-related disease with high mortality rates. Given the limitations of existing treatments, there is a pressing need to explore novel therapeutic agents and understand their mechanisms of action. Through this network, we delved into the anti-hypoxia mechanism of sesamoside, we can identify critical nodes and pathways, which may be the key factors for sesamoside exerting anti-hypoxic effects.
Our network pharmacology analysis identified multiple targets associated with sesamoside’s anti-hypoxic effects, primarily involved in glucose and lipid metabolism, nucleotide metabolism, and inflammatory processes. We predicted the core targets of sesamoside against high-altitude hypoxia, including AR, GAPDH, EGFR, IL-2, and others. Notably, AR emerged as a crucial target, given its role in the polyol metabolic pathway and regulation of oxidative stress and inflammation. Therefore, it can be inferred that the role of AR is not limited to being a rate-limiting enzyme in the polyol pathway, but is likely a crucial factor affecting the entire body’s energy supply and sugar and lipid metabolism. 31 This hypothesis is consistent with our GO analysis results. Combined with the KEGG pathway enrichment analysis, the primary pathways involved in prevention and treatment include cancer, pathogenic Escherichia coli infection, lipid and atherosclerosis, nucleotide metabolism, and the IL-17 inflammation signaling pathway. These signaling pathways are related to human immunity and inflammation, suggesting that they might be the path signals through which sesamoside exerts its anti-hypoxic effects by inhibiting the activation of inflammatory factors, thus mitigating excessive immune responses and exerting anti-inflammatory effects. TNF-α is crucial in inflammation, cell proliferation, and cell death. It is a primary inflammatory cytokine activating the NF-κB pathway. When the NF-κB pathway is activated, various inflammatory cytokines and related enzymes, including TNF-α and IL-6, are induced. These inflammatory mediators, in turn, stimulate macrophages themselves, forming an inflammatory cascade waterfall reaction, exacerbating inflammation development. 32 TNF-α initiates systemic inflammatory responses, binding to receptors in lung tissue after a series of inflammatory reactions, causing a storm of lung inflammation and inducing more cytokines production, leading to lysosomal damage and leakage, ultimately causing lung injury. The concentration of IL-6 in the serum of HAPE patients significantly increases throughout the disease process. 33 These results are consistent with our KEGG analysis. Molecular docking studies further corroborated the strong binding activity between sesamoside and AR, with a binding energy of −5.5 kcal/mol, indicating a stable and favorable interaction. Our in vitro validation experiments also indicate that sesamoside pre-treatment significantly reduced the expression of AR, ERK, and VEGF-α that were up-regulated by hypoxia. At the same time, sesamoside pre-treatment improved the transcription and secretion of cytokines TNFα- and IL-6 induced by hypoxia.
Our findings shed light on the anti-hypoxic mechanism of sesamoside, providing a theoretical basis and experimental foundation for its potential application in the prevention and treatment of hypoxic diseases, such as HAPE. The combination of network pharmacology and in vitro validation experiments has allowed us to identify critical targets and pathways through which sesamoside exerts its beneficial effects. While our study offers valuable insights, it is important to acknowledge the limitations of network pharmacology and the need for further experimental validation in animal models and clinical trials. Future research should focus on elucidating the precise molecular mechanisms underlying sesamoside’s anti-hypoxia effects and exploring its potential as a therapeutic agent for various hypoxic-related diseases.
Conclusion
In conclusion, by constructing a network model, predicting and validating target interactions, and integrating multiple omics data, the targets, signaling pathways, and biological functions of sesame glycosides in combating high-altitude hypoxia were explored and predicted. Our study underscores the potential of sesamoside as a novel anti-hypoxic agent with pleiotropic effects. The identification of AR as a key target of sesamoside’s actions sheds light on the underlying mechanisms of its anti-hypoxic effects. Experimental validation confirmed that sesamoside significantly modulates AR expression and reduces inflammatory cytokines, thereby mitigating hypoxia-induced cellular damage. This work provides a comprehensive theoretical basis and experimental foundation for the potential application of sesamoside in the prevention and treatment of hypoxic diseases.
