Abstract
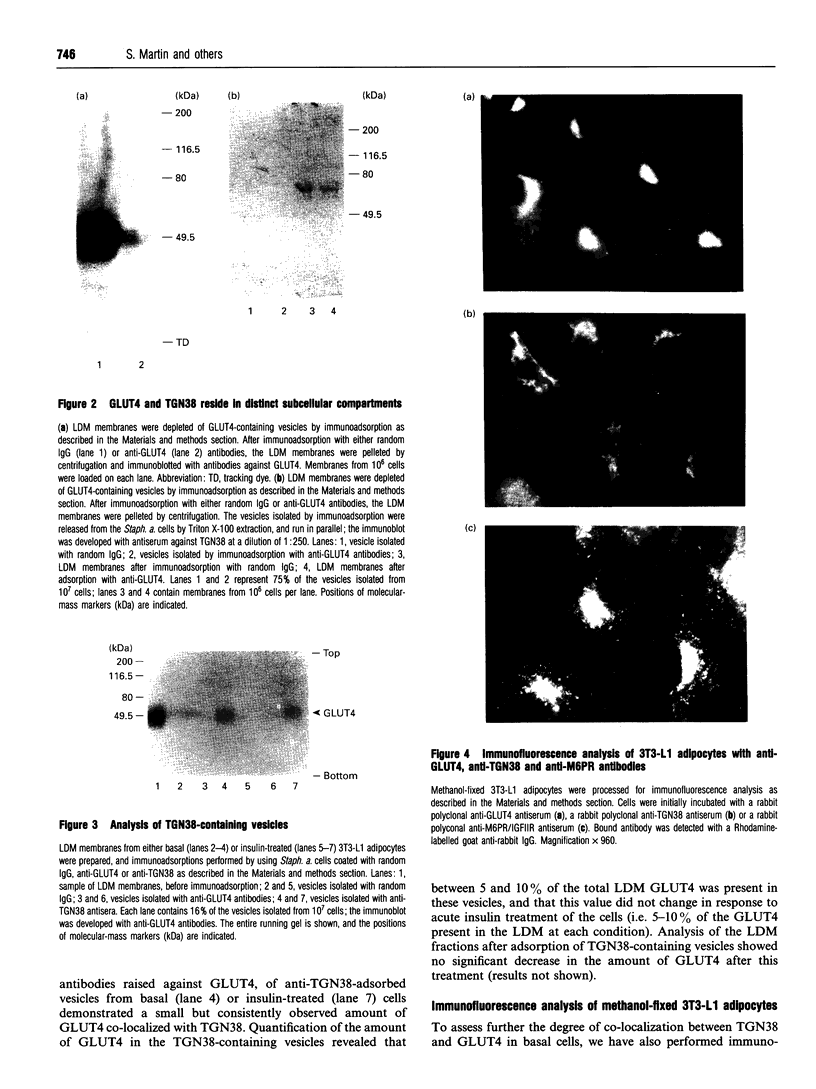
The exposure of isolated adipocytes to insulin results in an approximately 20-fold increase in the rate of glucose transport into the cell. This increase is mediated by the movement of a pool of intracellular vesicles containing the so-called insulin-responsive glucose transporter (GLUT4) to the cell surface. In the resting state, most of the GLUT4 molecules are sequestered inside the adipocyte in an as yet unidentified intracellular compartment. TGN38 is an integral membrane protein which has been shown to be predominantly localized to the trans Golgi network [Luzio, Brake, Banting, Howell, Braghetta and Stanley (1990) Biochem. J. 270, 97-102]. Here we investigate whether GLUT4 and TGN38 are co-localized in the murine 3T3-L1 adipocyte cell line. Immuno-adsorption of intracellular vesicles containing GLUT4 with an anti-peptide antibody specific for this isoform did not deplete the low-density microsomal fraction of TGN38 in these cells; moreover, no TGN38 was detected in the GLUT4-containing vesicles by immunoblotting with a TGN38-specific antiserum. Immuno-adsorption of TGN38-containing vesicles and subsequent analysis of the proteins in these vesicles revealed that a detectable amount of GLUT4 (5-10%) did co-localise with TGN38. The amount of GLUT4 in the TGN38-containing vesicles did not change in response to insulin. Immunofluorescence analysis of TGN38 and GLUT4 in these cells revealed markedly different staining patterns. Reversal of insulin-stimulated glucose transport and subsequent analysis of the TGN38-containing vesicles demonstrated that during the re-cycling of GLUT4 to the intracellular storage site there was no increase in the amount of GLUT4 co-localized with TGN38. Taken together, these results suggest that the trans Golgi network is not the major site of the intracellular GLUT4 pool within 3T3-L1 adipocytes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appell K. C., Simpson I. A., Cushman S. W. Characterization of the stimulatory action of insulin on insulin-like growth factor II binding to rat adipose cells. Differences in the mechanism of insulin action on insulin-like growth factor II receptors and glucose transporters. J Biol Chem. 1988 Aug 5;263(22):10824–10829. [PubMed] [Google Scholar]
- Bell G. I., Kayano T., Buse J. B., Burant C. F., Takeda J., Lin D., Fukumoto H., Seino S. Molecular biology of mammalian glucose transporters. Diabetes Care. 1990 Mar;13(3):198–208. doi: 10.2337/diacare.13.3.198. [DOI] [PubMed] [Google Scholar]
- Biber J. W., Lienhard G. E. Isolation of vesicles containing insulin-responsive, intracellular glucose transporters from 3T3-L1 adipocytes. J Biol Chem. 1986 Dec 5;261(34):16180–16184. [PubMed] [Google Scholar]
- Blok J., Gibbs E. M., Lienhard G. E., Slot J. W., Geuze H. J. Insulin-induced translocation of glucose transporters from post-Golgi compartments to the plasma membrane of 3T3-L1 adipocytes. J Cell Biol. 1988 Jan;106(1):69–76. doi: 10.1083/jcb.106.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brant A. M., McCoid S., Thomas H. M., Baldwin S. A., Davies A., Parker J. C., Gibbs E. M., Gould G. W. Analysis of the glucose transporter content of islet cell lines: implications for glucose-stimulated insulin release. Cell Signal. 1992 Nov;4(6):641–650. doi: 10.1016/0898-6568(92)90045-a. [DOI] [PubMed] [Google Scholar]
