Abstract
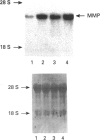
Chicken embryo fibroblasts secrete a 72 kDa progelatinase that displays all of the characteristics of a matrix metalloproteinase. Employing reverse-transcription PCR and degenerate oligonucleotide primers that are specific for two highly conserved sequences found in all matrix metalloproteinases, a DNA fragment specific for the chicken gelatinase was generated. Using this PCR product as a probe, cDNA clones were isolated from a chicken embryo cDNA library and the entire protein coding sequence was determined. The chicken progelatinase is 84% identical, at the amino acid level, with human and mouse 72 kDa progelatinase/type-IV procollagenase, with the greatest degree of similarity occurring in the propeptide and catalytic domains. The avian and mammalian proteinases diverge significantly in the C-terminal, hemopexin-like domain. The last 100 residues of the chicken gelatinase are only 66% identical with mammalian gelatinases. Mouse 72 kDa progelatinase, however, does not diverge significantly (> 98% identity) from human progelatinase in the hemopexin-like domain. The divergence in this domain of the chicken progelatinase may explain some of the distinct catalytic and inhibitory properties of the 72 kDa chicken progelatinase. Northern-blot analysis reveals that steady-state levels of the chicken progelatinase mRNA are increased 5-fold upon malignant transformation of chicken embryo fibroblasts with Rous sarcoma virus (RSV) and 3-fold by treatment with the tumour-promoting phorbol ester, phorbol 12-myristate 13-acetate (PMA). This represents the first reported cloning of an avian matrix metalloproteinase. The increased expression of the chicken progelatinase by RSV transformation and the tumour promoter PMA suggests that the progelatinase is regulated differently in chicken cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Basset P., Bellocq J. P., Wolf C., Stoll I., Hutin P., Limacher J. M., Podhajcer O. L., Chenard M. P., Rio M. C., Chambon P. A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature. 1990 Dec 20;348(6303):699–704. doi: 10.1038/348699a0. [DOI] [PubMed] [Google Scholar]
- Case J. P., Lafyatis R., Kumkumian G. K., Remmers E. F., Wilder R. L. IL-1 regulation of transin/stromelysin transcription in rheumatoid synovial fibroblasts appears to involve two antagonistic transduction pathways, an inhibitory, prostaglandin-dependent pathway mediated by cAMP, and a stimulatory, protein kinase C-dependent pathway. J Immunol. 1990 Dec 1;145(11):3755–3761. [PubMed] [Google Scholar]
- Chen J. M., Aimes R. T., Ward G. R., Youngleib G. L., Quigley J. P. Isolation and characterization of a 70-kDa metalloprotease (gelatinase) that is elevated in Rous sarcoma virus-transformed chicken embryo fibroblasts. J Biol Chem. 1991 Mar 15;266(8):5113–5121. [PubMed] [Google Scholar]
- Chin J. R., Murphy G., Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. Biosynthesis, isolation, characterization, and substrates. J Biol Chem. 1985 Oct 5;260(22):12367–12376. [PubMed] [Google Scholar]
- Clark I. M., Cawston T. E. Fragments of human fibroblast collagenase. Purification and characterization. Biochem J. 1989 Oct 1;263(1):201–206. doi: 10.1042/bj2630201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier I. E., Wilhelm S. M., Eisen A. Z., Marmer B. L., Grant G. A., Seltzer J. L., Kronberger A., He C. S., Bauer E. A., Goldberg G. I. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem. 1988 May 15;263(14):6579–6587. [PubMed] [Google Scholar]
- D'Aquila R. T., Bechtel L. J., Videler J. A., Eron J. J., Gorczyca P., Kaplan J. C. Maximizing sensitivity and specificity of PCR by pre-amplification heating. Nucleic Acids Res. 1991 Jul 11;19(13):3749–3749. doi: 10.1093/nar/19.13.3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty A. J., Murphy G. The tissue metalloproteinase family and the inhibitor TIMP: a study using cDNAs and recombinant proteins. Ann Rheum Dis. 1990 Jun;49 (Suppl 1):469–479. [PubMed] [Google Scholar]
- Docherty A. J., O'Connell J., Crabbe T., Angal S., Murphy G. The matrix metalloproteinases and their natural inhibitors: prospects for treating degenerative tissue diseases. Trends Biotechnol. 1992 Jun;10(6):200–207. doi: 10.1016/0167-7799(92)90214-g. [DOI] [PubMed] [Google Scholar]
