Abstract
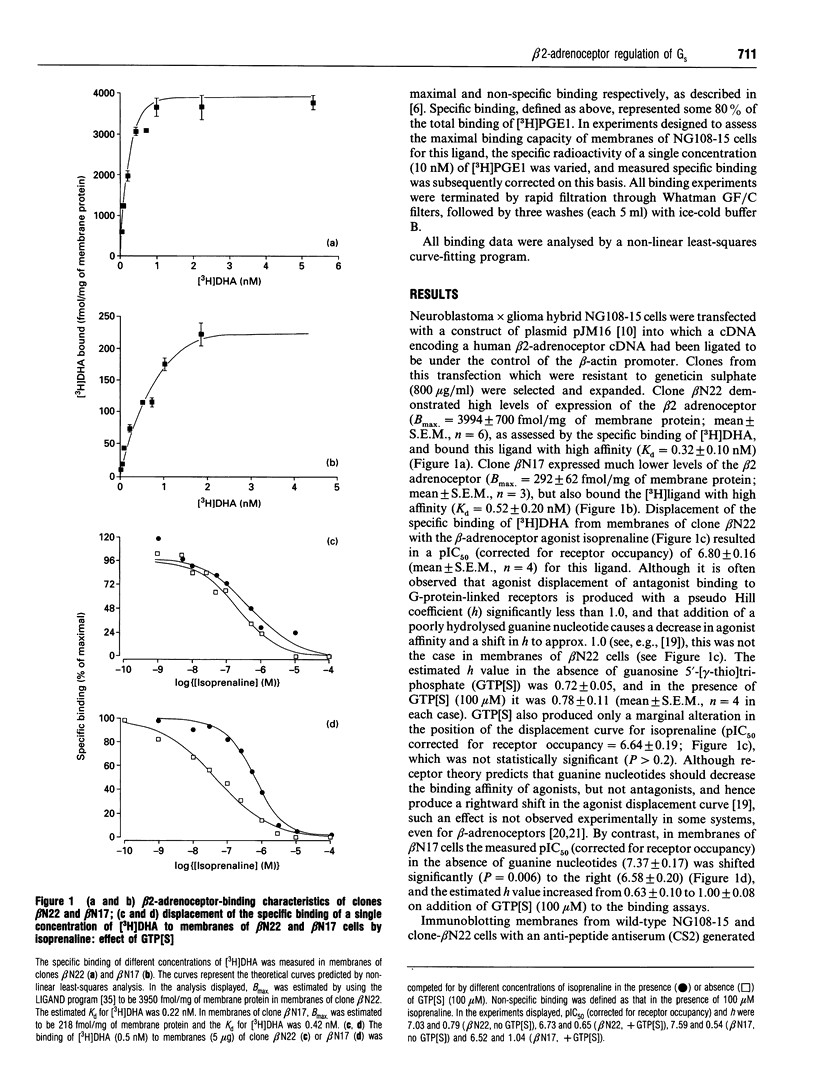
Neuroblastoma x glioma hybrid NG108-15 cells endogenously express at least three receptors which activate adenylate cyclase via the intermediacy of the stimulatory G-protein, Gs. Sustained exposure of the cells to agonists at the IP prostanoid receptor results in a substantial decrease in cellular levels of the alpha-subunit of Gs (Gs alpha) [McKenzie and Milligan (1990) J. Biol. Chem. 265, 17084-17093; Adie, Mullaney, McKenzie and Milligan (1992) Biochem J. 285, 529-536]. By contrast, equivalent treatments of the cells with agonists at either the A2 adenosine receptor or the secretin receptor have no measurable effect on cellular amounts of Gs alpha. To examine whether this is a feature specific to the IP prostanoid receptor or is related to the level of expression of the individual receptors, NG108-15 cells were transfected with a construct containing a human beta 2-adrenoceptor cDNA under the control of the beta-actin promoter. Two clones of these cells were examined in detail, beta N22, which expressed some 4000 fmol/mg of membrane protein, and clone beta N17, which expressed approx. 300 fmol/mg of membrane protein of the receptor. Exposure of beta N22 cells to the beta-adrenergic agonist isoprenaline resulted maximally in some 55% decrease in membrane-associated levels of Gs alpha, without effect on membrane levels of Gi2 alpha, Gi3 alpha, G(o) alpha or Gq alpha/G11 alpha. Dose-response curves to isoprenaline in beta N22 cells indicated that half-maximal down-regulation of Gs alpha was produced by approx. 1 nM agonist. Equivalent exposure of beta N17 cells to isoprenaline did not significantly modify levels of any of the G-protein alpha subunits, including Gs alpha. In beta N22 cells the IP prostanoid receptor was expressed at similar levels to those in wild-type NG108-15 cells, and treatment with iloprost resulted in a similar down-regulation of cellular Gs alpha levels. Iloprost was also effective in causing down-regulation of Gs alpha levels in clone beta N17. Concurrent addition of both isoprenaline and iloprost to clone beta N22 resulted in less than additive down-regulation of Gs alpha. These results demonstrate that the phenomenon of agonist-induced specific G-protein down-regulation is determined by the levels of expression of the receptor.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adie E. J., Milligan G. Agonist regulation of cellular levels of the stimulatory guanine nucleotide-binding protein, Gs, in wild type and transfected neuroblastoma-glioma hybrid NG108-15 cells. Biochem Soc Trans. 1993 May;21(2):432–435. doi: 10.1042/bst0210432. [DOI] [PubMed] [Google Scholar]
- Adie E. J., Mullaney I., McKenzie F. R., Milligan G. Concurrent down-regulation of IP prostanoid receptors and the alpha-subunit of the stimulatory guanine-nucleotide-binding protein (Gs) during prolonged exposure of neuroblastoma x glioma cells to prostanoid agonists. Quantification and functional implications. Biochem J. 1992 Jul 15;285(Pt 2):529–536. doi: 10.1042/bj2850529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P. T., Hnatowich M., O'Dowd B. F., Caron M. G., Lefkowitz R. J., Hausdorff W. P. Mutations of the human beta 2-adrenergic receptor that impair coupling to Gs interfere with receptor down-regulation but not sequestration. Mol Pharmacol. 1991 Feb;39(2):192–198. [PubMed] [Google Scholar]