- Breitfeld P. P., Simmons C. F., Jr, Strous G. J., Geuze H. J., Schwartz A. L. Cell biology of the asialoglycoprotein receptor system: a model of receptor-mediated endocytosis. Int Rev Cytol. 1985;97:47–95. doi: 10.1016/s0074-7696(08)62348-7. [DOI] [PubMed] [Google Scholar]
- Brown S. J., Gould G. W., Davies A., Baldwin S. A., Lienhard G. E., Gibbs E. M. Characterization of vesicles containing insulin-responsive intracellular glucose transporters isolated from 3T3-L1 adipocytes by an improved procedure. Biochim Biophys Acta. 1988 Oct 7;971(3):339–350. doi: 10.1016/0167-4889(88)90150-4. [DOI] [PubMed] [Google Scholar]
- Cain C. C., Trimble W. S., Lienhard G. E. Members of the VAMP family of synaptic vesicle proteins are components of glucose transporter-containing vesicles from rat adipocytes. J Biol Chem. 1992 Jun 15;267(17):11681–11684. [PubMed] [Google Scholar]
- Calderhead D. M., Kitagawa K., Tanner L. I., Holman G. D., Lienhard G. E. Insulin regulation of the two glucose transporters in 3T3-L1 adipocytes. J Biol Chem. 1990 Aug 15;265(23):13801–13808. [PubMed] [Google Scholar]
- Chege N. W., Pfeffer S. R. Compartmentation of the Golgi complex: brefeldin-A distinguishes trans-Golgi cisternae from the trans-Golgi network. J Cell Biol. 1990 Sep;111(3):893–899. doi: 10.1083/jcb.111.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormont M., Tanti J. F., Grémeaux T., Van Obberghen E., Le Marchand-Brustel Y. Subcellular distribution of low molecular weight guanosine triphosphate-binding proteins in adipocytes: colocalization with the glucose transporter Glut 4. Endocrinology. 1991 Dec;129(6):3343–3350. doi: 10.1210/endo-129-6-3343. [DOI] [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- Davis R. J., Corvera S., Czech M. P. Insulin stimulates cellular iron uptake and causes the redistribution of intracellular transferrin receptors to the plasma membrane. J Biol Chem. 1986 Jul 5;261(19):8708–8711. [PubMed] [Google Scholar]
- Duncan J. R., Kornfeld S. Intracellular movement of two mannose 6-phosphate receptors: return to the Golgi apparatus. J Cell Biol. 1988 Mar;106(3):617–628. doi: 10.1083/jcb.106.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost S. C., Lane M. D. Evidence for the involvement of vicinal sulfhydryl groups in insulin-activated hexose transport by 3T3-L1 adipocytes. J Biol Chem. 1985 Mar 10;260(5):2646–2652. [PubMed] [Google Scholar]
- Gibbs E. M., Allard W. J., Lienhard G. E. The glucose transporter in 3T3-L1 adipocytes is phosphorylated in response to phorbol ester but not in response to insulin. J Biol Chem. 1986 Dec 15;261(35):16597–16603. [PubMed] [Google Scholar]
- Gibbs E. M., Lienhard G. E., Gould G. W. Insulin-induced translocation of glucose transporters to the plasma membrane precedes full stimulation of hexose transport. Biochemistry. 1988 Sep 6;27(18):6681–6685. doi: 10.1021/bi00418a006. [DOI] [PubMed] [Google Scholar]
- Gould G. W., Bell G. I. Facilitative glucose transporters: an expanding family. Trends Biochem Sci. 1990 Jan;15(1):18–23. doi: 10.1016/0968-0004(90)90125-u. [DOI] [PubMed] [Google Scholar]
- Gould G. W., Brant A. M., Kahn B. B., Shepherd P. R., McCoid S. C., Gibbs E. M. Expression of the brain-type glucose transporter is restricted to brain and neuronal cells in mice. Diabetologia. 1992 Apr;35(4):304–309. doi: 10.1007/BF00401196. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Simons K. The trans Golgi network: sorting at the exit site of the Golgi complex. Science. 1986 Oct 24;234(4775):438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- Holman G. D., Kozka I. J., Clark A. E., Flower C. J., Saltis J., Habberfield A. D., Simpson I. A., Cushman S. W. Cell surface labeling of glucose transporter isoform GLUT4 by bis-mannose photolabel. Correlation with stimulation of glucose transport in rat adipose cells by insulin and phorbol ester. J Biol Chem. 1990 Oct 25;265(30):18172–18179. [PubMed] [Google Scholar]