- Fairbairn S., Gilbert R., Ojakian G., Schwimmer R., Quigley J. P. The extracellular matrix of normal chick embryo fibroblasts: its effect on transformed chick fibroblasts and its proteolytic degradation by the transformants. J Cell Biol. 1985 Nov;101(5 Pt 1):1790–1798. doi: 10.1083/jcb.101.5.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg G. I., Marmer B. L., Grant G. A., Eisen A. Z., Wilhelm S., He C. S. Human 72-kilodalton type IV collagenase forms a complex with a tissue inhibitor of metalloproteases designated TIMP-2. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8207–8211. doi: 10.1073/pnas.86.21.8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg G. I., Wilhelm S. M., Kronberger A., Bauer E. A., Grant G. A., Eisen A. Z. Human fibroblast collagenase. Complete primary structure and homology to an oncogene transformation-induced rat protein. J Biol Chem. 1986 May 15;261(14):6600–6605. [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Howard E. W., Bullen E. C., Banda M. J. Regulation of the autoactivation of human 72-kDa progelatinase by tissue inhibitor of metalloproteinases-2. J Biol Chem. 1991 Jul 15;266(20):13064–13069. [PubMed] [Google Scholar]
- Huhtala P., Chow L. T., Tryggvason K. Structure of the human type IV collagenase gene. J Biol Chem. 1990 Jul 5;265(19):11077–11082. [PubMed] [Google Scholar]
- Huhtala P., Tuuttila A., Chow L. T., Lohi J., Keski-Oja J., Tryggvason K. Complete structure of the human gene for 92-kDa type IV collagenase. Divergent regulation of expression for the 92- and 72-kilodalton enzyme genes in HT-1080 cells. J Biol Chem. 1991 Sep 5;266(25):16485–16490. [PubMed] [Google Scholar]
- Lee C. C., Wu X. W., Gibbs R. A., Cook R. G., Muzny D. M., Caskey C. T. Generation of cDNA probes directed by amino acid sequence: cloning of urate oxidase. Science. 1988 Mar 11;239(4845):1288–1291. doi: 10.1126/science.3344434. [DOI] [PubMed] [Google Scholar]
- Librach C. L., Werb Z., Fitzgerald M. L., Chiu K., Corwin N. M., Esteves R. A., Grobelny D., Galardy R., Damsky C. H., Fisher S. J. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J Cell Biol. 1991 Apr;113(2):437–449. doi: 10.1083/jcb.113.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Steeg P. S., Stetler-Stevenson W. G. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991 Jan 25;64(2):327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- Macartney H. W., Tschesche H. Latent and active human polymorphonuclear leukocyte collagenases. Isolation, purification and characterisation. Eur J Biochem. 1983 Jan 17;130(1):71–78. doi: 10.1111/j.1432-1033.1983.tb07118.x. [DOI] [PubMed] [Google Scholar]
- Mackay A. R., Ballin M., Pelina M. D., Farina A. R., Nason A. M., Hartzler J. L., Thorgeirsson U. P. Effect of phorbol ester and cytokines on matrix metalloproteinase and tissue inhibitor of metalloproteinase expression in tumor and normal cell lines. Invasion Metastasis. 1992;12(3-4):168–184. [PubMed] [Google Scholar]
- Marcy A. I., Eiberger L. L., Harrison R., Chan H. K., Hutchinson N. I., Hagmann W. K., Cameron P. M., Boulton D. A., Hermes J. D. Human fibroblast stromelysin catalytic domain: expression, purification, and characterization of a C-terminally truncated form. Biochemistry. 1991 Jul 2;30(26):6476–6483. doi: 10.1021/bi00240a018. [DOI] [PubMed] [Google Scholar]
- Marti H. P., McNeil L., Davies M., Martin J., Lovett D. H. Homology cloning of rat 72 kDa type IV collagenase: cytokine and second-messenger inducibility in glomerular mesangial cells. Biochem J. 1993 Apr 15;291(Pt 2):441–446. doi: 10.1042/bj2910441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M., Glaichenhaus N., Gesnel M. C., Breathnach R. Epidermal growth factor and oncogenes induce transcription of the same cellular mRNA in rat fibroblasts. EMBO J. 1985 Jun;4(6):1435–1440. doi: 10.1002/j.1460-2075.1985.tb03799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M., Leroy P., Ruhlmann C., Gesnel M. C., Breathnach R. Isolation of the oncogene and epidermal growth factor-induced transin gene: complex control in rat fibroblasts. Mol Cell Biol. 1986 May;6(5):1679–1686. doi: 10.1128/mcb.6.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead D. A., Pey N. K., Herrnstadt C., Marcil R. A., Smith L. M. A universal method for the direct cloning of PCR amplified nucleic acid. Biotechnology (N Y) 1991 Jul;9(7):657–663. doi: 10.1038/nbt0791-657. [DOI] [PubMed] [Google Scholar]
