Abstract
Background
Even with significant advancements, treating multiple myeloma (MM) remains difficult. At present, the main treatment methods include combined treatment of stem cell transplantation, drug treatment, etc. With the clarification of the molecular biological mechanism of MM, as well as the in-depth study of the internal signal of myeloma cells and the microenvironment of MM patients, more and more new drugs targeting myeloma and microenvironment are gradually used in clinical maintenance treatment, such as inhibit the proteosome: ixazomib, bortezomib and carfilzomib, immune - modulators: thalidomide and lenalidomide, monoclonal antibodies, etc. have made great progress in MM maintenance treatment. With the continuous development of proteasome inhibitor maintenance treatment in MM, the prognosis of the disease has been significantly improved. Our aim is to evaluate the effectiveness and adverse reactions of proteasome inhibitors in maintenance therapy for multiple myeloma, providing new ideas for clinical medication.
Methods
Four databases containing randomized controlled studies on the effectiveness and safety of proteasome inhibitors in the maintenance therapy of multiple myeloma are retrieved by the computer. Once the quality of the literature has been thoroughly evaluated, run the data via the RevMan 5.3 software.
Results
Eventually 8 studies were added in this systematic review. Compared with the placebo group, proteasome inhibitor in maintenance treatment of multiple myeloma patients with prolonged the survival without progression and overall existence. 5 studies reported the peripheral neuropathy of multiple myeloma in the treatment group compared to placebo group, which was remarkably greater (OR: 1.98; 95 % Cl: 1.35, 2.92; P < 0.001) compared to placebo group, Serious adverse events (OR: 1.60; 95 % Cl: 1.19, 2.14; P < 0.01), Rash (OR: 2.23; 95 % Cl: 1.62, 3.05; P < 0.001) and Vomiting (OR: 5.12; 95 % Cl: 3.36, 7.80; P < 0.001). The Serious adverse events of the treatment group were remarkably greater compared with the untreated group (OR: 1.60; 95 % Cl: 1.19, 2.14; P < 0.01).
Conclusion
The study results proposed that proteasome inhibitors are effective in the multiple myeloma maintenance treatment compared with the placebo group. Bortezomib has certain advantages in prolonging PFS, followed by ixazomib and carfilzomib in terms of efficacy. Bortezombib may be superior to carfilzombib in extending OS. However, the adverse reactions caused by proteasome inhibitors, such as Peripheral neuropathy, Serious adverse events, Rash, Vomiting, etc., should be paid enough attention.
Keywords: Proteasome inhibitors, Multiple myeloma, Maintenance treatment, Safety, Meta-analysis
1. Introduction
Multiple myeloma (MM) is one of the three common types of blood cancer. It is a Plasma cell malignant tumor mainly existing in the bone marrow. Excessive production of protein is its main feature [1]. MM is a neoplasm of clonal plasma cells that originate from the post-germinal lymphoid B-cell lineage and develop after lineage commitment in the bone marrow of progenitor cells. The main clinical symptoms of MM include osteolytic bone injury, kidney injury, anemia, hypercalcemia, and recurrent infections [2]. The median age of MM is 69 years old. About 63 % of patients identified with MM are elder than 65 years old [3]. The rate of prevalence is high in men than women, an incidence rate is increasing year by year [4]. Globally about 588161 people are detected with Multiple myeloma every year. According to data from 2014 to 2018, there are 7.1 new cases of MM per 100000 males and females per year. According to data from 2015 to 2019, the age adjusted mortality rate for MM is 3.2 cases per 100000 people per year. In 2021, newly identified MM accounted for 1.8 % of all newly diagnosed cancers, and the mortality rate accounted for 2 % of all cancers. According to data from 2011 to 2017. For MM, the five-year survival rate is 55.6 % [5].
The etiology and pathogenesis of MM are currently unclear. Epidemiological investigations have shown that high-risk factors for MM include males, firefighters, obesity, exposure to dioxins/orange agents, etc. [6,7]. MM may also be related to ionizing radiation, genetic, industrial or agricultural exposure to toxins, environmental factors, viral infections, recurrent chronic infections, or antigen stimulation [8]. In the United States, the incidence rate of blacks (14 per 100000 people) is higher than that of whites (6.1 per 100000 people). Numerous studies have shown that chromosomal abnormalities or highly unstable chromosomal structures are common in most MM patients, with chromosomal abnormalities including hyperdiploid and non hyperdiploid occurring in over 90 % of MM patients, suggesting that chromosomal abnormalities may be an important pathogenesis of MM; Moreover, chromosomal abnormalities can also lead to abnormal expression of cancer promoting or tumor suppressor genes, leading to poor prognosis in MM patients [9,10].