- James D. E., Strube M., Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989 Mar 2;338(6210):83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- Jhun B. H., Rampal A. L., Liu H., Lachaal M., Jung C. Y. Effects of insulin on steady state kinetics of GLUT4 subcellular distribution in rat adipocytes. Evidence of constitutive GLUT4 recycling. J Biol Chem. 1992 Sep 5;267(25):17710–17715. [PubMed] [Google Scholar]
- Ladinsky M. S., Howell K. E. The trans-Golgi network can be dissected structurally and functionally from the cisternae of the Golgi complex by brefeldin A. Eur J Cell Biol. 1992 Oct;59(1):92–105. [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L., Tipper C., Amherdt M., Orci L., Klausner R. D. Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 1991 Nov 1;67(3):601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- Luzio J. P., Banting G. Eukaryotic membrane traffic: retrieval and retention mechanisms to achieve organelle residence. Trends Biochem Sci. 1993 Oct;18(10):395–398. doi: 10.1016/0968-0004(93)90097-7. [DOI] [PubMed] [Google Scholar]
- Luzio J. P., Brake B., Banting G., Howell K. E., Braghetta P., Stanley K. K. Identification, sequencing and expression of an integral membrane protein of the trans-Golgi network (TGN38). Biochem J. 1990 Aug 15;270(1):97–102. doi: 10.1042/bj2700097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. O., Edman J. C., Standring D. N., Fried V. A., Smith M. C., Roth R. A., Rutter W. J. Insulin-like growth factor II receptor as a multifunctional binding protein. Nature. 1987 Sep 24;329(6137):301–307. doi: 10.1038/329301a0. [DOI] [PubMed] [Google Scholar]
- Méresse S., Hoflack B. Phosphorylation of the cation-independent mannose 6-phosphate receptor is closely associated with its exit from the trans-Golgi network. J Cell Biol. 1993 Jan;120(1):67–75. doi: 10.1083/jcb.120.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka Y., Mottola C., Oppenheimer C. L., Czech M. P. Insulin activates the appearance of insulin-like growth factor II receptors on the adipocyte cell surface. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4028–4032. doi: 10.1073/pnas.81.13.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer C. L., Pessin J. E., Massague J., Gitomer W., Czech M. P. Insulin action rapidly modulates the apparent affinity of the insulin-like growth factor II receptor. J Biol Chem. 1983 Apr 25;258(8):4824–4830. [PubMed] [Google Scholar]
- Piper R. C., Hess L. J., James D. E. Differential sorting of two glucose transporters expressed in insulin-sensitive cells. Am J Physiol. 1991 Mar;260(3 Pt 1):C570–C580. doi: 10.1152/ajpcell.1991.260.3.C570. [DOI] [PubMed] [Google Scholar]
