Abstract

A promising route toward room-temperature ironmaking is electrowinning, where iron ore dissolution is coupled with cation electrodeposition to grow pure iron. However, poor faradaic efficiencies against the hydrogen evolution reaction (HER) is a major bottleneck. To develop a mechanistic picture of this technology, we conduct a first-principles thermodynamic analysis of the Fe110 aqueous electrochemical interface. Constructing a surface Pourbaix diagram, we predict that the iron surface will always drive toward adsorbate coverage. We calculate theoretical overpotentials for terrace and step sites and predict that growth at the step sites are likely to dominate. Investigating the hydrogen surface phases, we model several hydrogen absorption mechanisms, all of which are predicted to be endothermic. Additionally, for HER we identify step sites as being more reactive than on the terrace and with competitive limiting potentials to iron plating. The results presented here further motivate electrolyte design toward HER suppression.
Introduction
Steel production is responsible for 7–9% of global CO2 emissions.1 For every ton of steel produced, 2 tons of CO2 is released using current methods.1,2 In this process, extraction of iron from iron ore using a carbothermic blast furnace contributes 70% of these emissions.3 This has led to enormous interest in identifying pathways to decarbonize reduction of iron ore to iron.4 Electrochemical approaches offer an energy-efficient route to decarbonization through pairing with renewable electrochemistry. Several industries are adopting this approach, including cement and chemicals production, thereby creating a potential for synergy from concerted electrification across domains.5−7
For electrochemical ironmaking, two leading direct electrochemical pathways exist: molten oxide electrolysis (MOE) and electrowinning. MOE is a high-temperature process producing liquid iron and parallels the existing aluminum production process.8−12 Despite decades of investment and research, technical challenges remain associated with designing an inert anode that can withstand the harsh thermal and electrochemical conditions needed.13 Alternatively, room-temperature electrowinning of iron from iron ore, usually hematite (Fe2O3), is the second approach where iron plates are grown on the cathode in an electrochemical cell.14−16 Rather than using elevated temperatures like in MOE, here Fe–O bond breakage in the iron ore is driven via an applied potential to extract the iron.
Overall, electrowinning can be summarized as a two-step procedure: (i) dissolution of the iron ore and (ii) selective electrodeposition. First, the iron ore is dissolved to extract the iron cations and break the Fe–O bonds. Mechanistically, the exact details of the dissolution process remain an area of active investigation.14,17−20 While there has been some suggestion that a bulk conversion is possible for adsorbed iron ore particles, thus bypassing dissolution altogether, this approach is restricted by ore particle size.21,22 Thus, we frame the rest of this study within the context of efficient promotion of ore dissolution as the first step. The specific products of the dissolution will be pH dependent (e.g., Fe2O3 + 6H+ ⇌ 2Fe3+ + 3H2O under acidic conditions23). It is worth highlighting, however, that our subsequent discussion and analysis is equally valid for other iron cation sources beyond Fe2O3 such as Fe3O4, α-FeOOH, and iron salts (e.g., Fe2(SO4)3).
Following the initial dissolution step, a potential is applied within the cell to drive electrodeposition of the extracted iron cations. This grows high-purity iron plates on the cathode, which can then be further processed to produce other products of interest, such as varying types of steel.4 However, this electrodeposition step is hindered by competition with the hydrogen evolution reaction (HER).14−16 This challenge bears a resemblance to selectivity challenges observed in many electrocatalysis applications, where details of adsorbate–surface interactions can have an impact, such as the reduction of CO2 or N2.24−28 Thus, efficient and selective electrodeposition is a challenge moving forward.
From a holistic viewpoint, these two steps have fundamentally different environmental requirements. Dissolution is thermodynamically optimized under acidic conditions,23 but HER also becomes thermodynamically feasible. On the other hand, alkaline conditions suppress HER but are unfavorable for dissolution. This trade-off leaves an optimization challenge of the electrochemical environment conditions.
Motivated by HER’s suppression under alkaline conditions, it was a major research thrust in the Ultra-Low CO2 Steelmaking (ULCOS) project.14,15,21,22,29 This was a large collaborative effort from 2004 to 2010 spanning 48 European companies and headed by ArcelorMittal.29 Carrying the torch in 2017, the SIDERWIN project, once again coordinated by ArcelorMittal, set out to explore electrowinning of ceramic suspensions in alkaline conditions beyond solely hematite.16,30−32 While these projects were monumental in laying the groundwork for this field, the emphasis on alkaline conditions has left broader questions on the nature of the interface under acidic conditions. Recent experimental work has revisited acidic iron electrowinning,33,34 as well as the use of pH gradients,35 highlighting questions about the underlying electrochemistry.
Thus, general fundamental insights of the electrochemical iron interface is needed to further advance the electrowinning process. While there is extensive surface science literature on the interaction of iron with water and its constituent atoms,36−45 as well as established bulk iron–water Pourbaix diagrams,23,46−48 a full unified electrochemical mapping in the form of a surface Pourbaix diagram has yet to be proposed. In the electrocatalysis field where similar challenges of unification have arisen, surface Pourbaix diagrams have been a powerful tool to predict the surface as a function of electrochemical environment and thereby inform selectivity trends (e.g., chlorine evolution vs oxygen evolution).49−53 These diagrams map the thermodynamic preference for surface phase formation as a function of pH and applied potential relative to the standard hydrogen electrode (USHE).
In addition to developing a static thermodynamic model of the surface, investigating the dynamics of iron electrodeposition can unlock further insights into the system. Considering similar systems, free energy diagrams have previously enabled predicting the underlying electrochemical growth mechanisms and theoretical overpotentials key to charging Li–air batteries,54,55 Zn–air batteries,56 and metal anodes.57 Moreover, hydrogen embrittlement is a key challenge in ironmaking, and hydrogen absorption has been the subject of several mechanistic studies.45,58−60 Continued investigation of hydrogen interactions with iron, but within this electrowinning context in terms of absorption and HER, can shed further light into predicting overall system behavior and plate quality.
In this work, we employ surface Pourbaix diagrams to investigate the thermodynamically most favorable surface phases of O*, OH*, and H* on Fe110 as a function of pH and USHE. Interestingly, our results show that there does not exist a region in Pourbaix space where Fe110 is thermodynamically predicted to be both stable toward dissolution and free from any adsorbates. Employing free energy diagrams, we calculate the theoretical overpotentials for iron electrodeposition through terrace (Fe110) and step (Fe210) growth mechanisms. From these diagrams, we emphasize the importance of understanding hydrogen–iron interactions and probe the thermodynamics of absorption and theoretical HER overpotentials. This analysis paves the pathway for potential routes to electrowinning under acidic conditions.
Methods
To facilitate the thermodynamic analysis presented here, spin-polarized density functional theory (DFT) calculations were conducted using GPAW61,62 via the Atomic Simulation Environment package.63 Projector-augmented wave potentials were used along with the RPBE exchange-correlation functional.64 AutoCat was used to generate all initial structures.65 Surface coverage was incorporated via a combination of periodic supercell dimensions and explicit inclusion of the adsorbates. All terrace slabs were four layers (unless otherwise noted) and stepped slabs eight layers. Additional computational details are provided in the Supporting Information.
Modeling this aqueous environment, the following water oxidation reactions can occur on the surface forming either O* or OH*:
| 1 |
| 2 |
where * indicates an unoccupied surface site and X* is an X molecule adsorbed on the surface.