- Dohlman H. G., Thorner J., Caron M. G., Lefkowitz R. J. Model systems for the study of seven-transmembrane-segment receptors. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- Donnelly L. E., Boyd R. S., MacDermot J. Gs alpha is a substrate for mono(ADP-ribosyl)transferase of NG108-15 cells. ADP-ribosylation regulates Gs alpha activity and abundance. Biochem J. 1992 Nov 15;288(Pt 1):331–336. doi: 10.1042/bj2880331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith P., Gierschik P., Milligan G., Unson C. G., Vinitsky R., Malech H. L., Spiegel A. M. Antibodies directed against synthetic peptides distinguish between GTP-binding proteins in neutrophil and brain. J Biol Chem. 1987 Oct 25;262(30):14683–14688. [PubMed] [Google Scholar]
- Gonzales J. M., O'Donnell J. K., Stadel J. M., Sweet R. W., Molinoff P. B. Down-regulation of beta-adrenergic receptors by pindolol in Gs alpha-transfected S49 cyc- murine lymphoma cells. J Neurochem. 1992 Mar;58(3):1093–1103. doi: 10.1111/j.1471-4159.1992.tb09367.x. [DOI] [PubMed] [Google Scholar]
- Green A., Johnson J. L., Milligan G. Down-regulation of Gi sub-types by prolonged incubation of adipocytes with an A1 adenosine receptor agonist. J Biol Chem. 1990 Mar 25;265(9):5206–5210. [PubMed] [Google Scholar]
- Green A., Milligan G., Dobias S. B. Gi down-regulation as a mechanism for heterologous desensitization in adipocytes. J Biol Chem. 1992 Feb 15;267(5):3223–3229. [PubMed] [Google Scholar]
- Gunning P., Leavitt J., Muscat G., Ng S. Y., Kedes L. A human beta-actin expression vector system directs high-level accumulation of antisense transcripts. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4831–4835. doi: 10.1073/pnas.84.14.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamprecht B., Glaser T., Reiser G., Bayer E., Propst F. Culture and characteristics of hormone-responsive neuroblastoma X glioma hybrid cells. Methods Enzymol. 1985;109:316–341. doi: 10.1016/0076-6879(85)09096-6. [DOI] [PubMed] [Google Scholar]
- Keen M., Kelly E., MacDermot J. Guanine nucleotide sensitivity of [3H]iloprost binding to prostacyclin receptors. Eur J Pharmacol. 1991 Jun 19;207(2):111–117. doi: 10.1016/0922-4106(91)90085-v. [DOI] [PubMed] [Google Scholar]
- Kelly E., Keen M., Nobbs P., MacDermot J. Segregation of discrete GS alpha-mediated responses that accompany homologous or heterologous desensitization in two related somatic hybrids. Br J Pharmacol. 1990 Feb;99(2):309–316. doi: 10.1111/j.1476-5381.1990.tb14700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G. D., Adie E. J., Milligan G. Quantitative stoichiometry of the proteins of the stimulatory arm of the adenylyl cyclase cascade in neuroblastoma x glioma hybrid, NG108-15 cells. Eur J Biochem. 1994 Jan 15;219(1-2):135–143. doi: 10.1111/j.1432-1033.1994.tb19923.x. [DOI] [PubMed] [Google Scholar]
- Levis M. J., Bourne H. R. Activation of the alpha subunit of Gs in intact cells alters its abundance, rate of degradation, and membrane avidity. J Cell Biol. 1992 Dec;119(5):1297–1307. doi: 10.1083/jcb.119.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liggett S. B., Ostrowski J., Chesnut L. C., Kurose H., Raymond J. R., Caron M. G., Lefkowitz R. J. Sites in the third intracellular loop of the alpha 2A-adrenergic receptor confer short term agonist-promoted desensitization. Evidence for a receptor kinase-mediated mechanism. J Biol Chem. 1992 Mar 5;267(7):4740–4746. [PubMed] [Google Scholar]
- Longabaugh J. P., Didsbury J., Spiegel A., Stiles G. L. Modification of the rat adipocyte A1 adenosine receptor-adenylate cyclase system during chronic exposure to an A1 adenosine receptor agonist: alterations in the quantity of GS alpha and Gi alpha are not associated with changes in their mRNAs. Mol Pharmacol. 1989 Nov;36(5):681–688. [PubMed] [Google Scholar]
- McKenzie F. R., Adie E. J., Milligan G. Prostanoid-mediated downregulation of Gs in NG108-15 cells. Biochem Soc Trans. 1991 Apr;19(2):81S–81S. doi: 10.1042/bst019081s. [DOI] [PubMed] [Google Scholar]
