Abstract
Allergen immunotherapies are often successful at desensitizing allergic patients but can require life-long dosing and suffer from frequent adverse events including instances of systemic anaphylaxis, leading to poor patient compliance and high cost. Allergen vaccines, in turn, can generate more durable immunological allergen desensitization with far fewer doses. However, like immunotherapies, allergen vaccines are often highly reactogenic in allergic patients – hampering their use in therapeutic settings. In this work, we utilize a peptide-based self-assembling nanofiber platform to design allergen vaccines against allergen B cell epitopes that do not elicit systemic anaphylaxis when administered subcutaneously to allergic mice. We show that, in contrast to protein vaccines, nanofiber vaccines prevent leakage of allergen material into the vascular compartment – a feature that likely underpins their reduced systemic reactogenicity. Further, we show that our allergen vaccine platform elicits therapeutic IgG antibody responses capable of desensitizing allergic mice to allergen-induced Type I hypersensitivity reactions. Finally, we have demonstrated proof-of-concept for the therapeutic potential of nanofiber-based peanut allergen vaccines directed against peanut allergen-derived epitopes.
Keywords: Allergy, vaccine, nanofiber, nanomaterial
Graphical Abstract

Introduction
Allergic disease incidence is steadily rising,1–6 yet allergen-desensitizing treatment options remain limited. Allergen immunotherapies (AIT)7–12 are the most clinically advanced treatment modalities but can require life-long dosing and suffer from frequent adverse events including instances of systemic anaphylaxis, leading to poor patient compliance and high cost. Although the therapeutic mechanism(s) of AIT remain incompletely understood, it is generally appreciated that an increase in allergen specific IgG (sIgG) antibody is a major mechanistic component.13, 14 Indeed, recent development of therapeutic sIgG monoclonal antibody therapies suggest that sIgG can achieve significant allergen desensitization.15, 16 Type I hypersensitivity reactions are primarily driven by mast cells and basophils, which capture allergen-specific IgE antibody (sIgE) via the high affinity IgE receptor (FcεRI).17, 18 Allergen exposure triggers FcεRI crosslinking and rapid cellular activation, culminating in the release of effector molecules that drive allergic symptoms. sIgG facilitates desensitization through two pathways, both of which act to limit activation of allergic effector cells: 1) sIgG directly neutralizes allergen by competing with sIgE for access to allergen epitopes and 2) once incorporated into allergen immune complexes, sIgG engages the inhibitory Fc receptor (FcγRIIb) on effector cells thereby inhibiting cellular activation through FcεRI.19–21 Given the desensitizing nature of sIgG and the efficacy of sIgG monoclonal antibody therapeutics, efforts are being made to develop allergen B cell vaccines.22–24 These approaches aim to exploit vaccine technologies to generate robust and long-lived sIgG responses with only a few doses, thus addressing major disadvantages of AIT. However, like AIT, allergen vaccines are often highly reactogenic in allergic patients, hampering their use in therapeutic settings.23–26 Overcoming this barrier is critical for the clinical translation of allergen vaccines.
We have developed a supramolecular nanofiber vaccine system based on the self-assembling peptide Q11 (Ac-QQKFQFQFEQQ-Am) which can be easily fused to B and T cell epitopes for incorporation into peptide nanofiber immunogens upon self-assembly in salt-containing aqueous conditions.27 Q11 nanofibers are self-adjuvanting, although additional adjuvants and surface molecules can be included to tailor the immune response28, 29 or enable various routes of administration.30, 31 While the exact mechanism of Q11 nanofiber self-adjuvancy and immunogenicity remains to be elucidated, we have shown that these fiber immunogens are minimally inflammatory and elicit lower levels of inflammatory cytokine responses compared to other traditional adjuvants, such as alum.32–34 Here we explore the use of this supramolecular nanofiber vaccine system as an allergen vaccine platform.
Because of the safety concerns associated with allergen vaccination, we focused on characterizing allergic responses to the vaccine. We demonstrate that in contrast to protein-based allergen vaccines, our nanofiber platform does not induce systemic allergic responses after subcutaneous injection. Mechanistically, we show that unlike protein vaccines, nanofiber vaccines prevent leakage of allergen material into the vascular compartment, a feature that likely underpins their reduced systemic reactogenicity. Further, we show that our allergen vaccine platform elicits therapeutic IgG antibody responses capable of desensitizing allergic mice to allergen-induced Type I hypersensitivity reactions, and we demonstrate proof-of-concept that our platform can raise therapeutic sIgG responses to broadly reactive epitopes from major peanut allergens. Together these data suggest that a nanofiber allergen vaccine platform may be effective in achieving allergen desensitization while limiting vaccine-related adverse events, warranting further development of nanofiber allergen vaccines.
Materials and Methods
Peptide synthesis
All peptides were synthesized using Fmoc solid-phase chemistry on a CEM Liberty Blue microwave-assisted synthesizer and purified with high-performance liquid chromatography (HPLC) and matrix-assisted laser desorption/ionization–mass spectrometry (MALDI-MS), as previously reoprted.31 Biotinylated peptides were synthesized on-resin by reacting Biotin-ONp (33755-53-2, Novabiochem) with amine-terminated peptides in a threefold excess overnight in dimethyl sulfoxide (DMSO). Alexafluor-647-labeled peptides were synthesized on-resin by reacting 5 mg Alexa Fluor™ 647 NHS Ester (Succinimidyl Ester) (ThermoFisher, A20006) with 0.05 mmol amine-terminated peptide overnight in DMSO. Peptides were cleaved for 2 hours at room temperature in a 95/2.5/2.5 trifluoroacetic acid/triisopropylsilane/water cocktail, followed by washing with cold diethyl ether. Peptides were purified by reversed-phase high-performance liquid chromatography using a C18 column and lyophilized. See Supplementary Table 1 for a complete list of peptides used in this study.
Nanofiber vaccine formulation
Q11 nanofibers were formed as previously described.29,30,31 Briefly, lyophilized peptides were dissolved at 8 mM in sterile water and incubated at 4°C overnight. The solutions were then brought to a final peptide concentration of 2mM in 1× phosphate-buffered saline (PBS) by addition of sterile water and sterile 10× PBS and incubated at room temperature for 3 hours before use to allow for fibrillization. Unless otherwise specified, (OVA61–68)Q11 nanofibers were formulated with 25% (OVA61–68)Q11 peptide content. All peanut epitope nanofiber formulations were formulated with 25% (Epitope)Q11 peptide content. Nanofiber formulations used in immunization experiments included 2.5% PADRE-Q11 peptide to provide a helper T cell epitope. For adjuvanted formulations, CpG (InvivoGen, tlrl-1826) was added just before fibrillization corresponding to a concentration of 100 μg/mL, or a dose of 10 μg of CpG per mouse.
Transmission electron microscopy (TEM)
To visualize nanofiber morphology by transmission electron microscopy, nanofiber solutions were diluted to 0.2 mM in 1× PBS and deposited onto Formvar/carbon-coated 400 mesh copper grids (Electron Microscopy Sciences, EMS400-Cu) for 1 min, rinsed with ultrapure water, and negatively stained for 1 min with 1% (w/v) uranyl acetate (EMS, 22400–1) before wicking away with filter paper, as previously described.31 Samples were imaged on an FEI Tecnai G2 Twin electron microscope at 120 kV.
Model protein allergen formulation
To formulate model protein allergen, equal masses of biotinylated OVA61–68 peptide and purified streptavidin (Biolegend, 405151) were incubated at a 1 mg/mL streptavidin concentration in PBS. Excess unbound peptide was removed via centrifugal filtration using 10 kDa cutoff Amicon Ultra-0.5 Centrifugal Filter Units (MilliporeSigma, UFC501096) according to the manufacturer’s instructions.
Animal experiments
Animal experiments were performed using 8–12 week old age- and sex-matched BALB/c (Strain cAnNHsd) female mice purchased from Envigo and housed at the animal facility of Duke University. All animal procedures were performed in accordance with and approved by the Institutional Animal Care and Use Committee of Duke University under protocol #A199-21-09.