Additionally, the formation of H* species on the surface can occur through a Volmer process, the first step of HER:
| 3 |
In predicting the
most thermodynamically favorable surface phases
of Fe110, the energetics of the above processes are key. Calculation
of these free energies are done by invoking the computational hydrogen
electrode (CHE).66 This approach sets the
reference potential to be that for which the reaction 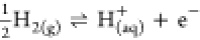 is in equilibrium. From
this choice, surface
phase formation energies as a function of pH and USHE can be extracted, taking into account the number of
consumed or produced electrons and protons. For example, the free
energy change upon formation of an O* phase formed via eq 2 can be expressed as
is in equilibrium. From
this choice, surface
phase formation energies as a function of pH and USHE can be extracted, taking into account the number of
consumed or produced electrons and protons. For example, the free
energy change upon formation of an O* phase formed via eq 2 can be expressed as
| 4 |
where kB is the Boltzmann constant, e is the fundamental charge, and ΔG0O* is the energy upon formation at 0 V (vs SHE) and pH = 0. ΔG0O* is obtained directly from DFT, and similar energy expressions can be derived for surface phases involving H* and OH*. Moreover, to allow for comparisons across monolayer (ML) coverages, these calculated energies are subsequently normalized to be per surface atom. We refer the reader to the Supporting Information for more details on the calculation of these energetics.
These energies are not only governed by the strength of adsorbate interactions with Fe110 relative to their reference phases but also adsorbate coverage and patterns on the surface. At higher coverages, steric effects can become more pronounced, and at a given coverage, there may exist multiple configurations. As will be discussed in greater detail in the following section, we thus modeled several supercell sizes, number of adsorbates, and adsorbate placements to obtain a variety of surface phases.
In addition to the surface phases, stability of the Fe110 surface is also important and we consider the following iron dissolution reaction:
| 5 |
Following a similar logic as for the adsorbate phases, the energy change upon dissolution at aFe2+ = 1 can be expressed as
| 6 |
where U⊖Fe is the redox potential for this process at standard conditions (−0.45 V vs SHE67). It is worth noting that as this process does not produce or consume H+, it is independent of the pH of the environment. Dissolution of the iron surface forming HFeO2– is also possible, but we assume this will have a considerable kinetic barrier and thus exclude it from our analysis.
Free energy diagrams for iron deposition were calculated by sequentially adding iron atoms either on the terrace of a Fe110 slab or a Fe210 stepped surface. Energy differences from depositing a single Fe atom are calculated via the following equation:
| 7 |
where GFe* is the total energy after deposition, G* is the total energy before deposition, and μ⊖Fe is the energy per bulk iron atom. For these iron deposition calculations, the vibrational contributions to energy were neglected. To ensure consistent k-point sampling between the surface and bulk iron energy cells, the linear fitting method68 was used to calculate μ⊖Fe. For fitting this value, we used primitive surface slabs with odd numbers of layers ranging from 3 to 19 to converge the bulk energy to within 0.05 eV. We refer the reader to the Supporting Information for additional methodological details including computational parameters and calculating energies with CHE.
Results and Discussion
Surface Phases of Fe110
To model the iron surface at the cathode in an electrowinning cell, we use a body-centered cubic (bcc) Fe110 slab model. Previously, this surface facet was shown to possess the lowest surface energy.69−71 In addition, the 110 facet has been previously identified for electrodeposited iron films using both atomic force microscopy (AFM) and X-ray diffraction (XRD).72−74 Moreover, even with electrodeposition on a face-centered cubic substrate, a bcc transition was observed upon increasing layers deposited, and the 110 facet was observed to emerge.73,74 Thus, through the selection of this bcc structure, the analysis presented in this work focuses on growth after the initial nucleation stage. As the environment is aqueous, we consider the energetics of water oxidation and Volmer processes to form OH*, O*, and H* on the surface. These adsorbates form surface phases based on their coverage and pattern on the Fe110 terrace. The energies of forming these phases are not only governed by the strength of adsorbate–surface interactions but also adsorbate coverage and patterns on the surface. By modeling several supercell sizes, number of adsorbates, and adsorbate placements we obtain a variety of surface phases that can be considered.
Identifying the most favorable adsorption sites for O*, H*, and OH*, we calculate adsorption energies at 1/4 ML coverage by relaxing the adsorbates at all unique surface sites on the 110 terrace facet (Figure S1): hollow, long bridge, short bridge, and ontop. These calculated adsorption energy values are given in Table 1. It is observed that for H* at this coverage, the hollow site yielded the most negative adsorption energy. Much of the previous literature, both computational and experimental, identifies either the hollow or long bridge as the most favorable site,36−44 and we highlight the small geometric distance distinguishing these two sites. Similarly, OH* and O* were also observed to prefer the hollow site, and for all other coverages on the terrace, we assume that this site will be most favorable.
Table 1. Adsorption Energies of H, O, and OH at 1/4 ML Coverage on Fe110.
|
ΔG0 (eV) |
||||
|---|---|---|---|---|
| adsorbate | hollow | on top | long bridge | short bridge |
| H | –0.60 | 0.40 | –0.29 | –0.42 |
| O | –0.99 | 0.82 | –0.67 | –0.52 |
| OH | –0.59 | 0.31 | –0.30 | –0.39 |
To develop as complete a picture of the surface as possible, we modeled several different surface coverages and phases. In the following paragraphs we discuss the phases considered, with particular emphasis on coverages that have multiple different patterns.
At 1/2 ML coverage for all three adsorbates, we considered two phases, as described by Jiang and Carter:37 (2 × 1) and (2 × 2)-2H. The former has a rectangular pattern on the surface whereas the latter forms a hexagonal pattern, as shown in Figure 1a. H* and O* show a slight preference toward the hexagonal configuration at 1/2 ML coverage, in agreement with previous work.37,75 OH* also shows a slight preference toward the hexagonal phase at this coverage. On a per-surface atom basis, this preference is at most a difference of approximately 30 meV for the three adsorbates.
Figure 1.

(a) Two surface configurations of H at 1/2 ML coverage (top: 2 × 1; bottom (2 × 2)-2H). (b) Two surface configurations of O at 1/6 ML coverage (top: orthogonal unit cell; bottom: nonorthogonal unit cell). (c) Two surface configurations of OH at 1/3 ML coverage (top: orthogonal unit cell; bottom: nonorthogonal unit cell).
At 1/6 ML and 1/3 ML coverages, we considered two different configurations for each. One is formed by using an orthogonal cell where the other uses a conventional nonorthogonal cell in the xy plane (Figure 1b,c). H* shows a preference toward the orthogonal configuration at both 1/6 ML and 1/3 ML coverage but by only ∼5 meV/surface atom. The differing behavior of H* may be rationalized by decreased steric effects, as evidenced by the vertical OH* orientations on the surface. Both OH* and O* prefer the pattern formed via the nonorthogonal cell at these coverages.
In addition to these single adsorbate phases, we also considered mixed OH* + H* phases, as they can be formed via the chemical dissociation of water. At a total of 1/2 ML, we model both configurations ((2 × 1) and (2 × 2)-2H) in the mixed phase at this coverage with 1/4 ML OH* + 1/4 ML H* (Figure S3). Again, we observe minimal energetic difference, with a marginal preference toward the hexagonal phase of ∼5 meV/surface atom. We also model the higher coverage mixed phase 1/2 ML OH* + 1/2 ML H* to fully cover the surface (Figure S3). Interestingly this phase is predicted to be thermodynamically stable, unlike the 1 ML O* and 1 ML OH* phases, possibly through the OH–H hydrogen bonding as evidenced from the induced tilt of the OH moieties. Visualizations of all surface phases considered in this work are aggregated in Figures S2 and S3.
Mapping the Surface Pourbaix Diagram
Aggregating the above calculations and corresponding pH and USHE dependencies, we visualize ΔG as a function of USHE for a selection of phases in Figure 2. In the acidic and neutral regimes (Figure 2a,b), we observe that for negative applied potentials the surface is predicted to be driven toward full hydrogenation. Increasing the potential is predicted to lead to dissolution of the Fe110 surface beyond approximately −0.20 V (vs SHE) at pH = 0.0 and −0.28 V (vs SHE) at pH = 7.0.