Footnotes
Author Contributions: DS designed and organized the study, reviewing and editing, and provide fund support. MJ W performed the network pharmacology and experiments, prepared the original draft. YY Z performed the experiments, and provide fund support. XJ Z helped with data analysis and provide fund support. YR Z, HY Y and LZ helped with data acquisition and experimental materials prepar.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (Grant No. 32160165), the Natural Science Foundation of Tibet Autonomous Region (Grant No. XZ202201ZR0065G), Youth project of Xizang Minzu University (Grant No. 24MDQ06), Graduate Research Innovation and Practice Project of Xizang Minzu University (Grant No. Y2024012, Y2024004).
ORCID iD
References
- 1.Ahluwalia A, Underwood PJ. Acute Mountain Sickness Score. Treasure Island, FL: StatPearls; 2024. ineligible companies. Disclosure: Philipp Underwood declares no relevant financial relationships with ineligible companies. [PubMed] [Google Scholar]
- 2.Scherrer U, Rexhaj E, Jayet PY, Allemann Y, Sartori C. New insights in the pathogenesis of high-altitude pulmonary edema. Prog Cardiovasc Dis. 2010;52(6):485-492. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Zhang Y, Zhang Y. Research advances in pathogenesis and prophylactic measures of acute high altitude illness. Respir Med. 2018;145:145-152. [DOI] [PubMed] [Google Scholar]
- 4.Peterson DC, Hamel RN. Corneal Reflex. Treasure Island, FL: StatPearls; 2024. ineligible companies. Disclosure: Renee Hamel declares no relevant financial relationships with ineligible companies. [PubMed] [Google Scholar]
- 5.Johnson NJ, Luks AM. High-altitude medicine. Med Clin. 2016;100(2):357-369. [DOI] [PubMed] [Google Scholar]
- 6.Jensen JD, Vincent AL. High Altitude Pulmonary Edema. Treasure Island, FL: StatPearls; 2024. ineligible companies. Disclosure: Andrew Vincent declares no relevant financial relationships with ineligible companies. [PubMed] [Google Scholar]
- 7.Woods P, Alcock J. High-altitude pulmonary edema. Evol Med Public Health. 2021;9(1):118-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soree P, Gupta RK, Singh K, et al. Raised HIF1α during normoxia in high altitude pulmonary edema susceptible non-mountaineers. Sci Rep. 2016;6:26468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Xia Y, Huang Z, et al. Novel HIF-1-target gene isthmin1 contributes to hypoxia-induced hyperpermeability of pulmonary microvascular endothelial cells monolayers. Am J Physiol Cell Physiol. 2021;321(4):C671-C680. [DOI] [PubMed] [Google Scholar]
- 10.Sharma K, Mishra A, Singh HN, et al. High-altitude pulmonary edema is aggravated by risk loci and associated transcription factors in HIF-prolyl hydroxylases. Hum Mol Genet. 2021;30(18):1734-1749. [DOI] [PubMed] [Google Scholar]
- 11.Sharma S, Sandhir R, Ganju L, Kumar B, Singh Y. Unique mutations in mitochondrial DNA and associated pathways involved in high altitude pulmonary edema susceptibility in Indian lowlanders. J Biomol Struct Dyn. 2023;41(11):5183-5198. [DOI] [PubMed] [Google Scholar]
- 12.Hanaoka M, Droma Y, Naramoto A, Honda T, Kobayashi T, Kubo K. Vascular endothelial growth factor in patients with high-altitude pulmonary edema. J Appl Physiol (1985). 2003;94(5):1836-1840. [DOI] [PubMed] [Google Scholar]
- 13.Ali Z, Mishra A, Kumar R, et al. Interactions among vascular-tone modulators contribute to high altitude pulmonary edema and augmented vasoreactivity in highlanders. PLoS One. 2012;7(9):e44049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanazawa F, Nakanishi K, Osada H, et al. Expression of endothelin-1 in the brain and lung of rats exposed to permanent hypobaric hypoxia. Brain Res. 2005;1036(1–2):145-154. [DOI] [PubMed] [Google Scholar]