It is a very difficult problem to treat MM clinically. At present, the main treatment methods include combined treatment of stem cell transplantation, drug treatment, etc. With the clarification of the molecular biological mechanism of MM, as well as the in-depth study of the internal signal of myeloma cells and the microenvironment of MM patients, more and more new drugs targeting myeloma and microenvironment are gradually used in clinical maintenance treatment, such as inhibit the proteosome: carfilzomib, bortezomib and ixazomib; immunomodulators: lenalidomide and thalidomide, monoclonal antibodies, etc. have made great progress in MM maintenance treatment.
The treatment of MM with stem cell transplantation is gradually moving towards clinical practice, which can be divided into two categories: autologous stem cell transplantation (ASCT) and allogeneic stem cell transplantation (Allo SCT). Although ASCT cannot cure MM, compared to standard chemotherapy, transplantation with high-dose myeloablative therapy in ASCT can enhance the complete response rate and prolong the median survival period of nearly 1 year, with a mortality rate of 1 %–2%, making it the main treatment option for MM [11]. Patients who are suitable for ASCT treatment need to thoroughly evaluate factors such as age, toxicity, and side effects. Studies have shown that after receiving ASCT treatment, 3 %–10 % of patients have complete remission and maintain it for more than 10 years. However, conducting two ASCTs has better clinical efficacy compared to one ASCT, but there is no remarkable variation in the total survival period of MM patients [12]. Allo SCT is considered the only method to cure MM, but it is limited by factors such as receptors, donors, adverse reactions, high mortality, and end-organ damage, which affect the use of allogeneic stem cells in clinical practice [13]. With in-depth research on its pathogenesis and the development of new drugs, the degree of remission and survival of patients have significantly improved. The application of proteasome inhibitors is the significant milestone in the multiple myeloma treatment. The first appearance of bortezomib has significant clinical benefits. As the first oral proteasome inhibitor, Ixazomib provides a more convenient treatment method for clinical use. The new generation of Carfilzomab can achieve a higher benefit risk ratio due to improved molecular structure and optimized mechanism of action. Therefore, it is urgent to systematically evaluate the effectiveness and adverse reactions of proteasome inhibitors in maintenance therapy for multiple myeloma. At present, there are also some meta-analyses on the treatment of MM with proteasome inhibition, but our study systematically reviews and compares the efficacy and survival cycle of various proteasome inhibitors.
2. Materials and methods
2.1. Types of studies
Study Design Type RCTs that have been published on the efficacy and adverse reactions of Maintenance treatment of proteasome inhibitors in multiple myeloma patients. However, the pre-clinical trials were exempted.
2.2. Types of participants
Patients with multiple myeloma were excluded if they had primary refractory multiple myeloma according to International Myeloma Working Group (IMWG) response criteria, serum-free light chain measurable disease only, or Eastern Cooperative Oncology Group performance status greater than 2. Patients were excluded if they received anti-myeloma treatment within 14 days of randomization, previous treatment with carfilzomib, were refractory to anti-CD38 antibody therapy, or had a contraindication to dexamethasone. Patients with estimated glomerular filtration rate (eGFR) of less than 15 mL/min per 1·73 m2 according to the modification of diet in renal disease formula or left ventricular ejection fraction less than 40 % were excluded. Patients with previous pulmonary comorbidities, including chronic obstructive pulmonary disease, could be enrolled.
All patients had adequate hepatic, hematologic, and renal function (creatinine clearance, ≥50 mL per minute) at screening. Patients were excluded if they had grade 3 or 4 peripheral neuropathy (or grade 2 with pain) within 14 days before randomization or New York Heart Association class III or IV heart failure.
2.3. Types of interventions
The treatment group received proteasome inhibitors in the maintenance treatment of MM patients, and the untreated group received placeboin the maintenance treatment of MM patients.
2.4. Types of outcomes
Effect Measures of multiple myeloma patients; Based on research, the tools for assessing the effectiveness and adverse reactions of proteasome inhibitors in multiple myeloma patients are: ① Survival without progression (SWP); ② Overall survival (OS); ③ Peripheral neuropathy; ④ Serious adverse events; ⑤ Rash; ⑥ Vomiting. At least one of the aforementioned scales was used in the literature reviewed for this study to assess outcome measures.
