Abstract
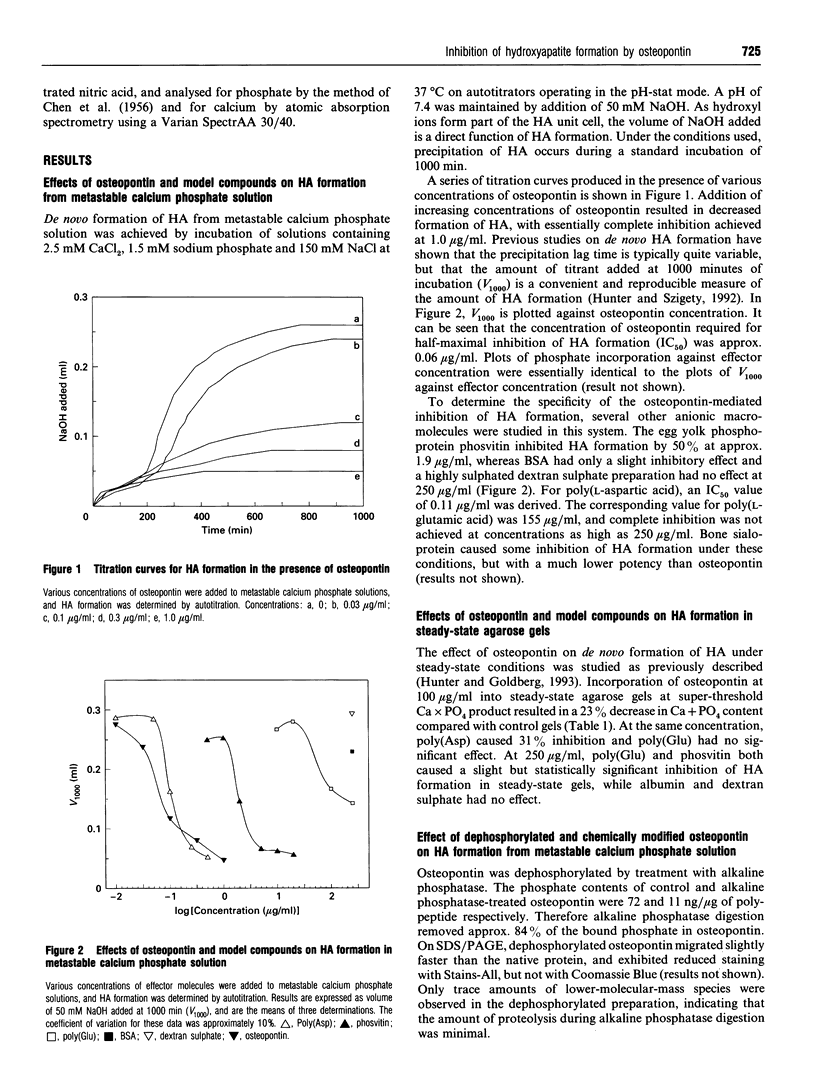
Osteopontin is a phosphorylated sialoprotein containing a conserved sequence of contiguous aspartic acid residues. This protein is expressed at high levels in mineralized tissues and has previously been shown to inhibit the in vitro formation of hydroxyapatite (HA). In the present study, protein modification and model compound studies have been used to identify the structural features of osteopontin that are responsible for its crystal-modulating properties. Using metastable calcium phosphate solutions buffered by autotitration, osteopontin caused half-maximal inhibition of HA formation at a concentration (IC50) of 0.06 microgram/ml. The hen egg yolk phosphoprotein phosvitin was a much weaker inhibitor, while dextran sulphate had no effect. The synthetic polypeptide poly(aspartic acid) was almost as effective an inhibitor of HA formation as osteopontin (IC50 0.11 microgram/ml), whereas poly(glutamic acid) was more than a thousand times less potent (IC50 155 micrograms/ml). In a steady-state agarose gel system, much higher polypeptide concentrations were required for inhibition of HA formation, but a similar relative order of inhibitory effectiveness was observed. Treatment of osteopontin with alkaline phosphatase removed 84% of the covalently bound phosphate and reduced its HA-inhibiting activity by more than 40-fold. Treatment with glycine ethyl ester in the presence of carbodi-imide modified 86% of the carboxylate groups in osteopontin and reduced its inhibitory activity by 6-fold. These findings indicate that osteopontin is a potent inhibitor of HA formation. This activity requires phosphate and carboxylate groups, possibly including the conserved sequence of contiguous aspartic acid residues. Osteopontin may act as an inhibitor of phase separation in physiological fluids of high supersaturation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addadi L., Moradian J., Shay E., Maroudas N. G., Weiner S. A chemical model for the cooperation of sulfates and carboxylates in calcite crystal nucleation: Relevance to biomineralization. Proc Natl Acad Sci U S A. 1987 May;84(9):2732–2736. doi: 10.1073/pnas.84.9.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P., Fisher L. W., Young M. F., Termine J. D., Robey P. G. Expression of bone sialoprotein (BSP) in developing human tissues. Calcif Tissue Int. 1991 Dec;49(6):421–426. doi: 10.1007/BF02555854. [DOI] [PubMed] [Google Scholar]
- Boskey A. L., Maresca M., Doty S., Sabsay B., Veis A. Concentration-dependent effects of dentin phosphophoryn in the regulation of in vitro hydroxyapatite formation and growth. Bone Miner. 1990 Oct;11(1):55–65. doi: 10.1016/0169-6009(90)90015-8. [DOI] [PubMed] [Google Scholar]
- Boskey A. L., Maresca M., Ullrich W., Doty S. B., Butler W. T., Prince C. W. Osteopontin-hydroxyapatite interactions in vitro: inhibition of hydroxyapatite formation and growth in a gelatin-gel. Bone Miner. 1993 Aug;22(2):147–159. doi: 10.1016/s0169-6009(08)80225-5. [DOI] [PubMed] [Google Scholar]