Immunizations
Mice were anesthetized under isoflurane and immunized subcutaneously at the tail base with indicated solutions with 50 μl at each side of the tail base (for nanofiber vaccines, 200 nmol each peptide epitope per mouse). At indicated time points, blood samples were collected from submandibular vein to analyze for allergen-specific sera antibodies via ELISA. To purify IgG for subsequent analysis in BMMC activation assays, serum was pooled and 0.2 mL NAb™ Protein G Spin columns were used according to the manufacturer’s instructions. After IgG purification, the resulting purified IgG was buffer-exchanged to a balanced salt solution consisting of 135mM NaCl, 12.5mM MgCl2, 1.8mM CaCl2, 20mM HEPES, and 5.6mM Glucose using 10 kDa cutoff Amicon Ultra-0.5 Centrifugal Filter Units (MilliporeSigma, UFC501096) according to the manufacturer’s instructions.
Systemic vaccine exposure
Mice were immunized as described above with the indicated Alexa Fluor™ 647-labeled vaccines. Small blood samples (~10 μL) were collected via tail snip into heparinized capillary tubes. Blood was removed from capillary tubes and diluted 1:1.5 in 1x PBS. Samples were centrifuged at 600 rcf for 5 minutes to pellet cells. Supernatant was removed and transferred to a new tube. Fluorescence was measured on a Nanodrop 3300 (ThermoFisher) at an emission of 670 nm, values reported as relative fluorescence units (RFU).
Passive sensitization and allergen challenge
Mice were administered the indicated amount (either 50 or 55 μg) of E-C1 IgE mAb (Chondrex, 3006) in 100 μL of sterile PBS via tail vein injection. The next day, mice were challenged with 100 μL of the indicated allergen solutions via the indicated route (subcutaneous at the tail base or intraperitoneally). To monitor systemic allergic responses, body temperature was measured via rectal thermometer and serum was collected immediately before and 1 hour after challenge for analysis of mast cell protease 1 (MCPT-1) levels. Significant hypothermia (rectal temperatures below 32 °C) was considered a humane endpoint. Serum levels of MCPT-1 were analyzed using a MCPT-1 (mMCP-1) Mouse Uncoated ELISA Kit (ThermoFisher, 88-7503-22) according to manufacturer’s instructions.
Active allergen sensitization
For sensitization against crude peanut extract, mice were injected intraperitoneally with 100 μg of crude peanut extract (Stallergenes Greer, F171 peanut source material, XPF171D3A2.5, Lot# 352557) mixed with 200 μL of AlHydrogel adjuvant (InvivoGen, vac-alu-250) once a week for a total of 3 weeks. Serum was collected, pooled, and depleted of IgG using 1 mL NAb™ Protein G Spin columns (ThermoFisher, 89979). The IgG-depleted serum was then used to sensitize BMMC (see BMMC activation assays).
Peritoneal mast cell activation assay
Mice were sensitized to OVA61–68 via intraperitoneal injection of 25 μg E-C1 IgE mAb (Chondrex, 3006) in 250 μL of sterile PBS. The next day, 500uL of a 0.2 mM (OVA61–68)Q11 nanofiber solution or an 11 μg/mL OVA61–68 tetramer solution was injected intraperitoneally. One hour later, mice were sacrificed and a lavage of the peritoneal cavity was performed with 500uL of sterile PBS + 1% bovine serum albumin. Lavage fluid was filtered through a 70 μm cell strainer and centrifuged to pellet cells. Peritoneal mast cell activation (CD63 surface expression) was analyzed via flow cytometry. See Supplementary Figure 2 for flow cytometry gating strategy.
Enzyme-linked immunosorbent assays (ELISA)
For analysis of peptide epitope-specific antibody by ELISA, plates were coated with 20 μg/ml streptavidin (MilliporeSigma, 189730) overnight at 4°C. Plates were washed with Tween 20 (0.5 g/liter) in PBS (1x PBST). Biotinylated peptides (20 μg/ml) were captured for 30 minutes. For analysis of protein-specific antibody, ELISA plates were coated with 5 μg/mL crude peanut extract (Stallergenes Greer, F171 peanut source material, XPF171D3A2.5, Lot# 352557), natural Ara h 1 (InBio, NA-AH1–1), natural Ara h 2 (InBio, NA-AH2–1), natural Ara h 3 (InBio, NA-AH3–1), or recombinant Ara h 7 (RayBiotech, 230-00901-50). Coated plates were blocked with Super Block Blocking Buffer (ThermoFisher Scientific, 37515). Sera diluted in 1x PBST + 1% bovine serum albumin were added to the plate for 1.5 hours and plates were washed in 1x PBST. To detect antigen-specific IgG, horseradish peroxidase (HRP)–conjugated Fcγ fragment–specific goat anti-mouse IgG (Jackson ImmunoResearch, 15-035-071) was added to the plates. To detect antigen-specific IgE, HRP-conjugated goat anti-mouse IgE (Southern Biotech, 1110-05) was added to the plates. Plates were washed in 1x PBST and TMB substrate (Invitrogen, 00-4201-56) was added to the plates for 5 minutes. Development was stopped with 1M H3PO4 and absorbance was read at 450nm on a microplate reader. Results are reported as antibody titers calculated with an absorbance cutoff of 0.2 OD (optical density), or as background subtracted A450 (absorbance at 450 nm) values. Samples below the limit of detection were assigned a titer of zero.
BMMC culture
Bone marrow was isolated from mouse femurs and tibias and passed through a 70 μm cell strainer. Red blood cells were lysed using ACK cell lysis buffer (Gibco, A10492–01) and the remaining cells were cultured on tissue culture-treated cell culture flasks in BMMC culture media [RPMI 1640 with l-glutamine, 10 mM Hepes, 50 μM β-mercaptoethanol, 1 mM sodium pyruvate, 1 mM nonessential amino acids, and 10% fetal bovine serum (FBS) + Penicillin-Streptomycin] supplemented with 10 ng/mL recombinant mouse interleukin 3 (rmIL-3) (R&D Systems, 403-ML). Every 3–4 days, non-adherent cells were pelleted via centrifugation, resuspended in fresh BMMC culture media + 10 ng/mL rmIL-3, and moved to a new tissue culture flask. All BMMC experiments were performed with cells cultured for 4 to 8 weeks.
BMMC activation assays
For assays of BMMC activation in response to the OVA61–68 model allergen epitope, BMMC were sensitized for 16 hours with 0.1 μg/mL E-C1 IgE mAb (Chondrex, 3006) at a cell density of 500K/mL in BMMC culture media. BMMC were washed to remove excess IgE and plated at 500K/mL and stimulated with the indicated solutions. For assessment of sIgG-mediated allergen neutralization, 400 μg/mL purified IgG was pre-mixed with allergen for 30 minutes prior to addition to BMMC. BMMC were stimulated for 45 minutes before analysis of activation via flow cytometry. See Supplementary Figure 1 for flow cytometry gating strategy.
For assays of BMMC activation in response to peanut allergen proteins, BMMC were sensitized via 24-hour incubation in 10% IgG-depleted allergic serum (see active allergen sensitization section) in BMMC culture media supplemented with 10 ng/mL rmIL-3 (R&D Systems, 403-ML) at a cell density of 500K/mL. BMMC were then washed and plated in BMMC culture media overnight. BMMC were washed and plated at a cell density of 500K/mL with the indicated allergen solutions at a concentration of 10 ug/mL allergen. For assessment of sIgG-mediated allergen neutralization, 400 μg/mL purified IgG was pre-mixed with allergen for 30 minutes prior to addition to BMMC. BMMC were stimulated for 45 minutes before analysis of activation via flow cytometry.
To quantify BMMC activation, cells were stained for CD45, FcεRI, c-kit, and CD63 in FACS buffer (1x PBS + 1% bovine serum albumin + 1 mM EDTA) using FITC anti-mouse CD45 (Biolegend, 103108), PE anti-mouse FcεRIα (Biolegend, 134308), Brilliant Violet 510™ anti-mouse CD117 (c-kit) (Biolegend, 105839), and APC anti-mouse CD63 (Biolegend, 143906). DAPI was included as a viability dye at 1 μg/mL. BMMC were defined as viable CD45+FcεRI+cKIT+ cells and activation was quantified via CD63 expression (MFI, mean fluorescent intensity). CD63 MFI of unstimulated cells was subtracted as background signal.