2.5. Data sources and search strategy
We conducted electronic searches on Cochrane Library, Embase, PubMed, and Web of Science databases. The search term is “proteasome inhibitor”, “multiple myeloma”, “maintenance” and “randomized”. The time of search was from the library establishment until February 2023. Reference lists of eligible articles and citing articles were also screened to capture all relevant studies (via Google Scholar search engine). The procedures involved in doing a literature search are as follows: (1) look for pertinent publications in databases (English); (2) read the abstract, title and keywords to find out additional terms for search related to this topic; (2) Using a combination of subject words and keywords, the English database search employed “MeSH Terms” to determine the subject terms. All identified studies were combined in a single reference manager file (EndNote) and uploaded in an online software (Covidence).
2.6. Data retrieval and assessment of quality
Two researchers independently completed the process of screening the abstract first, then reviewing the full text to determine the findings of the literature screening. Until the outcomes are agreed upon, discuss opposing literature, consult a third researcher, or exchange screening results. Basic literature information, study type and object, size of sample, intervention content, effect measures and other details are among the information that was taken from the data.
2.7. Assessment of risk of bias
The risk of bias assessment of included studies was performed independently by four authors (S.J.H, L.J.W, K.A.T and M.A.S) according to the criteria and tools defined in the Cochrane Handbook for systematic review of interventions. Assessable domains included: selection bias, performance bias, detection bias, attrition and reporting bias. A risk category (low, high, unclear) was assigned to each.
2.8. Analysis of statistics
RevMan was employed to conduct this systematic review. Combining effects: All of the effect measures of the study were measured data and different assessment techniques were employed. Because there are variations in the scores, the standardized mean difference (SMD) and the 95 % confidence interval (CI) are utilized as effect indicators. Heterogeneity test: If P > 0.1 and I2<50 %, the inclusion studies were regarded to be more homogeneous. Chi-square tests are performed to assess whether there is heterogeneity among studies. Carry out a fixed-effects model systematic review; if P<0.1, I2> = 50 %, and the included studies indicated heterogeneity, Examine a variety of sources, Should clinical heterogeneity be absent, systematic review are performed with a random-effects model. Additionally, a subgroup analysis was carried out to examine potential variations in the qualitative characteristics.
3. Results
3.1. Outcomes of the search
In accordance with the search plan, 1095 references were found. Following the removal of redundant investigation, the abstract and title of 20 papers were investigated. Overall texts of 13 articles were further assessed. Five records were exempted following full text examination for the following reasons: lack of data (n = 3) and duplicate literature (n = 2). In the end, this systematic review contained 8 studies [[14], [15], [16], [17], [18], [19], [20], [21]] (Table 1). This procedure is given in the PRISMA statement flow chart (Fig. 1).
Table 1.
The basic characteristics of the included studies: ① Progression-free survival (PFS); ② Overall survival (OS); ③ Peripheral neuropathy; ④ Serious adverse events; ⑤ Rash; ⑥ Vomiting. PBO: Placebo.
| Reference | Total cases | Proteasome inhibitors/control | Man/Woman | Median age (years) | Period | Clinical setting | Main Outcomes |
|---|---|---|---|---|---|---|---|
| Goldschmidt, 2017 [14] | 827 | 413/414 | None | None | Jul. 2005 to Jul. 2008 | Bortezomib vs PBO | ①② |
| Dimopoulos, 2018 | 656 | 395/261 | 414/242 | 58 (52–63)/60 (54–64) | Jul. 2014 to Mar. 2016 | Ixazomib vs PBO | ①③④⑤⑥ |
| Dimopoulos, 2020 [16] | 706 | 425/281 | 377/329 | 72 (42–89)/73 (52–90) | Apr. 2015 to Oct. 2018 | Ixazomib vs PBO | ①③④⑤⑥ |
| Gregersen, 2021 [17] | 168 | 82/86 | 96/72 | 60 (53–64)/62 (58–67) | Jan. 2015 to Apr. 2018 | Carfilzomib vs PBO | ④ |
| Yong, 2021 [18] | 141 | 69/72 | 85/56 | 65 (35–80)/69 (48–83) | Feb. 2013 to Sept. 2016 | Carfilzomib vs PBO | ①②④ |
| Rosiñol, 2012 [19] | 257 | 130/127 | 140/117 | 56/56 | Aug. 2009 to Aug. 2011 | Bortezomib vs PBO | ①②③ |
| Rosiñol, 2017 [20] | 179 | 91/88 | 94/85 | 56/59 | Apr. 2006 to Aug. 2009 | Bortezomib vs PBO | ①③ |
| Sonneveld, 2012 [21] | 827 | 413/414 | 500/327 | 57 (31–65)/57 (25–65) | May 2005 to May 2008 | Bortezomib vs PBO | ①②③ |
Fig. 1.