Figure 2.
Surface Pourbaix slices for pH values of (a) 0.0, (b) 7.0, and (c) 14.0. (d) Full surface Pourbaix diagram.
Moving into the alkaline regime with a pH of 14.0 (Figure 2c), it is predicted that oxygenated surface phases will emerge that prevent dissolution. Previous targeted surface science works have observed the formation of oxygen covered phases on Fe110, thereby demonstrating a general predisposition for the formation of these phases.38,39
Continuing this analysis and treating pH as a continuous variable, we plot the surface Pourbaix diagram in Figure 2d. This diagram maps the surface to predict the thermodynamically most favorable phases as a function of electrochemical environment. Within the context of iron electrodeposition, this enables prediction of surface coverage of the grown iron plates under relevant electrochemical conditions. As this approach does not incorporate changes in the bulk, the pH and USHE range is selected to mostly coincide with stable Fe from the bulk Pourbaix diagram.23,46−48 Throughout this space there is no region where a clean Fe110 surface is thermodynamically preferred. For most negative potentials, 1 ML H* is the favored phase. At potentials that are near 0 V (vs SHE) or are positive, either O* phases are predicted to form or dissolution of the surface is favored depending on the pH. We highlight that much of these O phases overlaps with oxide phases in the bulk Pourbaix, which implies bulk changes could occur in the iron. With the aim of promoting pure iron growth, and identifying promising environmental conditions for this process, optimizing for low coverage surface phases may be beneficial for ease of deposition due to increased exposed surface area. Thus, this diagram aligns with previous motivation toward exploring alkaline conditions.
Focusing on the acidic to neutral region, we additionally calculate a surface Pourbaix diagram for the Fe210 step modeling 1/5 ML, 3/5 ML, and 1 ML coverage of the steps by O and H. Since the terrace surface Pourbaix diagram predicts a drive toward 1 ML H coverage, we cover the terrace region of this step model with hydrogens (Figure S6). Here we predict that for all regions where the surface is predicted to be stable, there will be a thermodynamic drive toward keeping the step free from adsorbates (Figure S7).
We note that this analysis does not include solvation in calculating the thermodynamics of these surface phases. Through hydrogen-bonding, the water molecules from the aqueous environment can stabilize the surface adsorbates. For example, the water layer structure on metallic interfaces is an area of extensive study.76−79 Of particular relevance here, OH has been observed to incorporate itself into the water layer network on Pt which can stabilize it on the surface.80 On the other hand, solvation has a negligible effect on atomic O adsorbates.66
To gauge how this effect may change the physical conclusions of our model, we focus on the neutral to acidic region and applied potentials leading to a stable surface. Here we investigate the stabilization required to displace the 1 ML H phase as most favored. Without solvation, the most stable phase of pure OH is the 1/4 ML OH phase, where the most stable mixed phase involving OH is the 1/4 ML OH + 1/4 ML H phase. Here we calculate the difference between the formation energy of these phases and the 1 ML H phase as a function of pH and applied potential. At pH = 0, the OH molecules would need to be stabilized by 10.51 eV for the entire 1 ML H region to be replaced by 1/4 OH. The difference at this pH is slightly smaller for the mixed 1/4 ML OH + 1/4 ML H phase, 8.32 eV, but remains substantial. For the OH phases to replace part of the 1 ML H phase, i.e., up until −0.5 V, the differences are 4.32 and 2.32 eV for the pure and mixed OH phases, respectively. Moving into the neutral regime, the difference is further decreased but there remains a large gap to overcome. At pH = 7 and for the OH phases to become more thermodynamically favorable until −0.5 V, the mixed phase is 0.65 eV less stable than 1 ML H. For context, OH is stabilized by 0.33 eV on Pt by the water layer,66 implying that solvation alone is unlikely to significantly alter our predicted surface Pourbaix diagram for the acidic to neutral regimes.
It is also worth noting that this analysis does not incorporate kinetics and is strictly based on thermodynamics. Thus, large activation barriers may hinder the formation of the predicted thermodynamically favored phases under given conditions. In light of this, this diagram represents phases the surface will be driven toward. Moreover, our analysis is restricted to including the surface phases calculated in the previous section. In reality, intermediate coverages or combinations of these surface phases may coexist on the surface. Methods toward addressing this limitation is an active area of research53,81 and is beyond the scope of this work.
Theoretical Overpotentials for Iron Electrodeposition
To understand the most relevant region of the surface Pourbaix for iron electrodeposition (the reverse reaction of eq 5), we next calculate the theoretical limiting potentials and overpotentials associated with plating. The theoretical limiting potential UL is the least negative applied potential for which all steps are downhill in a free energy landscape for a given reaction mechanism. Taking the difference between this limiting potential and the equilibrium potential U⊖Fe yields the theoretical overpotential.
For iron electrowinning, the reaction of interest is electrodeposition. The energetics of this process govern the efficiency of the growth of pure iron plates. In this work we consider two main growth mechanisms: (i) island nucleation on a Fe110 terrace and (ii) kink formation at a Fe210 step. Modeling both of these mechanisms was performed by sequentially placing one iron atom at a time until the terrace or step is reproduced. As a simplification, we model both of these surface models without the presence of H*, O*, and OH* adsorbates. Thus, the calculated potentials in this section can be interpreted as a scenario where adsorbate formation is blocked either kinetically or through electrolyte design. Adsorbates present on the surface are expected to generally have a detrimental impact on these overpotential values and possibly plate composition.
We first model the growth mechanism stemming from island nucleation
on a terrace (Figure 3). In this case, iron atoms are placed until another full layer of
iron is deposited on the surface. At the standard reduction potential
for iron, the first two iron deposition steps are endothermic and
the latter two steps are exothermic. Ideally, placement of the final
iron should return the energy to 0 eV as the terrace has been reproduced.
However, the observed deviance of 0.07 eV is close to our applied
convergence criteria for the bulk energy, 0.05 eV. Moreover, this
deviation may be a consequence of differing coordinations of the reference
bulk and surface iron atoms, as observed in other metals.57 Applying limiting potential analysis to this
free energy diagram, the most uphill step in this growth mechanism
is placement of the first iron atom with a value of 0.50 eV. The limiting
potential is calculated by 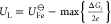 referenced to SHE. Since the theoretical
overpotential is the difference between the standard reduction potential
and limiting potential, it is calculated by
referenced to SHE. Since the theoretical
overpotential is the difference between the standard reduction potential
and limiting potential, it is calculated by  . Therefore, this initial island nucleation
step is the potential determining step, yielding a limiting potential UL of −0.70 V vs SHE. Propagating this
quantity to obtain the theoretical overpotential, we calculate this
value to be 0.25 V for deposition on a terrace.
. Therefore, this initial island nucleation
step is the potential determining step, yielding a limiting potential UL of −0.70 V vs SHE. Propagating this
quantity to obtain the theoretical overpotential, we calculate this
value to be 0.25 V for deposition on a terrace.
Figure 3.

Free energy diagram for sequential electrodeposition of iron atoms on an Fe110 terrace. Yellow atoms in the atomic images highlight the iron atoms deposited so far. The supercells have been repeated to visualize the overarching patterns.
Further investigating this terrace growth mechanism, we point out that for placing the second iron atom on the surface there are two symmetrically unique configurations (Figure S5). Their energies are comparable with values of 0.66 and 0.71 eV. Since this second step is not potential determining, both deposition paths are thermodynamically accessible when the potential applied is at least UL.