- Brown L. F., Berse B., Van de Water L., Papadopoulos-Sergiou A., Perruzzi C. A., Manseau E. J., Dvorak H. F., Senger D. R. Expression and distribution of osteopontin in human tissues: widespread association with luminal epithelial surfaces. Mol Biol Cell. 1992 Oct;3(10):1169–1180. doi: 10.1091/mbc.3.10.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Singh K., Mukherjee B. B., Sodek J. Developmental expression of osteopontin (OPN) mRNA in rat tissues: evidence for a role for OPN in bone formation and resorption. Matrix. 1993 Mar;13(2):113–123. doi: 10.1016/s0934-8832(11)80070-3. [DOI] [PubMed] [Google Scholar]
- Doi Y., Horiguchi T., Kim S. H., Moriwaki Y., Wakamatsu N., Adachi M., Ibaraki K., Moriyama K., Sasaki S., Shimokawa H. Effects of non-collagenous proteins on the formation of apatite in calcium beta-glycerophosphate solutions. Arch Oral Biol. 1992 Jan;37(1):15–21. doi: 10.1016/0003-9969(92)90147-z. [DOI] [PubMed] [Google Scholar]
- Doi Y., Okuda R., Takezawa Y., Shibata S., Moriwaki Y., Wakamatsu N., Shimizu N., Moriyama K., Shimokawa H. Osteonectin inhibiting de novo formation of apatite in the presence of collagen. Calcif Tissue Int. 1989 Mar;44(3):200–208. doi: 10.1007/BF02556565. [DOI] [PubMed] [Google Scholar]
- Fisher L. W., Whitson S. W., Avioli L. V., Termine J. D. Matrix sialoprotein of developing bone. J Biol Chem. 1983 Oct 25;258(20):12723–12727. [PubMed] [Google Scholar]
- Franzén A., Heinegård D. Isolation and characterization of two sialoproteins present only in bone calcified matrix. Biochem J. 1985 Dec 15;232(3):715–724. doi: 10.1042/bj2320715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa R., Kuboki Y., Sasaki S. Effects of dentin phosphophoryn on precipitation of calcium phosphate in gel in vitro. Calcif Tissue Int. 1987 Jul;41(1):44–47. doi: 10.1007/BF02555130. [DOI] [PubMed] [Google Scholar]
- Garnett J., Dieppe P. The effects of serum and human albumin on calcium hydroxyapatite crystal growth. Biochem J. 1990 Mar 15;266(3):863–868. [PMC free article] [PubMed] [Google Scholar]
- Glimcher M. J. Mechanism of calcification: role of collagen fibrils and collagen-phosphoprotein complexes in vitro and in vivo. Anat Rec. 1989 Jun;224(2):139–153. doi: 10.1002/ar.1092240205. [DOI] [PubMed] [Google Scholar]
- Hay D. I., Carlson E. R., Schluckebier S. K., Moreno E. C., Schlesinger D. H. Inhibition of calcium phosphate precipitation by human salivary acidic proline-rich proteins: structure-activity relationships. Calcif Tissue Int. 1987 Mar;40(3):126–132. doi: 10.1007/BF02555696. [DOI] [PubMed] [Google Scholar]
- Hoare D. G., Koshland D. E., Jr A method for the quantitative modification and estimation of carboxylic acid groups in proteins. J Biol Chem. 1967 May 25;242(10):2447–2453. [PubMed] [Google Scholar]
- Hunter G. K., Goldberg H. A. Nucleation of hydroxyapatite by bone sialoprotein. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8562–8565. doi: 10.1073/pnas.90.18.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter G. K., Szigety S. K. Effects of proteoglycan on hydroxyapatite formation under non-steady-state and pseudo-steady-state conditions. Matrix. 1992 Nov;12(5):362–368. doi: 10.1016/s0934-8832(11)80032-6. [DOI] [PubMed] [Google Scholar]
- Kiefer M. C., Bauer D. M., Barr P. J. The cDNA and derived amino acid sequence for human osteopontin. Nucleic Acids Res. 1989 Apr 25;17(8):3306–3306. doi: 10.1093/nar/17.8.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohri K., Suzuki Y., Yoshida K., Yamamoto K., Amasaki N., Yamate T., Umekawa T., Iguchi M., Sinohara H., Kurita T. Molecular cloning and sequencing of cDNA encoding urinary stone protein, which is identical to osteopontin. Biochem Biophys Res Commun. 1992 Apr 30;184(2):859–864. doi: 10.1016/0006-291x(92)90669-c. [DOI] [PubMed] [Google Scholar]