Flow chart.
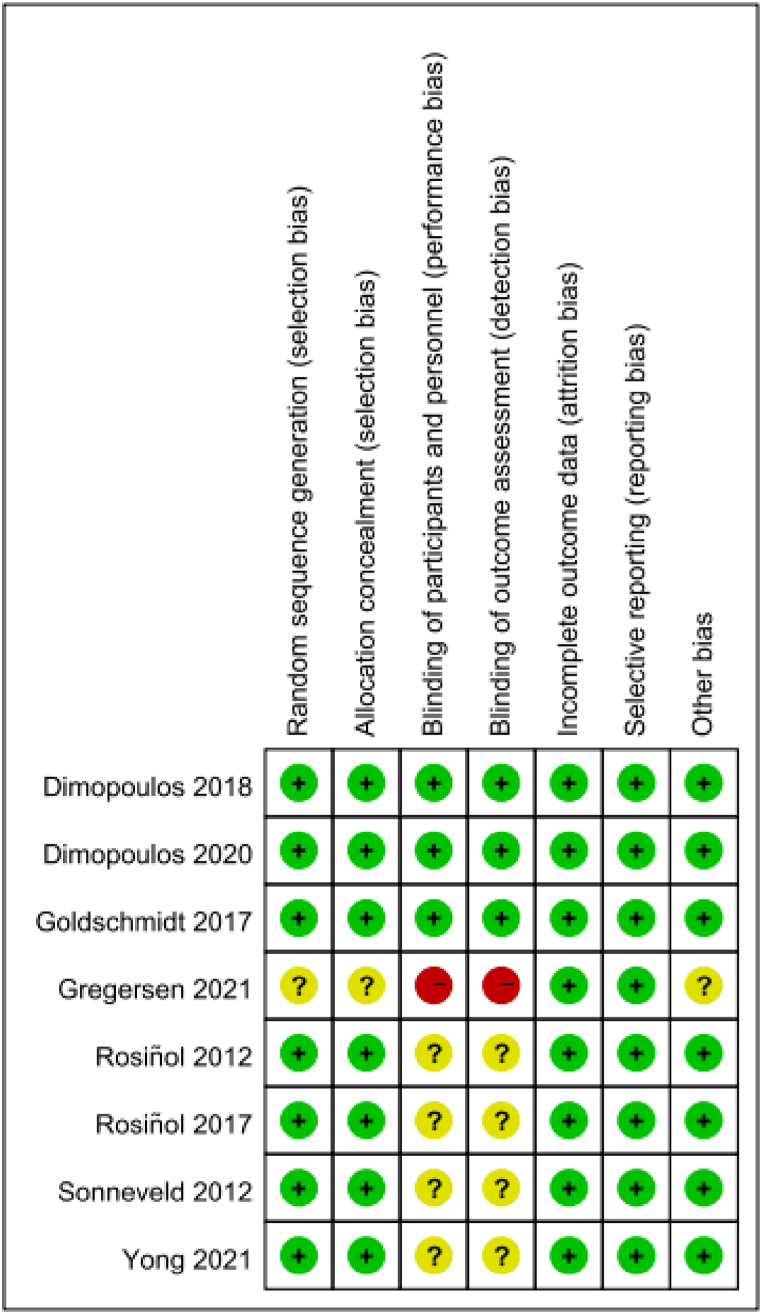
3.2. Bias assessment risk
All included studies were assessed for risk of bias according to the Cochrane collaboration's assessment tool. The literature quality added in this study is relatively high (Fig. 2, Fig. 3). All 8 articles are all randomized controlled trials. The risk of bias in most assessment areas is low. However, one of the studies [17] did not set up a blind method, including Blinding of participants and personnel and Blinding of outcome assessment. Four of the studies [[18], [19], [20], [21]] did not provide a detailed description of the blinding method.
Fig. 2.
Risk of bias summary.
Fig. 3.
Risk of bias graph.