Human peanut allergic serum
Serum from peanut allergic human donors was obtained from PlasmaLab International (Donor codes: 33599-KA, 32342-DG, 26730-AB, 25373-AD, 33454-PS, 34480-NV, 29927-MF, 20197-BH).
Humanized RBL-NFAT-DsRed culture
Parental RBL-NFAT-DsRed cells35 expressing the human FcεRIα and FcεRIγ chains were obtained from the University of Nottingham. Cells were maintained in RBL Culture Media (MEM with GlutaMAX™ Supplement + 10% FBS + Penicillin-Streptomycin). Every 3–4 passages, cells were treated with 1 mg/mL G418 sulfate, 20 μg/mL Blasticidin S.
Humanized RBL-NFAT-DsRed sorting
To improve the sensitivity of our assays, we sorted functional reporter cells from the parental humanized RBL-NFAT-DsRed cell line. Cells were plated in a 24 well plate at 7.5 × 104 cells per well and incubated overnight. The next day cells were sensitized with 0.2 μm sterile filtered 2.5% allergic human serum in RBL culture media. Prior to dilution in RBL culture media, allergic serum was heat inactivated via incubation at 56°C for 5 minutes. The next day, cells were washed and stimulated with 1 μg/mL goat anti-human IgE for 24 hours. Cells were washed, trypsinized, suspended in FACS buffer and DsRed+ cells were sorted using a Sony MA900 cytometer. The sorted cell population was diluted in RBL culture media to ~1 cell/100μL and plated in a 96-well plate to isolate and expand single cell clones. A single cell clonal line was selected for expansion and used for subsequent experiments.
Humanized RBL-NFAT-DsRed activation assay
RBL-NFAT-DsRed cells were plated in a 96-well plate at 1.25 × 104 cells per well and incubated overnight. The next day, cells were sensitized for 24 hours with 0.2 μm sterile filtered serum from peanut allergic donors at 5% allergic serum in RBL culture media. Prior to dilution in RBL culture media, allergic serum was heat inactivated via incubation at 56°C for 5 minutes. The following day, cells were washed and treated overnight with solutions of 0.05 mM peanut epitope nanofibers or Q11-only nanofibers, or with 1 μg/mL crude peanut extract as a positive control (Stallergenes Greer, F171 peanut source material, XPF171D3A2.5, Lot# 352557). Cells were washed, trypsinized, and suspended in FACS buffer for flow cytometry analysis of DsRed expression. DAPI was included as a viability dye. Flow cytometry was performed using BD Canto II instrument and analyzed using FlowJo software. Cells sensitized with serum from donor 20197-BH failed to respond to the positive control and were excluded from our analysis.
Results
Nanofibers are minimally reactogenic in allergic mice, even when displaying allergenic epitopes.
As a first step to evaluating our self-assembling peptide nanofiber vaccine system as an allergen vaccine platform we compared the in vivo reactogenicity of a model nanofiber allergen vaccine to a model protein allergen vaccine. To form our model nanofiber vaccines, we synthesized in tandem a model allergen B cell epitope (OVA61–68) and the self-assembling peptide Q11 (Fig. 1A,B, Supplementary Table 1). To form a comparative protein-based vaccine against the same model allergen epitope, we loaded streptavidin tetramers with biotinylated OVA61–68 peptide (Fig. 1C), a strategy used by others to generate multivalent model allergen proteins.36 It should be noted that we utilized allergen peptide tetramer as a model protein vaccine immunogen because mast cell activation through FcεRI requires receptor crosslinking by multivalent allergen. Protein ovalbumin (OVA) is not an appropriate comparative protein allergen vaccine immunogen, as it contains only a single copy of the OVA61–68 epitope.
Figure 1: Nanofiber allergen vaccines do not elicit systemic anaphylaxis after subcutaneous injection in a mouse model of passive systemic anaphylaxis.
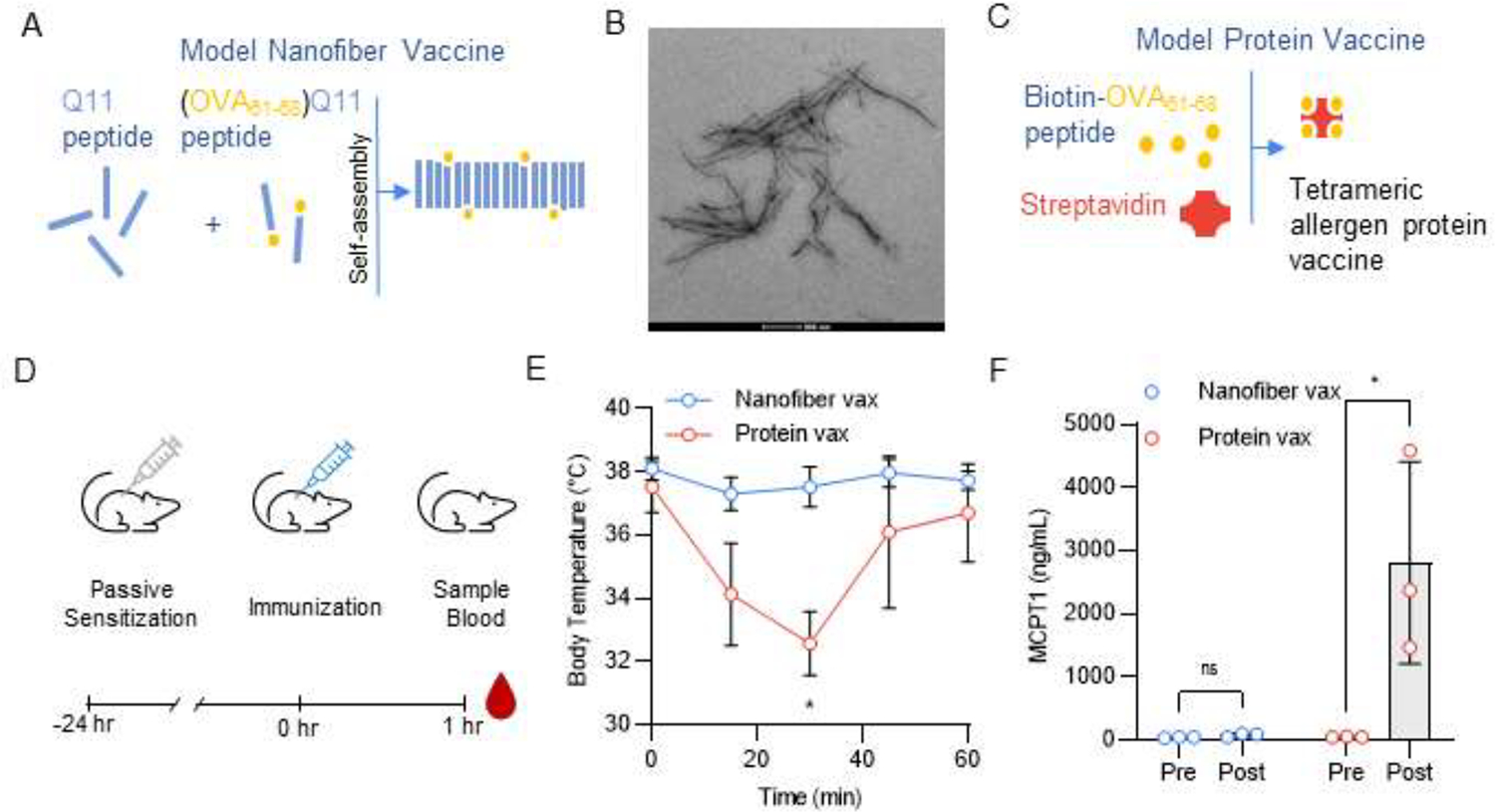
(A) Schematic of Q11 nanofiber allergen vaccine (Nanofiber Vax). (B) TEM images of (OVA61–68)Q11 nanofibers. Nanofiber formulation: 12.5% (OVA61–68)Q11, 87.5% Q11. (C) Schematic of model protein-based allergen vaccines (Protein Vax) formed by binding biotin-OVA61–68 to streptavidin. (D) Timeline for allergen vaccine challenge experiment. Mice were passively sensitized to the OVA61–68 allergen via intravenous injection of 50μg E-C1 IgE mAb and challenged via subcutaneous injection of nanofiber and protein vaccines 24 hours later. Nanofiber formulation: 12.5% (OVA61–68)Q11, 87.5% Q11. 2 mM nanofibers, 100 μL per mouse. Allergen tetramer dose: 100 μg in 100 μL per mouse. (E) Body temperature of mice after vaccine challenge. Mixed-effects analysis with Sidak’s multiple comparisons test, * p < 0.05, n = 3 mice. (F) MCPT1 serum levels before (Pre) and 1 hour after (Post) vaccine challenge. Two-way RM ANOVA with Sidak’s multiple comparisons test, * p < 0.05, n = 3 mice.