- Morodomi T., Ogata Y., Sasaguri Y., Morimatsu M., Nagase H. Purification and characterization of matrix metalloproteinase 9 from U937 monocytic leukaemia and HT1080 fibrosarcoma cells. Biochem J. 1992 Jul 15;285(Pt 2):603–611. doi: 10.1042/bj2850603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D., Quantin B., Gesnel M. C., Millon-Collard R., Abecassis J., Breathnach R. The collagenase gene family in humans consists of at least four members. Biochem J. 1988 Jul 1;253(1):187–192. doi: 10.1042/bj2530187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Allan J. A., Willenbrock F., Cockett M. I., O'Connell J. P., Docherty A. J. The role of the C-terminal domain in collagenase and stromelysin specificity. J Biol Chem. 1992 May 15;267(14):9612–9618. [PubMed] [Google Scholar]
- Murphy G., McAlpine C. G., Poll C. T., Reynolds J. J. Purification and characterization of a bone metalloproteinase that degrades gelatin and types IV and V collagen. Biochim Biophys Acta. 1985 Sep 20;831(1):49–58. doi: 10.1016/0167-4838(85)90148-7. [DOI] [PubMed] [Google Scholar]
- Murphy G., Willenbrock F., Ward R. V., Cockett M. I., Eaton D., Docherty A. J. The C-terminal domain of 72 kDa gelatinase A is not required for catalysis, but is essential for membrane activation and modulates interactions with tissue inhibitors of metalloproteinases. Biochem J. 1992 May 1;283(Pt 3):637–641. doi: 10.1042/bj2830637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H., Ogata Y., Suzuki K., Enghild J. J., Salvesen G. Substrate specificities and activation mechanisms of matrix metalloproteinases. Biochem Soc Trans. 1991 Aug;19(3):715–718. doi: 10.1042/bst0190715. [DOI] [PubMed] [Google Scholar]
- Nicholson R., Murphy G., Breathnach R. Human and rat malignant-tumor-associated mRNAs encode stromelysin-like metalloproteinases. Biochemistry. 1989 Jun 13;28(12):5195–5203. doi: 10.1021/bi00438a042. [DOI] [PubMed] [Google Scholar]
- Okada Y., Harris E. D., Jr, Nagase H. The precursor of a metalloendopeptidase from human rheumatoid synovial fibroblasts. Purification and mechanisms of activation by endopeptidases and 4-aminophenylmercuric acetate. Biochem J. 1988 Sep 15;254(3):731–741. doi: 10.1042/bj2540731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Morodomi T., Enghild J. J., Suzuki K., Yasui A., Nakanishi I., Salvesen G., Nagase H. Matrix metalloproteinase 2 from human rheumatoid synovial fibroblasts. Purification and activation of the precursor and enzymic properties. Eur J Biochem. 1990 Dec 27;194(3):721–730. doi: 10.1111/j.1432-1033.1990.tb19462.x. [DOI] [PubMed] [Google Scholar]
- Overall C. M., Wrana J. L., Sodek J. Transcriptional and post-transcriptional regulation of 72-kDa gelatinase/type IV collagenase by transforming growth factor-beta 1 in human fibroblasts. Comparisons with collagenase and tissue inhibitor of matrix metalloproteinase gene expression. J Biol Chem. 1991 Jul 25;266(21):14064–14071. [PubMed] [Google Scholar]
- Pavloff N., Staskus P. W., Kishnani N. S., Hawkes S. P. A new inhibitor of metalloproteinases from chicken: ChIMP-3. A third member of the TIMP family. J Biol Chem. 1992 Aug 25;267(24):17321–17326. [PubMed] [Google Scholar]
- Quantin B., Murphy G., Breathnach R. Pump-1 cDNA codes for a protein with characteristics similar to those of classical collagenase family members. Biochemistry. 1989 Jun 27;28(13):5327–5334. doi: 10.1021/bi00439a004. [DOI] [PubMed] [Google Scholar]
- Quigley J. P., Berkenpas M. B., Aimes R. T., Chen J. M. Serine protease and metallo protease cascade systems involved in pericellular proteolysis. Cell Differ Dev. 1990 Dec 2;32(3):263–275. doi: 10.1016/0922-3371(90)90039-y. [DOI] [PubMed] [Google Scholar]
- Quigley J. P., Gold L. I., Schwimmer R., Sullivan L. M. Limited cleavage of cellular fibronectin by plasminogen activator purified from transformed cells. Proc Natl Acad Sci U S A. 1987 May;84(9):2776–2780. doi: 10.1073/pnas.84.9.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988 Aug 5;241(4866):708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Reponen P., Sahlberg C., Huhtala P., Hurskainen T., Thesleff I., Tryggvason K. Molecular cloning of murine 72-kDa type IV collagenase and its expression during mouse development. J Biol Chem. 1992 Apr 15;267(11):7856–7862. [PubMed] [Google Scholar]