- Linde A., Lussi A., Crenshaw M. A. Mineral induction by immobilized polyanionic proteins. Calcif Tissue Int. 1989 Apr;44(4):286–295. doi: 10.1007/BF02553763. [DOI] [PubMed] [Google Scholar]
- McDiarmid R., Doty P. The spectrophotometric titration of polyacrylic, poly-L-aspartic, and poly-L-glutamic acids. J Phys Chem. 1966 Aug;70(8):2620–2627. doi: 10.1021/j100880a030. [DOI] [PubMed] [Google Scholar]
- McKee M. D., Farach-Carson M. C., Butler W. T., Hauschka P. V., Nanci A. Ultrastructural immunolocalization of noncollagenous (osteopontin and osteocalcin) and plasma (albumin and alpha 2HS-glycoprotein) proteins in rat bone. J Bone Miner Res. 1993 Apr;8(4):485–496. doi: 10.1002/jbmr.5650080413. [DOI] [PubMed] [Google Scholar]
- Menanteau J., Neuman W. F., Neuman M. W. A study of bone proteins which can prevent hydroxyapatite formation. Metab Bone Dis Relat Res. 1982;4(2):157–162. doi: 10.1016/0221-8747(82)90030-3. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y., Setoguchi M., Yoshida S., Higuchi Y., Akizuki S., Yamamoto S. Nucleotide sequence of cDNA for mouse osteopontin-like protein. Nucleic Acids Res. 1989 Apr 25;17(8):3298–3298. doi: 10.1093/nar/17.8.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. A., Gotoh Y., Rafidi K., Gerstenfeld L. C. Characterization of a cDNA for chicken osteopontin: expression during bone development, osteoblast differentiation, and tissue distribution. Biochemistry. 1991 Mar 5;30(9):2501–2508. doi: 10.1021/bi00223a029. [DOI] [PubMed] [Google Scholar]
- Mueller E., Sikes C. S. Adsorption and modification of calcium salt crystal growth by anionic peptides and spermine. Calcif Tissue Int. 1993 Jan;52(1):34–41. doi: 10.1007/BF00675624. [DOI] [PubMed] [Google Scholar]
- Nawrot C. F., Campbell D. J., Schroeder J. K., Van Valkenburg M. Dental phosphoprotein-induced formation of hydroxylapatite during in vitro synthesis of amorphous calcium phosphate. Biochemistry. 1976 Aug 10;15(16):3445–3449. doi: 10.1021/bi00661a008. [DOI] [PubMed] [Google Scholar]
- Nomura S., Wills A. J., Edwards D. R., Heath J. K., Hogan B. L. Developmental expression of 2ar (osteopontin) and SPARC (osteonectin) RNA as revealed by in situ hybridization. J Cell Biol. 1988 Feb;106(2):441–450. doi: 10.1083/jcb.106.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldberg A., Franzén A., Heinegård D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldberg A., Franzén A., Heinegård D. The primary structure of a cell-binding bone sialoprotein. J Biol Chem. 1988 Dec 25;263(36):19430–19432. [PubMed] [Google Scholar]
- Prince C. W., Oosawa T., Butler W. T., Tomana M., Bhown A. S., Bhown M., Schrohenloher R. E. Isolation, characterization, and biosynthesis of a phosphorylated glycoprotein from rat bone. J Biol Chem. 1987 Feb 25;262(6):2900–2907. [PubMed] [Google Scholar]
- Raj P. A., Johnsson M., Levine M. J., Nancollas G. H. Salivary statherin. Dependence on sequence, charge, hydrogen bonding potency, and helical conformation for adsorption to hydroxyapatite and inhibition of mineralization. J Biol Chem. 1992 Mar 25;267(9):5968–5976. [PubMed] [Google Scholar]