Turning now to electrodeposition at steps, we next investigate growth via kink formation. Here we use the Fe210 surface facet as our model. In alignment with previous low-energy electron diffraction experiments, we observe significant relaxation from the bulk with comparable interlayer spacings as shown in Table S1.82 Applying this model for the step growth mechanism at the standard reduction potential (Figure 4), the first step is energetically uphill to nucleate the kink with subsequent steps relatively flat in energy. Thus, as in growth at the terrace, we observe the first deposition step to be the PDS. In contrast to growth on the terrace, however, we observe the magnitude of this PDS to be relatively smaller (0.26 eV) leading to a UL of −0.58 V vs SHE. The theoretical overpotential for growth at the step site is calculated to be 0.13 V.
Figure 4.

Free energy diagram for sequential electrodeposition of iron atoms at an Fe210 step. Yellow atoms in the atomic images highlight the iron atoms deposited so far. The supercells have been repeated to visualize the overarching patterns.
Comparing the theoretical overpotentials between growth at the terrace and step sites, we predict through this idealized model for growth to mainly occur via kink formation. This is in alignment with previous conclusions drawn for electroplating of other metals.57 However, we reiterate that this neglects solvation effects through adsorbate formation as well as diffusion kinetics which could influence these growth mechanisms.
It is worth highlighting the limitations of this growth model. With the restricted cell size of our model, it currently focuses on the overall growth energetics. Previous epitaxial growth investigations have observed island formation followed by coalescence with increasing coverage.83−85 Specifically for electrodeposition, layer-by-layer growth with relatively flat deposits after island formation was also reported using this method.73,86 Thus, if island clusters are to form as an intermediate growth stage, our model will not capture this detail. Further investigations into expanding upon this model will be the subject of future investigation.
Additionally, we note that the calculated limiting potentials of both terrace and step site mechanisms both lie within the 1 ML H region of the surface Pourbaix diagram (Figure 2d). This observation implies that potentials required to electroplate the iron cations from solution will also thermodynamically drive hydrogen adsorbate formation. In other words, iron deposition may proceed on Fe110 in the presence of hydrogen adsorbates thereby introducing the possibility for more complicated interactions and growth mechanisms. A coupled analysis further investigating the interplay of hydrogen as the layer grows is necessary to gauge the likelihood of more intertwined deposition mechanisms. Such a comprehensive analysis is beyond the scope of this initial work but is another avenue for future study.
Hydrogen Evolution and Absorption
As the surface phases associated with hydrogen formation are most thermodynamically favorable under the required conditions for iron electrodeposition, we further investigate these iron–hydrogen interactions. Experimental investigations into the interplay of iron and hydrogen have proposed as early as the 1960s that the hydrogen adsorption state can lead to two further interaction modes: hydrogen absorption into the bulk and HER.87 Investigating each of these processes, we consider both terrace and steps to gauge impact stemming from different coordinations of the surface iron atoms. Previous work has highlighted increased reactivity of iron steps with both nitrogen and water,44,88 emphasizing the interplay between surface morphology and surface–adsorbate interactions.
For absorption, the hydrogen will first adsorb onto the surface at the hollow site via a Volmer process (Figure 5a). On the terrace, to allow for greater reconstruction of the slab to accommodate hydrogen entry into the subsurface, we increase the number of layers of our model from four to five with the top three layers free to move. In the x–y dimensions the terrace supercell model is kept at 2 × 2. With this thicker slab, we again relaxed hydrogen on the surface at the hollow site and calculate an adsorption enthalpy of −0.91 eV. To identify the most stable hydrogen site upon entry into the slab, we relaxed the hydrogen in the subsurface underneath the top site, hollow site, long bridge, and short bridge. For initial placement in the subsurface below the hollow and long bridge sites, the hydrogen atoms were spontaneously ejected to the surface. Calculating the enthalpies referenced to gas phase for placement under the short bridge and top site, we obtain values of 0.03 and 0.11 eV, respectively. This thermodynamic preference toward hydrogen absorption beneath the short bridge is in qualitative agreement with earlier reports,60 and the configuration is illustrated in Figure 5b. From these values, we calculate the enthalpy for terrace absorption to be 0.94 eV. This finding is in good agreement with previous reports of 0.98 eV60 and 1.00 eV.59 Moreover, we calculate the enthalpy of absorption to below the short bridge for 1 hydrogen from the fully hydrogenated Fe110 terrace (Figure S5a,b). We calculate this process to be slightly less endothermic (0.83 eV) than for the clean surface, indicating that neighboring hydrogens may help encourage this absorption process.
Figure 5.

Schematics illustrating absorption structures with hydrogen placed (a) at terrace surface hollow site (top and side views), (b) below terrace short bridge site (top and side views), (c) hollow site near step edge, (d) hollow site adjacent to step, and (e) below short bridge site near step edge. Outermost surface iron atoms are brown, subsurface iron atoms are gray, and hydrogen atoms are blue. (f) Summary of calculated absorption enthalpies for all paths considered, color-coded by mechanism.
Extending this analysis to include entry at step sites, we evaluated two scenarios for the clean 210 step: (i) absorption from near the step edge (Figure 5c) and (ii) absorption from adjacent to the step (Figure 5d). In both mechanisms explored, the hydrogen diffuses to below the short bridge near the step edge since it was most favorable in the terrace (Figure 5e). Starting from a hollow site near the step edge, we calculate an enthalpy of absorption to be 1.06 eV. Alternatively, if absorption starts from a hollow site directly adjacent to the step, we calculate an enthalpy of absorption of 0.75 eV. While this latter case exhibits a less uphill enthalpy for absorption, calculation of the kinetic barriers is required to determine whether it is predicted to be the more likely absorption mode. We additionally consider where the step is hydrogenated on the terrace and hydrogen enters from the step (Figure S5c,d). This process is less endothermic than the mechanism near the step edge but is comparable to entry from beside the step (0.78 eV). Similarly to the step edge, this indicates that nearby hydrogens may help hydrogen entry in some configurations.
As all absorption mechanisms investigated are endothermic (Figure 5f), there is an initial barrier for hydrogen entry into the pristine iron surface. However, defects and vacancies have been identified as key toward hydrogen embrittlement through the introduction of traps,45 and after initial absorption into the bulk, the hydrogen atoms are anticipated to exhibit increased mobility.59,60
In addition to entering the iron, adsorbed hydrogen on the surface may alternatively further react to form H2 via HER. After the initial Volmer step, HER may proceed via a Heyrovsky process as follows:
| 8 |
For HER, ΔGH ∼ 0 has generally been used as a descriptor toward catalytic activity.89,90 We thus calculate the adsorption energy of hydrogen on the terrace and several step configurations, which are summarized in Figure 6. All subsequent values are calculated for pH = 0. Starting with the terrace, our calculated adsorption energies used in constructing the surface Pourbaix are −0.60 eV/H atom and −0.28 eV/H atom for 1/4 and 1 ML, respectively. Of these two phases, we predict full coverage to be generally more dominant by the surface Pourbaix (Figure 2d). In comparison to the calculated limiting potential for iron growth on the terrace, −0.70 V, the −0.42 V limiting potential for HER is predicted to be competing with iron plating, as expected.
Figure 6.

Free energy diagrams for HER on the terrace sites (1/4 and 1 ML coverage), beside the step, near the step edge, and on a hydrogenated step. Values closer to zero are predicted to have higher HER activity. All sites are predicted to compete with iron electrodeposition
We next investigate HER at the Fe210 step by considering multiple possible active sites in the vicinity. Initially placing the hydrogen between two step iron atoms, the system relaxed to one of two sites as described in the absorption discussion: (i) beside the step above a hollow site (Figure 5c) or (ii) near the step edge (Figure 5d). For each of these configurations we calculate the ZPE to obtain more accurate vibrational corrections (Table S3). In addition to these two sites, we also relaxed the system starting with hydrogen singly coordinated to a step iron, and the system converged to a configuration beside the step but over a top site. Performing vibrational analysis on this configuration, however, revealed an imaginary mode from oscillating parallel to the step edge indicating it is metastable. Thus, we focus the analysis on the two stable sites.