We next assessed if either vaccine would elicit an allergic response when used to immunize allergic mice. We sensitized mice to OVA61–68 via intravenous injection of an anti-OVA61–68 IgE mAb (E-C1) and injected our model allergen vaccines subcutaneously the next day (Fig. 1D). We found that the model protein vaccine induced decreases in body temperature and spikes in serum levels of mast cell protease 1 (MCPT-1), indicating a systemic anaphylactic response. Contrastingly, mice receiving the model nanofiber vaccine showed no signs of a systemic allergic response (Fig. 1E,F).
We next asked if this difference in in vivo reactogenicity could be explained by differences in vaccine allergenicity. Here, we use the term “allergenicity” to mean the inherent ability of the vaccine to activate allergen-sensitized mast cells and trigger
Type I hypersensitivity responses. We found that both vaccines were similarly recognized by E-C1 IgE antibody (Fig. 2A,B) and that both vaccines elicited mast cell activation when exposed to E-C1-sensitized bone marrow-derived mast cells (BMMC) (Fig. 2C,D). Furthermore, we found that both vaccines activated peritoneal mast cells when injected directly into the peritoneal cavity of E-C1-sensitized mice (Fig. 2E,F). Thus, the observed differences in in vivo reactogenicity between the two vaccine types cannot be explained by differential vaccine allergenicity, as both the model nanofiber and protein vaccines were recognized by sIgE and activated allergen-sensitized mast cells.
Figure 2: Nanofiber and protein vaccines both exhibit allergenicity and activate allergen sensitized mast cells.

(A) Left; E-C1 IgE binding curves to nanofiber allergen vaccines (Nanofiber Vax) or Q11 nanofibers alone (Empty Nanofiber). Right; quantification of binding curve AUC. Unpaired t test, **** p < 0.0001, n = 3 technical replicates. (B) Left; E-C1 IgE binding curves to OVA61–68 tetrameric proteins (Protein Vax) and empty tetramers lacking the OVA61–68 peptide (Empty Protein). Right; quantification of binding curve AUC. Unpaired t test, **** p < 0.0001, n = 3 technical replicates. (C) Left; BMMC activation triggered by the nanofiber allergen vaccine as measured by the surface expression of CD63. Right; quantification of maximum BMMC activation. Unpaired t test, **** p < 0.0001, n = 3 technical replicates. (D) Left; BMMC activation triggered by the protein allergen vaccine as measured by the surface expression of CD63. Right; quantification of maximum BMMC activation. Unpaired t test, **** p < 0.0001, n = 3 technical replicates. (E) Timeline for peritoneal mast cell activation experiment. To assess peritoneal mast cell activation in response to nanofiber and protein allergen vaccines, mice were passively sensitized to the OVA61–68 allergen via intraperitoneal injection of 25 μg E-C1 IgE mAb and challenged via intraperitoneal injection of nanofiber and protein vaccines 24 hours later. Mice were sacrificed 1 hour later, and a lavage of the intraperitoneal space was performed. (F) Histograms depicting intraperitoneal mast cell activation quantified by CD63 surface expression. (G) Quantification of activated peritoneal mast cell as a percentage of all peritoneal mast cells. Unpaired t test, * p < 0.05, *** p < 0.001, n = 3 mice.
Nanofiber vaccines prevent systemic allergen exposure.
Because both model nanofiber and protein allergen vaccines activate mast cells ex vivo and when injected intraperitoneally, but only protein allergen vaccines induce systemic allergic responses after subcutaneous injection, we asked if this difference could be explained by reduced systemic exposure. To track systemic vaccine exposure, we labeled nanofiber and protein vaccine with fluorescent dye and injected fluorescence-matched vaccines subcutaneously into mice. We collected blood samples over a 24-hour period and found that nanofiber vaccines had drastically reduced accumulation in the vascular compartment compared to protein vaccines (Fig. 3A–C). Local allergic responses can increase vascular permeability37 and may affect levels of systemic vaccine exposure, so we also tracked the presence of vaccine material in the bloodstream after injection into E-C1-sensitized mice (Fig. 3D). Protein allergen vaccines induced systemic anaphylactic reactions that were fatal in 60% of mice, whereas nanofiber vaccines did not, despite both vaccines delivering equimolar doses of allergen (Fig. 3E–G). Nanofiber vaccines again drastically reduced systemic vaccine exposure compared to protein vaccines, even in the allergic setting (Fig. 3H,I).
Figure 3: Nanofiber allergen vaccines prevent leakage of vaccine material into the vascular compartment.

(A) Timeline for systemic vaccine exposure experiment in naïve mice. Fluorescence-matched vaccines labeled with Alexafluor 647 (AF647) dye were injected subcutaneously into mice and blood samples were collected over a 24-hour period. (B) Serum fluorescence over time. (C) AUC quantification of serum fluorescence over time. Unpaired t test, **** p < 0.0001. (D) Timeline for systemic vaccine exposure experiment in passively sensitized mice. Mice were passively sensitized to the OVA61–68 allergen via intravenous injection of 50μg E-C1 IgE mAb and challenged via subcutaneous injection of fluorescence-matched AF647-labeled nanofiber and protein vaccines 24 hours later. Nanofiber formulation: 6.25% (OVA61–68)Q11, 10% AF647-Q11 and 83.75% Q11. Tetrameric allergen protein (172 μg per mouse) contained equimolar OVA61–68 dose to the nanofiber vaccine. (E) Body temperature of mice after vaccine challenge. Mixed-effects analysis with Sidak’s multiple comparisons test, * p < 0.05, ** p < 0.001. (F) MCPT1 serum levels before (Pre) and 1 hour after (Post) vaccine challenge. Two-way RM ANOVA with Sidak’s multiple comparisons test, **** p < 0.0001. (G) Survival curves of mice after vaccine challenge. Endpoint criteria was set at a body temperature of 32°C or lower. Mantel Cox test, * p < 0.05. (H) Serum fluorescence over time. (I) AUC quantification of serum fluorescence over time. Unpaired t test, analysis performed on surviving mice only, **** p < 0.0001. n = 5 mice for all experiments.
A model nanofiber allergen vaccine is immunogenic and protects against allergen challenge.
A key feature of a successful allergen vaccine is the ability to generate therapeutic IgG antibody that can neutralize the allergen and prevent Type I hypersensitivity reactions after allergen exposure. Here, we evaluated the ability of our nanofiber vaccine platform to raise therapeutic sIgG responses. (OVA61–68)Q11 nanofiber vaccines alone or together with CpG adjuvant raised high titers of sIgG after only two immunizations (Fig. 4A–C). IgG purified from the serum of mice immunized with CpG-adjuvanted (OVA61–68)Q11 vaccines desensitized BMMC to allergen exposure ex vivo, while IgG purified from the serum of naïve mice or mice immunized with epitope-free Q11 nanofibers had no desensitizing effect (Fig. 4D,E). In this assay, IgG purified from the serum of mice immunized with unadjuvanted (OVA61–68)Q11 reduced BMMC activation but not to a statistically significant level, potentially due to the lower titer of sIgG in this group compared to the CpG-adjuvanted group. Given the allergen-neutralizing ability of sIgG from mice immunized with CpG-adjuvanted (OVA61–68)Q11 vaccines, we asked if immunized mice would be protected from allergen challenge. We passively sensitized the mice to OVA61–68 and challenged them via intraperitoneal injection of model protein. allergen (Fig. 4F). Naïve mice with no sIgG showed elevated serum levels of MCPT-1 after allergen challenge, whereas mice that received the allergen vaccine did not, suggesting that nanofiber allergen vaccination can protect against Type I allergic responses after allergen exposure (Fig. 4G).