- Romberg R. W., Werness P. G., Riggs B. L., Mann K. G. Inhibition of hydroxyapatite crystal growth by bone-specific and other calcium-binding proteins. Biochemistry. 1986 Mar 11;25(5):1176–1180. doi: 10.1021/bi00353a035. [DOI] [PubMed] [Google Scholar]
- Senger D. R., Perruzzi C. A., Papadopoulos A., Tenen D. G. Purification of a human milk protein closely similar to tumor-secreted phosphoproteins and osteopontin. Biochim Biophys Acta. 1989 Jun 13;996(1-2):43–48. doi: 10.1016/0167-4838(89)90092-7. [DOI] [PubMed] [Google Scholar]
- Shiraga H., Min W., VanDusen W. J., Clayman M. D., Miner D., Terrell C. H., Sherbotie J. R., Foreman J. W., Przysiecki C., Neilson E. G. Inhibition of calcium oxalate crystal growth in vitro by uropontin: another member of the aspartic acid-rich protein superfamily. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):426–430. doi: 10.1073/pnas.89.1.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K., Mukherjee A. B., De Vouge M. W., Mukherjee B. B. Differential processing of osteopontin transcripts in rat kidney- and osteoblast-derived cell lines. J Biol Chem. 1992 Nov 25;267(33):23847–23851. [PubMed] [Google Scholar]
- Staros J. V., Wright R. W., Swingle D. M. Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal Biochem. 1986 Jul;156(1):220–222. doi: 10.1016/0003-2697(86)90176-4. [DOI] [PubMed] [Google Scholar]
- Taborsky G. Phosvitin. Adv Inorg Biochem. 1983;5:235–279. [PubMed] [Google Scholar]
- Termine J. D., Conn K. M. Inhibition of apatite formation by phosphorylated metabolites and macromolecules. Calcif Tissue Res. 1976 Dec 22;22(2):149–157. doi: 10.1007/BF02010354. [DOI] [PubMed] [Google Scholar]
- Termine J. D., Eanes E. D., Conn K. M. Phosphoprotein modulation of apatite crystallization. Calcif Tissue Int. 1980;31(3):247–251. doi: 10.1007/BF02407188. [DOI] [PubMed] [Google Scholar]
- Termine J. D., Kleinman H. K., Whitson S. W., Conn K. M., McGarvey M. L., Martin G. R. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981 Oct;26(1 Pt 1):99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- Van Veldhoven P. P., Mannaerts G. P. Inorganic and organic phosphate measurements in the nanomolar range. Anal Biochem. 1987 Feb 15;161(1):45–48. doi: 10.1016/0003-2697(87)90649-x. [DOI] [PubMed] [Google Scholar]
- White S. W., Hulmes D. J., Miller A., Timmins P. A. Collagen-mineral axial relationship in calcified turkey leg tendon by X-ray and neutron diffraction. Nature. 1977 Mar 31;266(5601):421–425. doi: 10.1038/266421a0. [DOI] [PubMed] [Google Scholar]
- Wrana J. L., Zhang Q., Sodek J. Full length cDNA sequence of porcine secreted phosphoprotein-I (SPP-I, osteopontin). Nucleic Acids Res. 1989 Dec 11;17(23):10119–10119. doi: 10.1093/nar/17.23.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M. F., Kerr J. M., Ibaraki K., Heegaard A. M., Robey P. G. Structure, expression, and regulation of the major noncollagenous matrix proteins of bone. Clin Orthop Relat Res. 1992 Aug;(281):275–294. [PubMed] [Google Scholar]
- Zhang Q., Domenicucci C., Goldberg H. A., Wrana J. L., Sodek J. Characterization of fetal porcine bone sialoproteins, secreted phosphoprotein I (SPPI, osteopontin), bone sialoprotein, and a 23-kDa glycoprotein. Demonstration that the 23-kDa glycoprotein is derived from the carboxyl terminus of SPPI. J Biol Chem. 1990 May 5;265(13):7583–7589. [PubMed] [Google Scholar]