The corresponding adsorption energies were calculated to be −0.09 eV/H atom beside the step and −0.34 eV/H atom near the step edge. With these values closer to zero than on the terrace, this enforces the viewpoint that the step sites may be more reactive than the terrace. Comparing these adsorption energies with the step growth limiting potential, −0.58 V, competition between HER and iron deposition is again predicted.
Under neutral to acidic conditions, the surface will be driven toward forming a hydrogen overlayer on the terrace. Thus, we additionally calculated the HER landscape on the 210 step where the terrace portion is covered by hydrogen (Figure 6). In this scenario, the hydrogen is stabilized on the step edge at the bridge between two step iron atoms. With the introduction of the nearby hydrogen overlayer in our model, the hydrogen binding at the step is weakened further to 0.02 eV/H atom. Therefore, hydrogen coverage on the terrace sites increases the reactivity of the steps toward HER even further.
As an alternative to the Heyrovsky step, two adsorbed hydrogens may chemically recombine via the Tafel process, 2H* → H2(g). Previous experimental studies into the mechanism have observed this Tafel step to be more prevalent at lower overpotentials and Heyrovsky at moderate to higher overpotentials.87,91 Thus, our thermodynamic analysis using the Heyrovsky step may be interpreted as an upper bound on HER performance. As the Tafel step is a largely kinetic process, a kinetic analysis is required for a more comprehensive view of HER on iron. Extending our analysis here to incorporate kinetics alongside hydrogen coverage will be the subject of future study. However, the results here emphasize HER as a challenge toward the efficient electrowinning of iron and requires electrolytes that can suppress it (e.g., water in salt electrolytes92,93).
Conclusions
In summary, we modeled several surface phases on Fe110 ranging in coverage from 1/6 to 1 ML and including mixed adsorbate phases. Using the associated calculated energies of these phases, we generated surface Pourbaix diagrams and observed that there is a thermodynamic preference for adsorbates in all regions of surface stability. Calculating the theoretical overpotentials for iron deposition at the terrace and step sites, we predict a thermodynamic preference toward growth at the steps. Further investigating the hydrogen–iron interactions we calculate enthalpies for multiple absorption processes via the terrace and step, all of which determined to be endothermic. In addition, we calculate terrace and step HER limiting potentials and reinforce the challenge selectivity. The analysis here presents an important step toward mapping the aqueous electrochemical interface of iron and could shape the design of sustainable ironmaking through acidic electrowinning.
Acknowledgments
L.K. acknowledges the support of the Natural Sciences and Engineering Research Council of Canada (NSERC). The authors thank Hongyi Lin and Victor Azumah for important discussions.
Data Availability Statement
All structures and data are available at the following GitHub repository: https://github.com/BattModels/iron_electrowinning_mapping.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jpcc.4c01867.
Additional discussion on computational details, calculating energetics with the computational hydrogen electrode, visualizations of all considered surface phases, and schematics for hydrogen absorption mechanisms for hydrogenated surfaces (PDF)
The authors declare the following competing financial interest(s): L.K. and V.V. have filed a provisional patent on the electrowinning of iron.
Special Issue
Published as part of The Journal of Physical Chemistry Cvirtual special issue “Jens K. Nørskov Festschrift”.
Supplementary Material
References
- World Steel Association . Climate change and the production of iron and steel. Brussels, Belgium, 2021.
- Daehn K.; Basuhi R.; Gregory J.; Berlinger M.; Somjit V.; Olivetti E. A. Innovations to decarbonize materials industries. Nature Reviews Materials 2022, 7, 275–294. 10.1038/s41578-021-00376-y. [DOI] [Google Scholar]
- Fan Z.; Friedmann S. J. Low-carbon production of iron and steel: Technology options, economic assessment, and policy. Joule 2021, 5, 829–862. 10.1016/j.joule.2021.02.018. [DOI] [Google Scholar]
- Raabe D. The Materials Science behind Sustainable Metals and Alloys. Chem. Rev. 2023, 123, 2436–2608. 10.1021/acs.chemrev.2c00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallapragada D. S.; Dvorkin Y.; Modestino M. A.; Esposito D. V.; Smith W. A.; Hodge B.-M.; Harold M. P.; Donnelly V. M.; Nuz A.; Bloomquist C.; et al. Decarbonization of the chemical industry through electrification: Barriers and opportunities. Joule 2023, 7, 23–41. 10.1016/j.joule.2022.12.008. [DOI] [Google Scholar]
- Ellis L. D.; Badel A. F.; Chiang M. L.; Park R. J.-Y.; Chiang Y.-M. Toward electrochemical synthesis of cement—An electrolyzer-based process for decarbonating CaCO3 while producing useful gas streams. Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 12584–12591. 10.1073/pnas.1821673116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchins G.; Viswanathan V.. Electrochemical Synthesis of Cementitious Compounds. US Patent Appl. 17/771,197, 2022.
- Allanore A.; Yin L.; Sadoway D. R. A new anode material for oxygen evolution in molten oxide electrolysis. Nature 2013, 497, 353–356. 10.1038/nature12134. [DOI] [PubMed] [Google Scholar]
- Kim H.; Paramore J.; Allanore A.; Sadoway D. R. Electrolysis of molten iron oxide with an iridium anode: the role of electrolyte basicity. J. Electrochem. Soc. 2011, 158, E101. 10.1149/1.3623446. [DOI] [Google Scholar]
- Tang D.; Zheng K.; Yin H.; Mao X.; Sadoway D. R.; Wang D. Electrochemical growth of a corrosion-resistant multi-layer scale to enable an oxygen-evolution inert anode in molten carbonate. Electrochimica acta 2018, 279, 250–257. 10.1016/j.electacta.2018.05.095. [DOI] [Google Scholar]
- Wang D.; Gmitter A. J.; Sadoway D. R. Production of oxygen gas and liquid metal by electrochemical decomposition of molten iron oxide. J. Electrochem. Soc. 2011, 158, E51. 10.1149/1.3560477. [DOI] [Google Scholar]
- Allanore A. Features and challenges of molten oxide electrolytes for metal extraction. J. Electrochem. Soc. 2015, 162, E13. 10.1149/2.0451501jes. [DOI] [Google Scholar]