- 15.Droma Y, Hayano T, Takabayashi Y, et al. Endothelin-1 and interleukin-8 in high altitude pulmonary oedema. Eur Respir J. 1996;9(9):1947-1949. [DOI] [PubMed] [Google Scholar]
- 16.Ashina K, Tsubosaka Y, Kobayashi K, Omori K, Murata T. VEGF-induced blood flow increase causes vascular hyper-permeability in vivo. Biochem Biophys Res Commun. 2015;464(2):590-595. [DOI] [PubMed] [Google Scholar]
- 17.Zheng X, Zhang L, Zhai J, Chen Y, Luo H, Hu X. The molecular basis for inhibition of sulindac and its metabolites towards human aldose reductase. FEBS Lett. 2012;586(1):55-59. [DOI] [PubMed] [Google Scholar]
- 18.Khayami R, Hashemi SR, Kerachian MA. Role of aldo-keto reductase family 1 member B1 (AKR1B1) in the cancer process and its therapeutic potential. J Cell Mol Med. 2020;24(16):8890-8902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatnagar A, Srivastava SK. Aldose reductase: congenial and injurious profiles of an enigmatic enzyme. Biochem Med Metab Biol. 1992;48(2):91-121. [DOI] [PubMed] [Google Scholar]
- 20.Choi SE, Park YS, Koh HC. NF-κB/p53-activated inflammatory response involves in diquat-induced mitochondrial dysfunction and apoptosis. Environ Toxicol. 2018;33(10):1005-1018. [DOI] [PubMed] [Google Scholar]
- 21.Wang C, La L, Feng H, et al. Aldose reductase inhibitor engeletin suppresses pelvic inflammatory disease by blocking the phospholipase C/protein kinase C-dependent/NF-κB and MAPK cascades. J Agric Food Chem. 2020;68(42):11747-11757. [DOI] [PubMed] [Google Scholar]
- 22.Sarada SKS, Veeramohan, Himadri P, Mathew T, Saumya S, Chitharanjan M. Nifedipine inhibits hypoxia induced transvascular leakage through down regulation of NFkB. Respir Physiol Neurobiol. 2012;183(1):26-34. [DOI] [PubMed] [Google Scholar]
- 23.Shukla D, Saxena S, Purushothaman J, et al. Hypoxic preconditioning with cobalt ameliorates hypobaric hypoxia induced pulmonary edema in rat. Eur J Pharmacol. 2011;656(1-3):101-109. [DOI] [PubMed] [Google Scholar]
- 24.Poudel S, Gautam S, Adhikari P, Zafren K. Physiological effects of sildenafil versus placebo at high altitude: a systematic review. High Alt Med Biol. 2024;25(1):16-25. [DOI] [PubMed] [Google Scholar]
- 25.Luan F, Li M, Han K, et al. Phenylethanoid glycosides of Phlomis younghusbandii Mukerjee ameliorate acute hypobaric hypoxia-induced brain impairment in rats. Mol Immunol. 2019;108:81-88. [DOI] [PubMed] [Google Scholar]
- 26.Li Q, Yang S, Yang S, Xin F, Wang M. Anti-inflammatory activity of phlomisoside F isolated from Phlomis younghusbandii Mukerjee. Int Immunopharm. 2015;28(1):724-730. [DOI] [PubMed] [Google Scholar]
- 27.Zhao B, Liang HX, Yu YF, Dong X. [A new furanolabdane diterpene glycoside from Phlomis younghusbandii Mukerjee]. Yao Xue Xue Bao. 2009;44(1):60-62. [PubMed] [Google Scholar]
- 28.UniProt Consortium . UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47(D1):D506-D515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Y, Zhou B, Pache L, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10(1):1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szklarczyk D, Morris JH, Cook H, et al. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45(D1):D362-D368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Andrea F, Sartini S, Piano I, et al. Oxy-imino saccharidic derivatives as a new structural class of aldose reductase inhibitors endowed with anti-oxidant activity. J Enzym Inhib Med Chem. 2020;35(1):1194-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayden MS, Ghosh S. Regulation of NF-κB by TNF family cytokines. Semin Immunol. 2014;26(3):253-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rai N, Shihan M, Seeger W, Schermuly RT, Novoyatleva T. Genetic delivery and gene therapy in pulmonary hypertension. Int J Mol Sci. 2021;22(3):1179. [DOI] [PMC free article] [PubMed] [Google Scholar]