- Sakanari J. A., Staunton C. E., Eakin A. E., Craik C. S., McKerrow J. H. Serine proteases from nematode and protozoan parasites: isolation of sequence homologs using generic molecular probes. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4863–4867. doi: 10.1073/pnas.86.13.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo T., Liotta L. A., Tryggvason K. Purification and characterization of a murine basement membrane collagen-degrading enzyme secreted by metastatic tumor cells. J Biol Chem. 1983 Mar 10;258(5):3058–3063. [PubMed] [Google Scholar]
- Sanchez-Lopez R., Alexander C. M., Behrendtsen O., Breathnach R., Werb Z. Role of zinc-binding- and hemopexin domain-encoded sequences in the substrate specificity of collagenase and stromelysin-2 as revealed by chimeric proteins. J Biol Chem. 1993 Apr 5;268(10):7238–7247. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer J. L., Adams S. A., Grant G. A., Eisen A. Z. Purification and properties of a gelatin-specific neutral protease from human skin. J Biol Chem. 1981 May 10;256(9):4662–4668. [PubMed] [Google Scholar]
- Shapiro S. D., Griffin G. L., Gilbert D. J., Jenkins N. A., Copeland N. G., Welgus H. G., Senior R. M., Ley T. J. Molecular cloning, chromosomal localization, and bacterial expression of a murine macrophage metalloelastase. J Biol Chem. 1992 Mar 5;267(7):4664–4671. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Springman E. B., Angleton E. L., Birkedal-Hansen H., Van Wart H. E. Multiple modes of activation of latent human fibroblast collagenase: evidence for the role of a Cys73 active-site zinc complex in latency and a "cysteine switch" mechanism for activation. Proc Natl Acad Sci U S A. 1990 Jan;87(1):364–368. doi: 10.1073/pnas.87.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Krutzsch H. C., Liotta L. A. Tissue inhibitor of metalloproteinase (TIMP-2). A new member of the metalloproteinase inhibitor family. J Biol Chem. 1989 Oct 15;264(29):17374–17378. [PubMed] [Google Scholar]
- Sullivan L. M., Quigley J. P. An anticatalytic monoclonal antibody to avian plasminogen activator: its effect on behavior of RSV-transformed chick fibroblasts. Cell. 1986 Jun 20;45(6):905–915. doi: 10.1016/0092-8674(86)90565-9. [DOI] [PubMed] [Google Scholar]
- Tryggvason K., Huhtala P., Tuuttila A., Chow L., Keski-Oja J., Lohi J. Structure and expression of type IV collagenase genes. Cell Differ Dev. 1990 Dec 2;32(3):307–312. doi: 10.1016/0922-3371(90)90044-w. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Auld D. S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 1990 Jun 19;29(24):5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- Van Wart H. E., Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S. M., Collier I. E., Marmer B. L., Eisen A. Z., Grant G. A., Goldberg G. I. SV40-transformed human lung fibroblasts secrete a 92-kDa type IV collagenase which is identical to that secreted by normal human macrophages. J Biol Chem. 1989 Oct 15;264(29):17213–17221. [PubMed] [Google Scholar]
- Willenbrock F., Crabbe T., Slocombe P. M., Sutton C. W., Docherty A. J., Cockett M. I., O'Shea M., Brocklehurst K., Phillips I. R., Murphy G. The activity of the tissue inhibitors of metalloproteinases is regulated by C-terminal domain interactions: a kinetic analysis of the inhibition of gelatinase A. Biochemistry. 1993 Apr 27;32(16):4330–4337. doi: 10.1021/bi00067a023. [DOI] [PubMed] [Google Scholar]
- Windsor L. J., Birkedal-Hansen H., Birkedal-Hansen B., Engler J. A. An internal cysteine plays a role in the maintenance of the latency of human fibroblast collagenase. Biochemistry. 1991 Jan 22;30(3):641–647. doi: 10.1021/bi00217a008. [DOI] [PubMed] [Google Scholar]
- Winyard P. G., Zhang Z., Chidwick K., Blake D. R., Carrell R. W., Murphy G. Proteolytic inactivation of human alpha 1 antitrypsin by human stromelysin. FEBS Lett. 1991 Feb 11;279(1):91–94. doi: 10.1016/0014-5793(91)80258-5. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]
- Woessner J. F., Jr, Taplin C. J. Purification and properties of a small latent matrix metalloproteinase of the rat uterus. J Biol Chem. 1988 Nov 15;263(32):16918–16925. [PubMed] [Google Scholar]