- Xia R.; Overa S.; Jiao F. Emerging electrochemical processes to decarbonize the chemical industry. JACS Au 2022, 2, 1054–1070. 10.1021/jacsau.2c00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allanore A.; Lavelaine H.; Valentin G.; Birat J.; Lapicque F. Electrodeposition of metal iron from dissolved species in alkaline media. J. Electrochem. Soc. 2007, 154, E187. 10.1149/1.2790285. [DOI] [Google Scholar]
- Haarberg G. M.; Kvalheim E.; Rolseth S.; Murakami T.; Pietrzyk S.; Wang S. Electrodeposition of iron from molten mixed chloride/fluoride electrolytes. ECS Trans. 2007, 3, 341. 10.1149/1.2798677. [DOI] [Google Scholar]
- Boehme C.; Starykevich M.; Constantinescu G.; Kovalevsky A. V.; Lopes D. V. Low-Temperature Electrowinning of Iron from Mixed Hematite-Magnetite Alkaline Suspensions. J. Electrochem. Soc. 2023, 170, 052502. 10.1149/1945-7111/acd085. [DOI] [Google Scholar]
- Vehmaanperä P.; Salmimies R.; Häkkinen A. Thermodynamic and kinetic studies of dissolution of hematite in mixtures of oxalic and sulfuric acid. Mining, Metallurgy & Exploration 2021, 38, 69–80. 10.1007/s42461-020-00308-4. [DOI] [Google Scholar]
- McBriarty M. E.; Stubbs J. E.; Eng P. J.; Rosso K. M. Reductive dissolution mechanisms at the hematite-electrolyte interface probed by in situ X-ray scattering. J. Phys. Chem. C 2019, 123, 8077–8085. 10.1021/acs.jpcc.8b07413. [DOI] [Google Scholar]
- Zhan G.; Fang Y.; Zhang M.; Cao S.; Xu T.; Ling C.; Gu H.; Liu X.; Zhang L. Oxalate promoted iron dissolution of hematite via proton coupled electron transfer. Environmental Science: Nano 2022, 9, 1770–1779. 10.1039/D1EN01190A. [DOI] [Google Scholar]
- Liu L.; Sassi M.; Zhang X.; Nakouzi E.; Kovarik L.; Xue S.; Jin B.; Rosso K. M.; De Yoreo J. J. Understanding the mechanisms of anisotropic dissolution in metal oxides by applying radiolysis simulations to liquid-phase TEM. Proc. Natl. Acad. Sci. U. S. A. 2023, 120, e2101243120. 10.1073/pnas.2101243120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allanore A.; Lavelaine H.; Valentin G.; Birat J.; Lapicque F. Iron metal production by bulk electrolysis of iron ore particles in aqueous media. J. Electrochem. Soc. 2008, 155, E125. 10.1149/1.2952547. [DOI] [Google Scholar]
- Yuan B.; Kongstein O. E.; Haarberg G. M. Electrowinning of iron in aqueous alkaline solution using a rotating cathode. J. Electrochem. Soc. 2009, 156, D64. 10.1149/1.3039998. [DOI] [Google Scholar]
- Pourbaix M.Atlas of Electrochemical Equilibria in Aqueous Solutions; NACE International: Houston, TX, 1974. [Google Scholar]
- Kavalsky L.; Viswanathan V. Robust active site design of single-atom catalysts for electrochemical ammonia synthesis. J. Phys. Chem. C 2020, 124, 23164–23176. 10.1021/acs.jpcc.0c06692. [DOI] [Google Scholar]
- Tsiverioti L. M.; Kavalsky L.; Viswanathan V. Robust Analysis of 4e-Versus 6e-Reduction of Nitrogen on Metal Surfaces and Single-Atom Alloys. J. Phys. Chem. C 2022, 126, 12994–13003. 10.1021/acs.jpcc.2c01630. [DOI] [Google Scholar]
- Montoya J. H.; Tsai C.; Vojvodic A.; Nørskov J. K. The challenge of electrochemical ammonia synthesis: a new perspective on the role of nitrogen scaling relations. ChemSusChem 2015, 8, 2180–2186. 10.1002/cssc.201500322. [DOI] [PubMed] [Google Scholar]
- Dinh C.-T.; Burdyny T.; Kibria M. G.; Seifitokaldani A.; Gabardo C. M.; García de Arquer F. P.; Kiani A.; Edwards J. P.; De Luna P.; Bushuyev O. S.; et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 2018, 360, 783–787. 10.1126/science.aas9100. [DOI] [PubMed] [Google Scholar]
- Gauthier J. A.; Stenlid J. H.; Abild-Pedersen F.; Head-Gordon M.; Bell A. T. The role of roughening to enhance selectivity to C2. products during CO2 electroreduction on copper. ACS Energy Letters 2021, 6, 3252–3260. 10.1021/acsenergylett.1c01485. [DOI] [Google Scholar]
- Meijer K.; Denys M.; Lasar J.; Birat J.-P.; Still G.; Overmaat B. ULCOS: ultra-low CO2 steelmaking. Ironmaking & Steelmaking 2009, 36, 249–251. 10.1179/174328109X439298. [DOI] [Google Scholar]
- Lopes D. V.; Lisenkov A. D.; Sergiienko S. A.; Constantinescu G.; Sarabando A.; Quina M. J.; Frade J. R.; Kovalevsky A. V. Alkaline Electrochemical Reduction of a Magnesium Ferrospinel into Metallic Iron for the Valorisation of Magnetite-Based Metallurgical Waste. J. Electrochem. Soc. 2021, 168, 073504. 10.1149/1945-7111/ac1490. [DOI] [Google Scholar]
- Lopes D. V.; Kovalevsky A. V.; Quina M. J.; Frade J. R. Electrochemical deposition of zero-valent iron from alkaline ceramic suspensions of Fe2- xAlxO3 for iron valorisation. J. Electrochem. Soc. 2020, 167, 102508. 10.1149/1945-7111/ab9a2b. [DOI] [Google Scholar]
- Lopes D. V.; Quina M. J.; Frade J. R.; Kovalevsky A. V. Prospects and challenges of the electrochemical reduction of iron oxides in alkaline media for steel production. Frontiers in Materials 2022, 9, 1010156. 10.3389/fmats.2022.1010156. [DOI] [Google Scholar]
- Lopes D. V.; Frade J. R.; Kovalevsky A. V. Communication—On the Possibility of Fe Electrowinning from Hematite Suspensions in Mixed-Acid Electrolytes. J. Electrochem. Soc. 2024, 171, 012502. 10.1149/1945-7111/ad11b1. [DOI] [Google Scholar]
- Majid A. I.; van Graefschepe N.; Finotello G.; van der Schaaf J.; Deen N. G.; Tang Y. Comparative study of electroreduction of iron oxide using acidic and alkaline electrolytes for sustainable iron production. Electrochim. Acta 2023, 467, 142942. 10.1016/j.electacta.2023.142942. [DOI] [Google Scholar]
- Noble B. B.; Konovalova A.; Moutarlier L. J.; Brogden V.; Kempler P. A. Electrochemical chlor-iron process for iron production from iron oxide and salt water. Joule 2024, 8, 714–727. 10.1016/j.joule.2024.01.001. [DOI] [Google Scholar]
- Xu L.; Kirvassilis D.; Bai Y.; Mavrikakis M. Atomic and molecular adsorption on Fe (110). Surf. Sci. 2018, 667, 54–65. 10.1016/j.susc.2017.09.002. [DOI] [Google Scholar]
- Jiang D.; Carter E. A. Adsorption and diffusion energetics of hydrogen atoms on Fe (1 1 0) from first principles. Surf. Sci. 2003, 547, 85–98. 10.1016/j.susc.2003.10.007. [DOI] [Google Scholar]
- Wight A.; Condon N.; Leibsle F.; Worthy G.; Hodgson A. Initial stages of Fe (110) oxidation at 300 K: kinetics and structure. Surface science 1995, 331, 133–137. 10.1016/0039-6028(95)00176-X. [DOI] [Google Scholar]