Figure 4: Nanofiber allergen vaccines are immunogenic and raise therapeutic sIgG responses that desensitize against allergen challenge in a mouse model of passive systemic anaphylaxis.
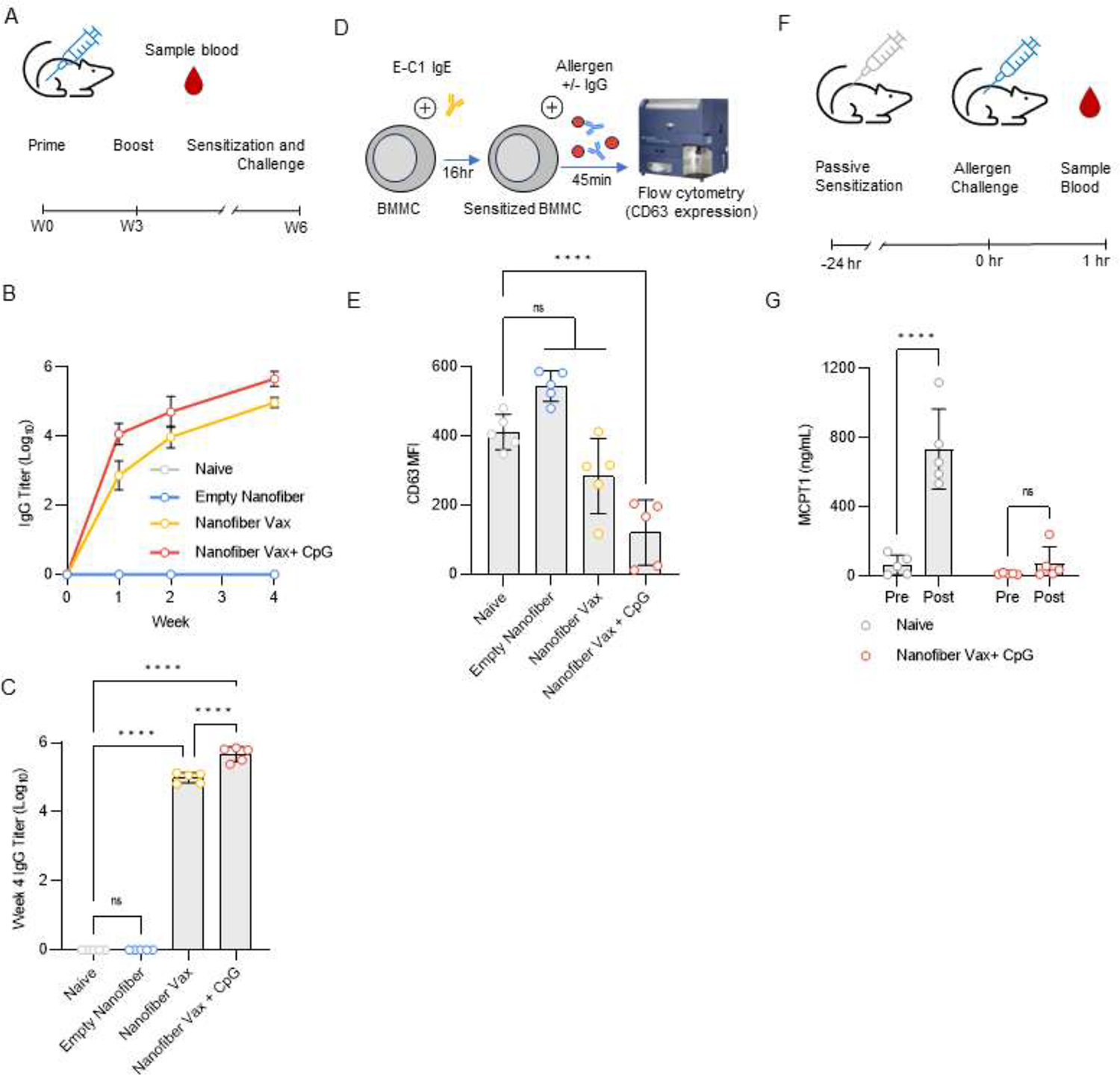
(A) Timeline of nanofiber allergen vaccine immunization. Mice were primed on Week 0 and boosted on Week 3. Blood samples were collected to assess antibody response. After 6 weeks, mice were sensitized and challenged (see Panels F,G). (B) anti-OVA61–68 IgG titers over time. (C) anti-OVA61–68 IgG titers at Week 4. One-way ANOVA with Dunnett’s multiple comparisons test, **** p < 0.0001, ns p > 0.05, n = 5 mice. (D) BMMC activation assay workflow. BMMC were sensitized via incubation with E-C1 IgE mAb. 24 hours later, BMMC were exposed to tetrameric OVA61–68 model allergen in the presence or absence of purified IgG. BMMC activation was quantified by CD63 surface expression (E). One-way ANOVA with Bonferroni multiple comparisons test against the Naïve group, ns p > 0.05, **** p < 0.0001, n = 5 biological replicates. (F) Timeline for allergen challenge experiment. Mice were passively sensitized to the OVA61–68 allergen via intravenous injection of 55 μg E-C1 IgE mAb and challenged via intraperitoneal injection of 5 μg tetrameric OVA61–68 model allergen 24 hours later. (G) MCPT1 serum levels before (Pre) and 1 hour after (Post) vaccine challenge. Two-way RM ANOVA with Sidak’s multiple comparisons test, **** p < 0.0001, n = 5 mice.
Nanofiber allergen vaccines directed against broadly reactive peanut allergen epitopes are immunogenic and generate therapeutic sIgG.
Motivated by our findings regarding the safety and efficacy of our model nanofiber allergen vaccines, we next aimed to identify and validate allergen epitope targets relevant to human allergic disease. Here, we focused on peanut allergy, one of the most life-threatening food allergies. To identify candidate epitope targets from the major peanut allergen proteins
Ara h 1, 2, 3, and 7, we selected six immunodominant epitopes from the literature38–42 (Supplementary Table 1) with broad IgE reactivity across allergic patients and validated their allergenicity using a humanized mast cell line sensitized with serum from peanut allergic human donors (Fig. 5A,B). Of the six epitopes, four were potently allergenic across all donors (AH1a, AH2a, AH2b, AH3a). The remaining two epitopes (AH1b and AH7a) also triggered detectable activation above baseline in this assay, but not to a statistically significant degree. Next, we confirmed the immunogenicity of nanofiber vaccines directed against the six candidate epitopes, with all vaccines raising robust IgG antibody responses that persisted for at least 25 weeks, which was the latest timepoint we assayed (Fig. 5C). Additionally, we confirmed that vaccine-induced sIgG recognized both the peptide antigen as well as the full protein allergen (Fig. 5D,E).
Figure 5: Nanofiber vaccines against B cell epitope allergen candidates from major peanut allergens raise sIgG responses with therapeutic potential.