- Piper R. C., Tai C., Slot J. W., Hahn C. S., Rice C. M., Huang H., James D. E. The efficient intracellular sequestration of the insulin-regulatable glucose transporter (GLUT-4) is conferred by the NH2 terminus. J Cell Biol. 1992 May;117(4):729–743. doi: 10.1083/jcb.117.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaves B., Banting G. Perturbation of the morphology of the trans-Golgi network following Brefeldin A treatment: redistribution of a TGN-specific integral membrane protein, TGN38. J Cell Biol. 1992 Jan;116(1):85–94. doi: 10.1083/jcb.116.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaves B., Horn M., Banting G. TGN38/41 recycles between the cell surface and the TGN: brefeldin A affects its rate of return to the TGN. Mol Biol Cell. 1993 Jan;4(1):93–105. doi: 10.1091/mbc.4.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J., Gigengack S., James D. E., Lienhard G. E. Translocation of the glucose transporter GLUT4 in cardiac myocytes of the rat. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7815–7819. doi: 10.1073/pnas.88.17.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J., Gigengack S., Lienhard G. E., James D. E. Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol. 1991 Apr;113(1):123–135. doi: 10.1083/jcb.113.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. M., Charron M. J., Shah N., Lodish H. F., Jarett L. Immunoelectron microscopic demonstration of insulin-stimulated translocation of glucose transporters to the plasma membrane of isolated rat adipocytes and masking of the carboxyl-terminal epitope of intracellular GLUT4. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6893–6897. doi: 10.1073/pnas.88.15.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel W., Geuze H. J., Griffith J. M., Schwartz A. L., Strous G. J. Relations between the intracellular pathways of the receptors for transferrin, asialoglycoprotein, and mannose 6-phosphate in human hepatoma cells. J Cell Biol. 1989 Jun;108(6):2137–2148. doi: 10.1083/jcb.108.6.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci U S A. 1980 May;77(5):2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner L. I., Lienhard G. E. Insulin elicits a redistribution of transferrin receptors in 3T3-L1 adipocytes through an increase in the rate constant for receptor externalization. J Biol Chem. 1987 Jul 5;262(19):8975–8980. [PubMed] [Google Scholar]
- Wardzala L. J., Simpson I. A., Rechler M. M., Cushman S. W. Potential mechanism of the stimulatory action of insulin on insulin-like growth factor II binding to the isolated rat adipose cell. Apparent redistribution of receptors cycling between a large intracellular pool and the plasma membrane. J Biol Chem. 1984 Jul 10;259(13):8378–8383. [PubMed] [Google Scholar]
- Wilde A., Reaves B., Banting G. Epitope mapping of two isoforms of a trans Golgi network specific integral membrane protein TGN38/41. FEBS Lett. 1992 Nov 30;313(3):235–238. doi: 10.1016/0014-5793(92)81199-v. [DOI] [PubMed] [Google Scholar]
- Yang J., Clark A. E., Harrison R., Kozka I. J., Holman G. D. Trafficking of glucose transporters in 3T3-L1 cells. Inhibition of trafficking by phenylarsine oxide implicates a slow dissociation of transporters from trafficking proteins. Biochem J. 1992 Feb 1;281(Pt 3):809–817. doi: 10.1042/bj2810809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Holman G. D. Comparison of GLUT4 and GLUT1 subcellular trafficking in basal and insulin-stimulated 3T3-L1 cells. J Biol Chem. 1993 Mar 5;268(7):4600–4603. [PubMed] [Google Scholar]
- Zorzano A., Wilkinson W., Kotliar N., Thoidis G., Wadzinkski B. E., Ruoho A. E., Pilch P. F. Insulin-regulated glucose uptake in rat adipocytes is mediated by two transporter isoforms present in at least two vesicle populations. J Biol Chem. 1989 Jul 25;264(21):12358–12363. [PubMed] [Google Scholar]