- Dwyer D.; Kelemen S.; Kaldor A. The water dissociation reaction on clean and oxidized iron (110). J. Chem. Phys. 1982, 76, 1832–1837. 10.1063/1.443154. [DOI] [Google Scholar]
- Yin X.; Wang H.; Han E.-H. Effects of solvation and applied potential on the adsorption behaviors of H, O, OH and H2O on Fe (110) surface. Surf. Sci. 2020, 691, 121504. 10.1016/j.susc.2019.121504. [DOI] [Google Scholar]
- Liu S.; Tian X.; Wang T.; Wen X.; Li Y.-W.; Wang J.; Jiao H. Coverage dependent water dissociative adsorption on Fe (110) from DFT computation. Phys. Chem. Chem. Phys. 2015, 17, 8811–8821. 10.1039/C5CP00044K. [DOI] [PubMed] [Google Scholar]
- Ossowski T.; Da Silva J. L.; Kiejna A. Water adsorption on the stoichiometric and defected Fe (110) surfaces. Surf. Sci. 2018, 668, 144–149. 10.1016/j.susc.2017.10.030. [DOI] [Google Scholar]
- Chohan U. K.; Jimenez-Melero E.; Koehler S. P. Surface atomic relaxation and magnetism on hydrogen-adsorbed Fe (110) surfaces from first principles. Appl. Surf. Sci. 2016, 387, 385–392. 10.1016/j.apsusc.2016.06.027. [DOI] [Google Scholar]
- White J. J.; Hinsch J. J.; Bennett W. W.; Wang Y. Theoretical understanding of water adsorption on stepped iron surfaces. Appl. Surf. Sci. 2022, 605, 154650. 10.1016/j.apsusc.2022.154650. [DOI] [Google Scholar]
- Juan A.; Hoffmann R. Hydrogen on the Fe (110) surface and near bulk bcc Fe vacancies: a comparative bonding study. Surface science 1999, 421, 1–16. 10.1016/S0039-6028(98)00780-8. [DOI] [Google Scholar]
- Beverskog B.; Puigdomenech I. Revised pourbaix diagrams for iron at 25–300 C. Corros. Sci. 1996, 38, 2121–2135. 10.1016/S0010-938X(96)00067-4. [DOI] [Google Scholar]
- Sripad S.; Krishnamurthy D.; Viswanathan V.. The Iron-Age of Storage Batteries: Techno-Economic Promises and Challenges. ECSarXiv 2021. [Google Scholar]
- Wang Z.; Guo X.; Montoya J.; Nørskov J. K. Predicting aqueous stability of solid with computed Pourbaix diagram using SCAN functional. npj Computational Materials 2020, 6, 160. 10.1038/s41524-020-00430-3. [DOI] [Google Scholar]
- Hansen H. A.; Rossmeisl J.; Nørskov J. K. Surface Pourbaix diagrams and oxygen reduction activity of Pt, Ag and Ni (111) surfaces studied by DFT. Phys. Chem. Chem. Phys. 2008, 10, 3722–3730. 10.1039/b803956a. [DOI] [PubMed] [Google Scholar]
- Sumaria V.; Krishnamurthy D.; Viswanathan V. Quantifying confidence in DFT predicted surface pourbaix diagrams and associated reaction pathways for chlorine evolution. ACS Catal. 2018, 8, 9034–9042. 10.1021/acscatal.8b01432. [DOI] [Google Scholar]
- Vinogradova O.; Krishnamurthy D.; Pande V.; Viswanathan V. Quantifying confidence in DFT-predicted surface pourbaix diagrams of transition-metal electrode-electrolyte interfaces. Langmuir 2018, 34, 12259–12269. 10.1021/acs.langmuir.8b02219. [DOI] [PubMed] [Google Scholar]
- Liu H.; Jia X.; Cao A.; Wei L.; D’agostino C.; Li H. The surface states of transition metal X-ides under electrocatalytic conditions. J. Chem. Phys. 2023, 158, 124705. 10.1063/5.0147123. [DOI] [PubMed] [Google Scholar]
- Ghanekar P. G.; Deshpande S.; Greeley J. Adsorbate chemical environment-based machine learning framework for heterogeneous catalysis. Nat. Commun. 2022, 13, 5788. 10.1038/s41467-022-33256-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummelshøj J. S.; Blomqvist J.; Datta S.; Vegge T.; Rossmeisl J.; Thygesen K. S.; Luntz A.; Jacobsen K. W.; Nørskov J. K. Communications: Elementary oxygen electrode reactions in the aprotic Li-air battery. J. Chem. Phys. 2010, 132, 071101. 10.1063/1.3298994. [DOI] [PubMed] [Google Scholar]
- Hummelshøj J.; Luntz A.; Nørskov J. Theoretical evidence for low kinetic overpotentials in Li-O2 electrochemistry. J. Chem. Phys. 2013, 138, 034703. 10.1063/1.4773242. [DOI] [PubMed] [Google Scholar]
- Siahrostami S.; Tripković V.; Lundgaard K. T.; Jensen K. E.; Hansen H. A.; Hummelshøj J. S.; Mỳrdal J. S.; Vegge T.; Nørskov J. K.; Rossmeisl J. First principles investigation of zinc-anode dissolution in zinc-air batteries. Phys. Chem. Chem. Phys. 2013, 15, 6416–6421. 10.1039/c3cp50349f. [DOI] [PubMed] [Google Scholar]
- Nagy K. S.; Kazemiabnavi S.; Thornton K.; Siegel D. J. Thermodynamic overpotentials and nucleation rates for electrodeposition on metal anodes. ACS Appl. Mater. Interfaces 2019, 11, 7954–7964. 10.1021/acsami.8b19787. [DOI] [PubMed] [Google Scholar]
- Li X.; Ma X.; Zhang J.; Akiyama E.; Wang Y.; Song X. Review of hydrogen embrittlement in metals: hydrogen diffusion, hydrogen characterization, hydrogen embrittlement mechanism and prevention. Acta Metallurgica Sinica (English Letters) 2020, 33, 759–773. 10.1007/s40195-020-01039-7. [DOI] [Google Scholar]
- Jiang D.; Carter E. A. Diffusion of interstitial hydrogen into and through bcc Fe from first principles. Phys. Rev. B 2004, 70, 064102. 10.1103/PhysRevB.70.064102. [DOI] [Google Scholar]
- Ferrin P.; Kandoi S.; Nilekar A. U.; Mavrikakis M. Hydrogen adsorption, absorption and diffusion on and in transition metal surfaces: A DFT study. Surface science 2012, 606, 679–689. 10.1016/j.susc.2011.12.017. [DOI] [Google Scholar]
- Mortensen J. J.; Hansen L. B.; Jacobsen K. W. Real-space grid implementation of the projector augmented wave method. Phys. Rev. B 2005, 71, 035109. 10.1103/PhysRevB.71.035109. [DOI] [Google Scholar]
- Enkovaara J.; Rostgaard C.; Mortensen J. J.; Chen J.; Dułak M.; Ferrighi L.; Gavnholt J.; Glinsvad C.; Haikola V.; Hansen H. A.; et al. Electronic structure calculations with GPAW: a real-space implementation of the projector augmented-wave method. J. Phys.: Condens. Matter 2010, 22, 253202. 10.1088/0953-8984/22/25/253202. [DOI] [PubMed] [Google Scholar]
- Larsen A. H.; Mortensen J. J.; Blomqvist J.; Castelli I. E.; Christensen R.; Dułak M.; Friis J.; Groves M. N.; Hammer B.; Hargus C.; et al. The atomic simulation environmenta Python library for working with atoms. J. Phys.: Condens. Matter 2017, 29, 273002. 10.1088/1361-648X/aa680e. [DOI] [PubMed] [Google Scholar]
- Hammer B.; Hansen L. B.; Nørskov J. K. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Phys. Rev. B 1999, 59, 7413. 10.1103/PhysRevB.59.7413. [DOI] [Google Scholar]
- Kavalsky L.; Hegde V. I.; Meredig B.; Viswanathan V. A multiobjective closed-loop approach towards autonomous discovery of electrocatalysts for nitrogen reduction. Digital Discovery 2024, 3, 999–1010. 10.1039/D3DD00244F. [DOI] [Google Scholar]