(A) Humanized mast cell line (RBL-NFAT-DsRed) activation assay workflow. RBL-NFAT-DsRed were sensitized via incubation with 5% heat-inactivated serum from 7 different peanut allergic human donors. 24 hours later, the cells were exposed to nanofiber vaccines displaying six different peanut allergen epitopes. 16 hours later, mast cell activation was assessed via quantification of DsRed expression. (B) DsRed expression of RBL-NFAT-DsRed cells after stimulation with peanut epitope nanofibers. Crude peanut extract (CE) was included as a positive control to illustrate maximal cell activation. Data is displayed as logarithmic MFI values which, for each donor, are normalized to a Q11-only control. One-way ANOVA with Holm-Sidak multiple comparisons test against the Q11 control group, p-values shown on plot. (C-E) Immunogenicity of peanut epitope nanofiber vaccines. (C) sIgG titers over time. Mice were primed on Week 0 and boosted on Weeks 3 and 5. sIgG titers assessed against crude peanut extract. (D) Week 8 sIgG binding to peanut epitopes. Titers assessed against peanut epitope nanofibers. (E) Week 8 sIgG binding to peanut allergen protein. Titers assessed against natural purified or recombinant allergen. (F) BMMC activation assay workflow. BMMC were sensitized via incubation with peanut allergic serum. 24 hours later, BMMC were exposed to protein peanut allergens in the presence or absence of purified IgG. BMMC activation was quantified by CD63 surface expression. (G,H) BMMC sensitized with serum from mice sensitized against crude peanut extract. (G) Effect of AH1a and AH1b sIgG on Ara h 1-induced BMMC activation. AH1b sIgG reduced BMMC activation. (H) Effect of AH3a sIgG on Ara h 3-induced BMMC activation. AH3a sIgG reduced BMMC activation. (I) Effect of AH7a sIgG on Ara h 7-induced BMMC activation. BMMC were sensitized with serum from mice sensitized against recombinant Ara h 7. AH7a sIgG reduced BMMC activation. (G-I) One-way ANOVA with Dunnett’s multiple comparisons test against the No IgG control group, ns p > 0.05, *** p < 0.001, **** p < 0.0001, n = 3 biological replicates.
To characterize therapeutic potential of sIgG for each target epitope, we again employed ex vivo BMMC activation assays (Fig. 5F). We first asked if vaccine-elicited sIgG could neutralize allergen and desensitize BMMC that were sensitized with serum from mice sensitized against crude peanut extract. When crude extract sensitized BMMC were exposed to Ara h 1 allergen protein, AH1b sIgG, but not AH1a sIgG, markedly reduced BMMC activation (Fig. 5G). When crude extract sensitized BMMC were exposed to Ara h 3 allergen protein, AH3a sIgG also markedly reduced BMMC activation (Fig. 5H). For the Ara h 2 and 7 allergens, in this assay the peanut-allergic mice did not raise sufficient IgE responses to sensitize BMMC (Supplementary Fig. 4B,C). So, to evaluate sIgG against Ara h 2 and 7, we sensitized mice specifically to these allergens and sensitized BMMC with the resulting serum. Here, we found that IgG from mice immunized against AH7a markedly desensitized BMMC to Ara h 7 exposure (Fig. 5I). We found that serum from mice sensitized to Ara h 2 was unable to sensitize BMMC to the allergen (Supplementary Fig. 4D) and were unable to evaluate the allergen neutralization of AH2a and AH2b sIgG. We also asked whether sIgG pooled from immunized mice could reduce BMMC responses to crude peanut extract but did not observe reduced BMMC activation in this setting (Supplementary Fig. 4E). This suggests that the sIgE response of crude extract-sensitized mice may be dominated by major allergens other than Ara h 1, 2, 3, and 7.
In sum, from our panel of broadly reactive IgE-binding epitopes, we found that epitopes AH1b, AH3a, and AH7a induced allergen neutralizing sIgG when used as immunogens on nanofiber allergen vaccines. Because our mice did not raise IgE responses to Ara h 2, we could not evaluate the therapeutic potential of AH2a or AH2b sIgG. Interestingly, we tested sIgG from each allergen epitope separately, indicating that sIgG directed against only a single epitope can potently neutralize allergen proteins and prevent mast cell activation even when the cells are sensitized with polyclonal serum. However, pooled sIgG against these targets could not neutralize the complex mixture of allergen proteins in crude peanut extract. These results confirm that peanut sIgG generated by nanofiber allergen vaccines has therapeutic potential to neutralize allergen and reduce mast cell activation, but optimization of major peanut allergen targets may be necessary to achieve adequate peanut desensitization.
Discussion
Motivated by the evidence of sIgG involvement in allergen immunotherapy and by promising reports of sIgG mAb therapy efficacy, allergen B cell vaccines aim to elicit long-lived allergen-neutralizing sIgG responses with only a few doses. However, like AIT, these technologies suffer from frequent adverse events caused by patient allergic responses to the vaccine. Here, we have performed an initial evaluation of a supramolecular nanofiber vaccine system as an allergen vaccine platform.
We found that nanofiber vaccines displaying a model B cell allergen epitope can be administered subcutaneously to allergen sensitized mice without inducing systemic allergic responses. In contrast, mice experience systemic anaphylactic reactions if the same allergen epitope is delivered via a protein carrier. Mechanistically, we found that this behavior is not explained by the inherent allergenicity of the two vaccine types, but rather that nanofiber vaccines prevent leakage of vaccine material into the blood stream. We posit that this feature underpins the reduced systemic reactogenicity of our nanofiber vaccines as systemic allergen exposure is thought to be required for induction of systemic anaphylaxis.43 Given that particle size is an important determinate of vascular access and lymphatic trafficking,44 our current hypothesis is that nanofiber vaccine particles are too large to cross the vascular endothelium and are restricted to the interstitial space at the injection site and to lymphatic vessels. Indeed, we have previously shown that Q11 nanofiber vaccines remain at the injection site for days.45 Exclusion from the vascular compartment is likely not unique to Q11 nanofiber vaccines and could affect the safety profile of other nanoparticle-based allergen vaccines - including those based on polymeric particles, liposomes, or VLPs. However, there is at least one example of a clinically tested liposomal allergen vaccine that saw high rates of systemic adverse events,25 although it is not clear if this was caused by contamination with soluble allergen, allergen adsorption to the liposomal surface, liposomal rupture and allergen release after injection, or by some other mechanism. Further, because our nanofiber vaccine retains allergenicity ex vivo and triggers local mast cell activation after intraperitoneal injection, it is likely that some level of local reactogenicity occurs after subcutaneous injection, although we did not characterize this in this study. Investigation into local reactions to nanofiber allergen vaccines is warranted and development of strategies to combat local reactions may be of interest.
For our studies of model vaccines against the OVA61–68 model allergen epitope, we employed a passive sensitization model in which mice were sensitized to the allergen via injection of monoclonal sIgE. We chose this model because of its excellent sensitivity to vaccine reactogenicity, as this was a major focus of this study. Active allergen sensitization models, while able to raise sIgE responses, also elicit extremely high levels of sIgG. Because of this, actively sensitized mice are significantly less sensitive to allergen exposure compared to passively sensitized mice that lack any preexisting sIgG. Indeed, anaphylaxis in active sensitization models requires allergen doses orders of magnitude higher than required to elicit anaphylaxis in a passively sensitized setting.17, 20 Further, anaphylaxis in actively sensitized mice is mediated in part through a IgG-dependent pathway involving the release of platelet activating factor (PAF) by macrophages activated through FcγRIII, whereas human anaphylaxis is thought to be primarily mediated by IgE-dependent Type I hypersensitivity. Although the passive sensitization model that we employed here has a number of advantages over alternative models, it also has its own limitations. Human allergy involves a polyclonal immune response to a complex mixture of allergen proteins mediated by both B and T cells. Much of this complexity is not captured in a passively sensitized model of allergy, which relies on a single interaction between monoclonal sIgE and a model allergen, and this simplification should be taken into account when interpreting the results of our study.
Our nanofiber vaccine system raises robust and long-lived IgG responses against peptide antigens. Here, we show that Q11 nanofiber vaccines directed against six different peptide B cell epitopes from major peanut allergens raise high sIgG titers that persist in mice for at least 25 weeks. For epitope targets from Ara h 1, 3, and 7, we found that sIgG from immunized mice significantly reduced activation of BMMC. Interestingly, we observed this effect despite BMMC being sensitized with polyclonal allergic sera and our sIgG being directed towards only a single epitope within the allergen protein. As such, our results suggest that an allergen B cell vaccine does not necessarily need to target every IgE-binding epitope on the allergen(s) and that sIgG directed against only a single allergen epitope can achieve desensitization to the entire protein. This effect could be mediated through engagement of inhibitory FcγRIIb on BMMC, which can inhibit FcεRI signaling even when FcεRI clusters lacking FcγRIIb co-engagement exist on the same mast cell.21 However, this effect does also seem to depend on the epitope target as sIgG directed against the AH1a epitope did not neutralize allergen. Interestingly, sIgE in the allergic serum used for this assay recognized the AH1b epitope, but not the AH1a epitope (Supplementary Fig. 3), suggesting that direct competition for sIgE epitopes is also an important mechanistic component of sIgG allergen neutralization. Unfortunately, we were unable to sensitize mice to the major peanut allergen Ara h 2 and thus could not evaluate therapeutic potential of vaccines against two of our target epitopes in this study. However, Ara h 2 is one of the most important allergens in human peanut allergy and these two epitope targets warrant further investigation as potential vaccine targets. It should also be noted that Ara h 6,46, 47 a peanut protein allergen that shares some homology with Ara h 2, is another important immunodominant peanut allergen which was not evaluated as a target in this study. Identification of candidate epitope targets within this important allergen may be a subject of future work.