3.3. Progression-free survival (PFS)
7 studies resulted that PFS of the test group and the untreated group and 1 study reported the Time To Progress (TTP) of the test group and the untreated group. Goldschmidt's [14] research suggests that the median PFS was 34 months (95 % CI: 30–38 months) in the test group and 28 months (95 % CI: 25–32 months) in the control group. Dimopoulos's [15,16] research suggests that median PFS of 26.5 months (95 % CI 23.7–33.8) in the ixazomib group versus 21.3 months (18.0–24.7) in the placebo group and median PFS since randomization was 17.4 months (95 % CI, 14.8–20.3 months) versus 9.4 months (95 % CI, 8.5–11.5 months). Gregersen's [17] research indicates that the carfilzomib-dexamethasone maintenance group had a median time to progression (TTP) of 25.1 months (22.5-NR) following randomization, while the control group had a median TTP of 16.7 months (14.4–21.8). According to Yong's research [18], PFS (median 11.9 months) for patients receiving carfilzomib maintenance was substantially longer than that of individuals not receiving maintenance medication (median 5.6 months). According to Rosiñol's [19,20] research, the survival without Progression (SWP) was remarkably longer in the treatment group compared to the untreated group (50.6 vs. 28.2 months, P = 0.03) after a median follow-up of 58.6 months. The median SWP for the entire series was 33.1 months, and it was remarkably greater in the treatment group compared to the untreated group (56.2 vs. 28.2 months, P < 0.01). Sonneveld's [21] research suggests that SWP was better in the test group at a median follow-up of 41 months (median of 28 months versus 35 months) as proteasome inhibitors are employed in the maintenance therapy of individuals with multiple myeloma, the rate of progression-free survival is higher as compared to the placebo group. Based on the aforementioned study findings, ixazomib offers some benefits in extending PFS, with bortezomib and carfilzomib following in terms of effectiveness (Fig. 4).
Fig. 4.
Subgroup analysis of the progression-free survival.
3.4. Overall survival (OS)
4 studies resulted that the OS of the treatment group and the untreated group. Goldschmidt's [14] research suggests that at three and five years, the OS probabilities in the test group were 72 % (95 % CI: 67–76 %) vs. 79 % (95 % CI: 74–82 %) and 59 % (95 % CI: 54–64 %) vs. 65 % (95 % CI: 60–70 %), respectively. According to Yong's research [18], the maintenance and observation groups had median OSs of 25.7 months (95 % CI: 20.8, upper limit not calculated) and 24.1 months (95 % CI: 21.5, upper limit not estimated) respectively from the time of maintenance randomization. According to Rosiñol's research [19], the test group's projected overall survival at 4 years after randomization was 74 %, whereas the control group's was 65 %. According to Sonneveld's research [21], the test group's overall survival was better in multivariate analysis (HR, 0.77; 95 % CI, 0.60 to 1.00; P = 0.049). Proteasome inhibitors extended overall survival in multiple myeloma maintenance treatment compared to placebo. Bortezombib may be superior to carfilzombib in extending OS (Fig. 5).
Fig. 5.
Subgroup analysis of the overall survival.
3.5. Peripheral neuropathy
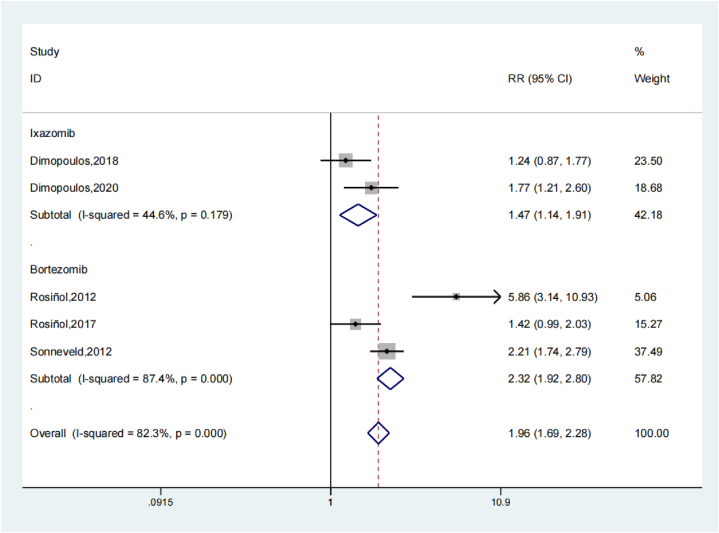
5 studies resulted that the Peripheral neuropathy of the treatment group and the untreated group. Systematic review revealed that the Peripheral neuropathy of the treatment group was remarkably greater compared to the untreated group (OR: 1.98; 95 % Cl: 1.35, 2.92; P < 0.001, Fig. 6). The trials results showed increased heterogeneity and sensitivity analysis was performed (Fig. 7). In contrast to the untreated group, proteasome inhibitors in multiple myeloma treatment increase the occurrence of peripheral neuropathy. The Begg's Test is 0.462 and the Egger's test is 0.659, so the results of this research are associated constantly and no overt publishing bias is present. The subgroup analysis results of peripheral neuropathy showed that the study results were relatively stable. The OR value of bortezomib related studies was 2.32 (95 % Cl: 1.92, 2.80), significantly higher than the ixazomib OR value of 1.47 (95 % Cl: 1.14, 1.91), indicating that maintenance therapy with bortezomib may lead to more peripheral neuropathy (Fig. 8).