- McKenzie F. R., Milligan G. Delta-opioid-receptor-mediated inhibition of adenylate cyclase is transduced specifically by the guanine-nucleotide-binding protein Gi2. Biochem J. 1990 Apr 15;267(2):391–398. doi: 10.1042/bj2670391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie F. R., Milligan G. Prostaglandin E1-mediated, cyclic AMP-independent, down-regulation of Gs alpha in neuroblastoma x glioma hybrid cells. J Biol Chem. 1990 Oct 5;265(28):17084–17093. [PubMed] [Google Scholar]
- Milligan G. Agonist regulation of cellular G protein levels and distribution: mechanisms and functional implications. Trends Pharmacol Sci. 1993 Nov;14(11):413–418. doi: 10.1016/0165-6147(93)90064-Q. [DOI] [PubMed] [Google Scholar]
- Milligan G. Foetal-calf serum stimulates a pertussis-toxin-sensitive high-affinity GTPase activity in rat glioma C6 BU1 cells. Biochem J. 1987 Jul 15;245(2):501–505. doi: 10.1042/bj2450501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G., Unson C. G. Persistent activation of the alpha subunit of Gs promotes its removal from the plasma membrane. Biochem J. 1989 Jun 15;260(3):837–841. doi: 10.1042/bj2600837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell F. M., Buckley N. J., Milligan G. Enhanced degradation of the phosphoinositidase C-linked guanine-nucleotide-binding protein Gq alpha/G11 alpha following activation of the human M1 muscarinic acetylcholine receptor expressed in CHO cells. Biochem J. 1993 Jul 15;293(Pt 2):495–499. doi: 10.1042/bj2930495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell F. M., Griffiths S. L., Saggerson E. D., Houslay M. D., Knowler J. T., Milligan G. Guanine-nucleotide-binding proteins expressed in rat white adipose tissue. Identification of both mRNAs and proteins corresponding to Gi1, Gi2 and Gi3. Biochem J. 1989 Sep 1;262(2):403–408. doi: 10.1042/bj2620403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell F. M., Mullaney I., Godfrey P. P., Arkinstall S. J., Wakelam M. J., Milligan G. Widespread distribution of Gq alpha/G11 alpha detected immunologically by an antipeptide antiserum directed against the predicted C-terminal decapeptide. FEBS Lett. 1991 Aug 5;287(1-2):171–174. doi: 10.1016/0014-5793(91)80043-3. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D., Chang F. H., Cheever L., Kim M., Diamond I., Gordon A. S. Chronic ethanol causes heterologous desensitization of receptors by reducing alpha s messenger RNA. Nature. 1988 Jun 30;333(6176):848–850. doi: 10.1038/333848a0. [DOI] [PubMed] [Google Scholar]
- Mullaney I., Dodd M. W., Buckley N., Milligan G. Agonist activation of transfected human M1 muscarinic acetylcholine receptors in CHO cells results in down-regulation of both the receptor and the alpha subunit of the G-protein Gq. Biochem J. 1993 Jan 1;289(Pt 1):125–131. doi: 10.1042/bj2890125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullaney I., Mitchell F. M., McCallum J. F., Buckley N. J., Milligan G. The human muscarinic M1 acetylcholine receptor, when express in CHO cells, activates and downregulates both Gq alpha and G11 alpha equally and non-selectively. FEBS Lett. 1993 Jun 14;324(2):241–245. doi: 10.1016/0014-5793(93)81401-k. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Maguire M. E., Sturgill T. W., Biltonen R. L., Gilman A. G. Relationship between the beta-adrenergic receptor and adenylate cyclase. J Biol Chem. 1977 Aug 25;252(16):5761–5775. [PubMed] [Google Scholar]
- Sibley D. R., Lefkowitz R. J. Molecular mechanisms of receptor desensitization using the beta-adrenergic receptor-coupled adenylate cyclase system as a model. Nature. 1985 Sep 12;317(6033):124–129. doi: 10.1038/317124a0. [DOI] [PubMed] [Google Scholar]
- Tolkovsky A. M., Levitzki A. Mode of coupling between the beta-adrenergic receptor and adenylate cyclase in turkey erythrocytes. Biochemistry. 1978 Sep 5;17(18):3795–3795. doi: 10.1021/bi00611a020. [DOI] [PubMed] [Google Scholar]