- Nørskov J. K.; Rossmeisl J.; Logadottir A.; Lindqvist L.; Kitchin J. R.; Bligaard T.; Jonsson H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. 10.1021/jp047349j. [DOI] [Google Scholar]
- Vanysek P.Electrochemical series. CRC handbook of chemistry and physics; CRC, 2000; Vol. 8, pp 8–33. [Google Scholar]
- Fiorentini V.; Methfessel M. Extracting convergent surface energies from slab calculations. J. Phys.: Condens. Matter 1996, 8, 6525. 10.1088/0953-8984/8/36/005. [DOI] [Google Scholar]
- Tran R.; Xu Z.; Radhakrishnan B.; Winston D.; Sun W.; Persson K. A.; Ong S. P. Surface energies of elemental crystals. Scientific data 2016, 3, 1–13. 10.1038/sdata.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Błoński P.; Kiejna A. Structural, electronic, and magnetic properties of bcc iron surfaces. Surface science 2007, 601, 123–133. 10.1016/j.susc.2006.09.013. [DOI] [Google Scholar]
- Dragoni D.; Daff T. D.; Csányi G.; Marzari N. Achieving DFT accuracy with a machine-learning interatomic potential: Thermomechanics and defects in bcc ferromagnetic iron. Physical Review Materials 2018, 2, 013808. 10.1103/PhysRevMaterials.2.013808. [DOI] [Google Scholar]
- Grujicic D.; Pesic B. Iron nucleation mechanisms on vitreous carbon during electrodeposition from sulfate and chloride solutions. Electrochimica acta 2005, 50, 4405–4418. 10.1016/j.electacta.2005.02.013. [DOI] [Google Scholar]
- Jurca H.; Damian A.; Gougaud C.; Thiaudière D.; Cortès R.; Maroun F.; Allongue P. Epitaxial electrodeposition of Fe on Au (111): structure, nucleation, and growth mechanisms. J. Phys. Chem. C 2016, 120, 16080–16089. 10.1021/acs.jpcc.5b12771. [DOI] [Google Scholar]
- Gündel A.; Devolder T.; Chappert C.; Schmidt J.; Cortes R.; Allongue P. Electrodeposition of Fe/Au (1 1 1) ultrathin layers with perpendicular magnetic anisotropy. Physica B: Condensed Matter 2004, 354, 282–285. 10.1016/j.physb.2004.09.068. [DOI] [Google Scholar]
- Błoński P.; Kiejna A.; Hafner J. Theoretical study of oxygen adsorption at the Fe (1 1 0) and (1 0 0) surfaces. Surface science 2005, 590, 88–100. 10.1016/j.susc.2005.06.011. [DOI] [Google Scholar]
- Ogasawara H.; Brena B.; Nordlund D.; Nyberg M.; Pelmenschikov A.; Pettersson L.; Nilsson A. Structure and bonding of water on Pt (111). Physical review letters 2002, 89, 276102. 10.1103/PhysRevLett.89.276102. [DOI] [PubMed] [Google Scholar]
- Björneholm O.; Hansen M. H.; Hodgson A.; Liu L.-M.; Limmer D. T.; Michaelides A.; Pedevilla P.; Rossmeisl J.; Shen H.; Tocci G.; et al. Water at interfaces. Chem. Rev. 2016, 116, 7698–7726. 10.1021/acs.chemrev.6b00045. [DOI] [PubMed] [Google Scholar]
- Henderson M. A. The interaction of water with solid surfaces: fundamental aspects revisited. Surf. Sci. Rep. 2002, 46, 1–308. 10.1016/S0167-5729(01)00020-6. [DOI] [Google Scholar]
- Li C.-Y.; Le J.-B.; Wang Y.-H.; Chen S.; Yang Z.-L.; Li J.-F.; Cheng J.; Tian Z.-Q. In situ probing electrified interfacial water structures at atomically flat surfaces. Nature materials 2019, 18, 697–701. 10.1038/s41563-019-0356-x. [DOI] [PubMed] [Google Scholar]
- Clay C.; Haq S.; Hodgson A. Hydrogen Bonding in Mixed O H+ H 2 O Overlayers on Pt (111). Physical review letters 2004, 92, 046102. 10.1103/PhysRevLett.92.046102. [DOI] [PubMed] [Google Scholar]
- Han S.; Lysgaard S.; Vegge T.; Hansen H. A. Rapid mapping of alloy surface phase diagrams via Bayesian evolutionary multitasking. npj Computational Materials 2023, 9, 139 10.1038/s41524-023-01087-4. [DOI] [Google Scholar]
- Sokolov J.; Jona F.; Marcus P. Multilayer relaxation of the Fe {210} surface. Phys. Rev. B 1985, 31, 1929. 10.1103/PhysRevB.31.1929. [DOI] [PubMed] [Google Scholar]
- Begley A.; Tian D.; Jona F.; Marcus P. Study of ultrathin Fe films on Pd {111)}, Ag {111} and A1 {111}. Surface science 1993, 280, 289–297. 10.1016/0039-6028(93)90682-A. [DOI] [Google Scholar]
- Fruchart O.; Jubert P.-O.; Eleoui M.; Cheynis F.; Borca B.; David P.; Santonacci V.; Liénard A.; Hasegawa M.; Meyer C. Growth modes of Fe (110) revisited: a contribution of self-assembly to magnetic materials. J. Phys.: Condens. Matter 2007, 19, 053001. 10.1088/0953-8984/19/5/053001. [DOI] [Google Scholar]
- Bethge H.; Heuer D.; Jensen C.; Reshöft K.; Köhler U. Misfit-related effects in the epitaxial growth of iron on W (110). Surface science 1995, 331, 878–884. 10.1016/0039-6028(95)00166-2. [DOI] [Google Scholar]
- Allongue P.; Maroun F. Electrodeposited magnetic layers in the ultrathin limit. MRS Bull. 2010, 35, 761–770. 10.1557/mrs2010.505. [DOI] [Google Scholar]
- Bockris J.; McBreen J.; Nanis L. The hydrogen evolution kinetics and hydrogen entry into a-iron. J. Electrochem. Soc. 1965, 112, 1025. 10.1149/1.2423335. [DOI] [Google Scholar]
- Egeberg R.; Dahl S.; Logadottir A.; Larsen J. H.; Nørskov J. K.; Chorkendorff I. N2 dissociation on Fe (1 1 0) and Fe/Ru (0 0 0 1): what is the role of steps?. Surface science 2001, 491, 183–194. 10.1016/S0039-6028(01)01397-8. [DOI] [Google Scholar]
- Greeley J.; Jaramillo T. F.; Bonde J.; Chorkendorff I.; Nørskov J. K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nature materials 2006, 5, 909–913. 10.1038/nmat1752. [DOI] [PubMed] [Google Scholar]
- Nørskov J. K.; Bligaard T.; Logadottir A.; Kitchin J.; Chen J. G.; Pandelov S.; Stimming U. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 2005, 152, J23. 10.1149/1.1856988. [DOI] [Google Scholar]
- Devanathan M.; Stachurski Z. The mechanism of hydrogen evolution on iron in acid solutions by determination of permeation rates. J. Electrochem. Soc. 1964, 111, 619. 10.1149/1.2426195. [DOI] [Google Scholar]
- Suo L.; Borodin O.; Gao T.; Olguin M.; Ho J.; Fan X.; Luo C.; Wang C.; Xu K. Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 2015, 350, 938–943. 10.1126/science.aab1595. [DOI] [PubMed] [Google Scholar]
- Dave A.; Mitchell J.; Kandasamy K.; Wang H.; Burke S.; Paria B.; Póczos B.; Whitacre J.; Viswanathan V. Autonomous discovery of battery electrolytes with robotic experimentation and machine learning. Cell Reports Physical Science 2020, 1, 100264. 10.1016/j.xcrp.2020.100264. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All structures and data are available at the following GitHub repository: https://github.com/BattModels/iron_electrowinning_mapping.