Fig. 6.
Forest illustration of the peripheral neuropathy.
Fig. 7.
Sensitivity analysis of the peripheral neuropathy.
Fig. 8.
Subgroup analysis of peripheral neuropathy.
3.6. Serious adverse events
Four studies reported the serious adverse events of the treatment group and the untreated group. Systematic review showed that the Serious adverse events of the treatment group was remarkably greater compared to the untreated group (OR: 1.60; 95 % Cl: 1.19, 2.14; P < 0.01, Fig. 9). The trials results showed moderate heterogeneity, and a sensitivity analysis was carried out (Fig. 10). In contrast to untreated group, proteasome inhibitor in the treatment of patients with multiple myeloma increases the occurrence of serious adverse events. The Begg's Test is 1.000 and the Egger's test is 0.431, so the results of the research are relatively constant and No overt publishing bias is present. The subgroup analysis results of serious adverse events showed that the results of this study were relatively constant. The OR value of Ixazomib related studies was 1.50 (95 % Cl: 1.25, 1.82), significantly lower than the Carfilzomib OR value of 1.90 (95 % Cl: 1.25, 2.88), indicating that maintenance therapy with Ixazomib may lead to less serious adverse events (Fig. 11). Lenalidomide is more effective than thalidomide, but causes more hematological adverse effects. Symptoms caused by peripheralneuropathy may occur as an adverse effect of treatment with bortezomib and thalidomide [22].
Fig. 9.
Forest illustration of the serious adverse events.
Fig. 10.
Sensitivity analysis of the serious adverse events.
Fig. 11.
Subgroup analysis of serious adverse events.
3.7. Rash
2 studies resulted that the Rash of the treatment group and the untreated group. Systematic review revealed that the Rash of the treatment group was remarkably greater compared to the untreated group (OR: 2.23; 95 % Cl: 1.62, 3.05; P < 0.001, Fig. 12). In contrast to the placebo group, proteasome inhibitor in treatment multiple myeloma treatment increase the occurrence of Rash.
Fig. 12.
Forest illustration of the rash.
3.8. Vomiting
2 studies resulted that the Vomiting of the treatment group and the untreated group. Systematic review revealed that the Vomiting of the treatment group was remarkably greater compared to untreated group (OR: 5.12; 95 % Cl: 3.36, 7.80; P < 0.001, Fig. 13). In contrast to the untreated group, proteasome inhibitor in the multiple myeloma treatment increases the occurrence of Vomiting.
Fig. 13.
Forest illustration of the vomiting.
4. Discussion
In the past decade we have seen four new agents approved by the US Food and Drug Administration for treatment of multiple myeloma: the proteasome inhibitor (PI) bortezomib (Velcade), the immunomodulatory agents: lenalidomide (Revlimid) and thalidomide (Thalomid), and liposomal doxorubicin. These are commonly used in the treatment of relapsed/refractory (R/R) multiple myeloma (MM), but there is no universally accepted standard treatment. Salvage therapy must be tailored according to an individual patient's clinical profile, with the risks and potential effects of treatment-related adverse events being major determinants of the choice of therapy [23].