T cell mediated Type IV hypersensitivity reactions also contribute to allergic disease. Our nanofiber vaccines included only allergen B cell epitopes for induction of sIgG, but it may be interesting to include a T cell component in future work. Indeed, AIT efficacy relies not only on sIgG induction, but also on a shift in allergen specific T cell phenotype away from the pro-allergic Th2 phenotype to a Th1/Treg phenotype.12, 14 Additionally, it may be worthwhile to consider a hybrid protocol where nanofiber allergen vaccines are administered first to achieve allergen desensitization, followed by AIT. In fact, the presence of pre-existing sIgG can enhance the efficacy of AIT by driving stronger shifts in T cell phenotype.19 Thus, pre-vaccination before AIT might enhance both the safety and efficacy of a subsequent AIT protocol.
Conclusions
Overall, our results support further investigation and development of nanofiber allergen vaccines. Allergen delivery via nanofiber vaccines prevents systemic exposure of vaccine material, potentially offering safety advantages over other vaccine platforms, particularly those employing soluble protein allergen. We have identified six epitope targets from peanut allergens that were selected for immunodominance and broad reactivity across peanut allergic individuals and have confirmed therapeutic potential of sIgG elicited by nanofiber allergen vaccines directed against three of these targets. In future work, we will continue to develop allergen vaccines using this platform.
Supplementary Material
Acknowledgements
We thank the University of Nottingham and Dr. Franco Falcone for providing the parental RBL-NFAT-DsRed cell line.
Funding
This work was supported by the National Institutes of Health under grant 1R21AI164740. B.J.C. was supported under grant T32GM008555.
Footnotes
Supporting Information Available
The following files are available free of charge: Additional Experimental Data including Flow Cytometry Gating Strategies - Supplementary Figures 1–4, Supplementary Table 1 (PDF).
Competing Interests Statement
JHC is an inventor on patents and patent applications associated with self-assembled peptide immunotherapies.
Animal Rights Statement
Animal experiments were performed using 8–12 week old age- and sex-matched BALB/c (Strain cAnNHsd) female mice purchased from Envigo and housed at the animal facility of Duke University. All animal procedures were performed in accordance with and approved by the Institutional Animal Care and Use Committee of Duke University under protocol #A199-21-09.
Data Availability
Data is available via the Duke University Libraries Digital Repository of Research Data under the same title and authors as this publication. https://research.repository.duke.edu/
References
- (1).Åberg N; Hesselmar B; Åberg B; Eriksson B Increase of asthma, allergic rhinitis and eczema in Swedish schoolchildren between 1979 and 1991. Clinical & Experimental Allergy 1995, 25 (9), 815–819. [DOI] [PubMed] [Google Scholar]
- (2).Lieberman J; Sublett J; Ali Y; Haselkorn T; Damle V; Chidambaram A; Rosen K; Mahr T Increased incidence and prevalence of peanut allergy in children and adolescents in the United States. Annals of Allergy, Asthma & Immunology 2018, 121 (5), S13. [Google Scholar]
- (3).Sicherer SH; Sampson HA Food allergy. Journal of allergy and clinical immunology 2010, 125 (2), S116–S125. [DOI] [PubMed] [Google Scholar]
- (4).Husain Z; Schwartz RA Peanut allergy: an increasingly common life-threatening disorder. Journal of the American Academy of Dermatology 2012, 66 (1), 136–143. [DOI] [PubMed] [Google Scholar]
- (5).Jackson KD; Howie LD; Akinbami OJ Trends in allergic conditions among children: United States, 1997–2011; US Department of Health and Human Services, Centers for Disease Control and …, 2013. [PubMed] [Google Scholar]
- (6).Lynch K Pediatric Peanut Allergy Prevalence Review. 2018.
- (7).Blumchen K; Trendelenburg V; Ahrens F; Gruebl A; Hamelmann E; Hansen G; Heinzmann A; Nemat K; Holzhauser T; Roeder M Efficacy, safety, and quality of life in a multicenter, randomized, placebo-controlled trial of low-dose peanut oral immunotherapy in children with peanut allergy. The Journal of Allergy and Clinical Immunology: In Practice 2019, 7 (2), 479–491. [DOI] [PubMed] [Google Scholar]
- (8).Chu DK; Wood RA; French S; Fiocchi A; Jordana M; Waserman S; Brożek JL; Schünemann HJ Oral immunotherapy for peanut allergy (PACE): a systematic review and meta-analysis of efficacy and safety. The Lancet 2019, 393 (10187), 2222–2232. [DOI] [PubMed] [Google Scholar]
- (9).Narisety SD; Frischmeyer-Guerrerio PA; Keet CA; Gorelik M; Schroeder J; Hamilton RG; Wood RA A randomized, double-blind, placebo-controlled pilot study of sublingual versus oral immunotherapy for the treatment of peanut allergy. Journal of Allergy and Clinical Immunology 2015, 135 (5), 1275–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Burks AW; Calderon MA; Casale T; Cox L; Demoly P; Jutel M; Nelson H; Akdis CA Update on allergy immunotherapy: American academy of allergy, asthma & immunology/European academy of allergy and clinical immunology/PRACTALL consensus report. Journal of Allergy and Clinical Immunology 2013, 131 (5), 1288–1296. [DOI] [PubMed] [Google Scholar]
- (11).Cox LS; Linnemann DL; Nolte H; Weldon D; Finegold I; Nelson HS Sublingual immunotherapy: a comprehensive review. Journal of Allergy and Clinical Immunology 2006, 117 (5), 1021–1035. [DOI] [PubMed] [Google Scholar]
- (12).Hochfelder JL; Ponda P Allergen immunotherapy: routes, safety, efficacy, and mode of action. ImmunoTargets and therapy 2013, 2, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Burton OT; Logsdon SL; Zhou JS; Medina-Tamayo J; Abdel-Gadir A; Rivas MN; Koleoglou KJ; Chatila TA; Schneider LC; Rachid R Oral immunotherapy induces IgG antibodies that act through FcγRIIb to suppress IgE-mediated hypersensitivity. Journal of allergy and clinical immunology 2014, 134 (6), 1310–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Larché M; Akdis CA; Valenta R Immunological mechanisms of allergen-specific immunotherapy. Nature Reviews Immunology 2006, 6 (10), 761–771. [DOI] [PubMed] [Google Scholar]
- (15).Orengo JM; Radin AR; Kamat V; Badithe A; Ben LH; Bennett BL; Zhong S; Birchard D; Limnander A; Rafique A Treating cat allergy with monoclonal IgG antibodies that bind allergen and prevent IgE engagement. Nature communications 2018, 9 (1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Storni F; Cabral-Miranda G; Roesti E; Zha L; Engeroff P; Zeltins A; Cragg M; Vogel M; Bachmann MF A Single Monoclonal Antibody against the Peanut Allergen Ara h 2 Protects against Systemic and Local Peanut Allergy. International Archives of Allergy and Immunology 2020, 181 (5), 334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Finkelman FD; Rothenberg ME; Brandt EB; Morris SC; Strait RT Molecular mechanisms of anaphylaxis: lessons from studies with murine models. Journal of Allergy and Clinical Immunology 2005, 115 (3), 449–457. [DOI] [PubMed] [Google Scholar]
- (18).Reber LL; Hernandez JD; Galli SJ The pathophysiology of anaphylaxis. Journal of Allergy and Clinical Immunology 2017, 140 (2), 335–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Burton OT; Tamayo JM; Stranks AJ; Koleoglou KJ; Oettgen HC Allergen-specific IgG antibody signaling through FcγRIIb promotes food tolerance. Journal of Allergy and Clinical Immunology 2018, 141 (1), 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Strait RT; Morris SC; Finkelman FD IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and FcγRIIb cross-linking. The Journal of clinical investigation 2006, 116 (3), 833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Malbec O; Cassard L; Albanesi M; Jönsson F; Mancardi D; Chicanne G; Payrastre B; Dubreuil P; Vivier E; Daëron M Trans-inhibition of activation and proliferation signals by Fc receptors in mast cells and basophils. Science signaling 2016, 9 (459), ra126–ra126. [DOI] [PubMed] [Google Scholar]
- (22).Dorofeeva Y; Shilovskiy I; Tulaeva I; Focke‐Tejkl M; Flicker S; Kudlay D; Khaitov M; Karsonova A; Riabova K; Karaulov A Past, present, and future of allergen immunotherapy vaccines. Allergy 2021, 76 (1), 131–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Bachmann MF; Mohsen MO; Kramer MF; Heath MD Vaccination against allergy: a paradigm shift? Trends in Molecular Medicine 2020, 26 (4), 357–368. [DOI] [PubMed] [Google Scholar]
- (24).Pohlit H; Bellinghausen I; Frey H; Saloga J Recent advances in the use of nanoparticles for allergen‐specific immunotherapy. Allergy 2017, 72 (10), 1461–1474. [DOI] [PubMed] [Google Scholar]
- (25).Galvain S; André C; Vatrinet C; Villet B Safety and efficacy studies of liposomes in specific immunotherapy. Current therapeutic research 1999, 60 (5), 278–294. [Google Scholar]
- (26).Wood RA; Sicherer SH; Burks AW; Grishin A; Henning AK; Lindblad R; Stablein D; Sampson HA A phase 1 study of heat/phenol‐killed, E. coli‐encapsulated, recombinant modified peanut proteins Ara h 1, Ara h 2, and Ara h 3 (EMP‐123) for the treatment of peanut allergy. Allergy 2013, 68 (6), 803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Rudra JS; Tian YF; Jung JP; Collier JH A self-assembling peptide acting as an immune adjuvant. Proceedings of the National Academy of Sciences 2010, 107 (2), 622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Shores LS; Kelly SH; Hainline KM; Suwanpradid J; MacLeod AS; Collier JH Multifactorial Design of a Supramolecular Peptide Anti-IL-17 Vaccine Toward the Treatment of Psoriasis. Front. Immunol 11: 1855. doi: 10.3389/fimmu 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Kelly SH; Cossette BJ; Varadhan AK; Wu Y; Collier JH Titrating Polyarginine into Nanofibers Enhances Cyclic-Dinucleotide Adjuvanticity in Vitro and after Sublingual Immunization. ACS biomaterials science & engineering 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Kelly SH; Wu Y; Varadhan AK; Curvino EJ; Chong AS; Collier JH Enabling sublingual peptide immunization with molecular self-assemblies. Biomaterials 2020, 119903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Kelly SH; Votaw NL; Cossette BJ; Wu Y; Shetty S; Shores LS; Issah LA; Collier JH A sublingual nanofiber vaccine to prevent urinary tract infections. Science Advances 2022, 8 (47), eabq4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Mora-Solano C; Wen Y; Han H; Chen J; Chong AS; Miller ML; Pompano RR; Collier JH Active immunotherapy for TNF-mediated inflammation using self-assembled peptide nanofibers. Biomaterials 2017, 149, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Pompano RR; Chen J; Verbus EA; Han H; Fridman A; McNeely T; Collier JH; Chong AS Titrating T‐cell epitopes within self‐assembled vaccines optimizes CD4+ helper T cell and antibody outputs. Advanced healthcare materials 2014, 3 (11), 1898–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Chen J; Pompano RR; Santiago FW; Maillat L; Sciammas R; Sun T; Han H; Topham DJ; Chong AS; Collier JH The use of self-adjuvanting nanofiber vaccines to elicit high-affinity B cell responses to peptide antigens without inflammation. Biomaterials 2013, 34 (34), 8776–8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Ali EA; Kalli M; Wan D; Nakamura R; Onion D; Alanine DGW; Alcocer MJC; Falcone FH Characterization of human FcεRIα chain expression and gene copy number in humanized rat basophilic leukaemia (RBL) reporter cell lines. Plos one 2019, 14 (8), e0221034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Bucaite G; Kang-Pettinger T; Moreira J; Gould HJ; James LK; Sutton BJ; McDonnell JM Interplay between Affinity and Valency in Effector Cell Degranulation: A Model System with Polcalcin Allergens and Human Patient–Derived IgE Antibodies. The Journal of Immunology 2019, 203 (7), 1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Nakamura T; Murata T Regulation of vascular permeability in anaphylaxis. British journal of pharmacology 2018, 175 (13), 2538–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Burks AW; Shin D; Cockrell G; Stanley JS; Helm RM; Bannon GA Mapping and mutational analysis of the IgE‐binding epitopes on Ara h 1, a legume vicilin protein and a major allergen in peanut hypersensitivity. European Journal of Biochemistry 1997, 245 (2), 334–339. [DOI] [PubMed] [Google Scholar]
- (39).Chen G; Shrock EL; Li MZ; Spergel JM; Nadeau KC; Pongracic JA; Umetsu DT; Rachid R; MacGinnitie AJ; Phipatanakul W High-resolution epitope mapping by AllerScan reveals relationships between IgE and IgG repertoires during peanut oral immunotherapy. Cell Reports Medicine 2021, 2 (10), 100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Bublin M; Kostadinova M; Radauer C; Hafner C; Szépfalusi Z; Varga E-M; Maleki SJ; Hoffmann-Sommergruber K; Breiteneder H IgE cross-reactivity between the major peanut allergen Ara h 2 and the nonhomologous allergens Ara h 1 and Ara h 3. Journal of Allergy and Clinical Immunology 2013, 132 (1), 118–124. [DOI] [PubMed] [Google Scholar]
- (41).Deak PE; Kim B; Qayum AA; Shin J; Vitalpur G; Kloepfer KM; Turner MJ; Smith N; Shreffler WG; Kiziltepe T Designer covalent heterobivalent inhibitors prevent IgE-dependent responses to peanut allergen. Proceedings of the National Academy of Sciences 2019, 116 (18), 8966–8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Bernard H; Guillon B; Drumare M-F; Paty E; Dreskin SC; Wal J-M; Adel-Patient K; Hazebrouck S Allergenicity of peanut component Ara h 2: Contribution of conformational versus linear hydroxyproline-containing epitopes. Journal of Allergy and Clinical Immunology 2015, 135 (5), 1267–1274. [DOI] [PubMed] [Google Scholar]
- (43).Strait RT; Mahler A; Hogan S; Khodoun M; Shibuya A; Finkelman FD Ingested allergens must be absorbed systemically to induce systemic anaphylaxis. Journal of allergy and clinical immunology 2011, 127 (4), 982–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Swartz MA The physiology of the lymphatic system. Advanced drug delivery reviews 2001, 50 (1–2), 3–20. [DOI] [PubMed] [Google Scholar]
- (45).Votaw NL; Collier L; Curvino EJ; Wu Y; Fries CN; Ojeda MT; Collier JH Randomized peptide assemblies for enhancing immune responses to nanomaterials. Biomaterials 2021, 273, 120825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Chen X; Zhuang Y; Wang Q; Moutsoglou D; Ruiz G; Yen SE; Dreskin SC Analysis of the effector activity of Ara h 2 and Ara h 6 by selective depletion from a crude peanut extract. Journal of immunological methods 2011, 372 (1–2), 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Otsu K; Guo R; Dreskin SC Epitope analysis of Ara h 2 and Ara h 6: characteristic patterns of IgE‐binding fingerprints among individuals with similar clinical histories. Clinical & Experimental Allergy 2015, 45 (2), 471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available via the Duke University Libraries Digital Repository of Research Data under the same title and authors as this publication. https://research.repository.duke.edu/