Bortezomib, a reversible Proteasome inhibitor, with US FDA approval in 2003 for the treatment of patients with advanced MM. Bortezomib reversibly sexual inhibition the chymotrypsin/trypsin activity of the Proteasome 26S subunit in mammalian cells by selectively binding to Threonine at the Proteasome Active site. Bortezomib has been highly used in the MM clinical treatment, and has been proved to be effective in alleviating the pathogenesis of MM. Bortezomib and NF-κB have been proved in tumor cells signaling pathway is closely related. In MM, Bortezomib can significantly reduce the degradation of nuclear factor inhibitor and inhibit the expression level of genes associated with proliferation of cell and apoptosis after specifically Sexual inhibition the activity of Proteasome, thereby reducing the myeloma cell growth factors secretion such as IL-6 and the expression of adhesion factors, and ultimately leading to apoptosis of MM cell [24].
Carfilzomib is an epoxyketone proteasome inhibitor. Carfilzomib binds selectively and irreversibly to its target and leads to antiproliferative and proapoptotic effects on cancer cells. This irreversible inhibition is dose- and time-dependent in vitro and in vivo. In phase 1 studies, a maximum tolerated dose was not established for carfilzomib monotherapy. However, on the basis of the overall observed side-effect profile, an initial dose of 20 mg per square meter of body-surface area with subsequent escalation to 27 mg per square meter was selected for further study [7,8]. This regimen of carfilzomib monotherapy was subsequently approved in the United States for use in patients with relapsed and refractory multiple myeloma on the basis of a phase 2 study that showed a 23.7 % overall response rate in this population. The US FDA approved MM treatment in 2012 for those who have received at least two types of bortezomib and IMID treatments but are ineffective [25]. Different from bortezomib, carfilzomib binds irreversibly to proteasome and binds irreversibly to the core subunit 20S of proteasomeβ5-subunit binding preferentially inhibits chymotrypsin like activity rather than caspase like or trypsin like activity, while high concentrations of carfilzomib can also inhibit chymotrypsin like activity by inhibiting β1, β2, β5 three catalytic subunits inhibit peptide glutamyl peptide hydrolysis and tryptase like activity, making proteasome inactivated [26]. Carfilzomib is generally considered well-tolerated, with a manageable toxicity profile for most patients (Table 2).
Table 2.
Management of adverse events (AEs) in MM patients receiving cafizomib.
| Toxicity | Recommended action |
|---|---|
| Hematological toxicity Neutropenia (grade 3/4) Thrombocytopenia (grade 4) |
|
Cardiac toxicity
|
|
| Pulmonary hypertension or Peripheral neuropathy (grad 3/4) |
|
| Renal toxicity Serum creatinine ≥2× baseline |
|
Ixazomib, also known as MLN-9708, is the first oral Proteasome inhibition approved by the US FDA for the treatment of MM in 2015. Its mechanism of action is identical to that of carfilzomib. Its mechanism of action is to inhibit chymotrypsin like activity to induce apoptosis of MM cells, block the connection between MM cells and BM microhabitat. In addition, new generation proteasome inhibitors such as marizomib, CEP-18770, ONX-0912 are undergoing clinical trials. It is believed that with the passage of time, the research and clinical application of proteasome inhibitors will reach a higher level.
Before 2018, the only medication authorized for use in post-transplant maintenance is lenalidomide. However, lenalidomide was not approved for use as post-ASCT maintenance treatment at the time of study design in early 2014. There was no standard of care in this setting, with the majority of patients’ worldwide not receiving maintenance treatment during the enrollment period from July 2014 to March 2016. Lenalidomide maintenance was found to have a significant overall survival benefit when compared to placebo or no maintenance, according to a 2017 meta-analysis of the CALGB 100104, GIMEMA RV-MM-PI-209, and IFM 2005-02 trials. The rates of discontinuation due to treatment-emergent adverse events were 29 % and 12 %, respectively. In February 2017, lenalidomide maintenance was authorized for use in post-transplant settings in the USA and Europe. While the approval of lenalidomide in this context represents a significant advancement in patient care, lenalidomide is linked to the emergence of recurrent primary cancers, and its efficacy varies among patients with high-risk characteristics, including but not limited to certain cytogenetic abnormalities and renal failure [15].
In the ENDEAVOR trial, the combination of carfilzomib and dexamethasone showed better OS and progression-free survival than the combination of bortezomib and dexamethasone. Next-generation proteasome inhibitors, such as carfilzomib, are frequently used to treat multiple myeloma that has relapsed or is resistant to treatment. Moreover, carfilzomib has shown clinical benefit in individuals who have previously been exposed to bortezomib as well as those who are resistant to it. It's crucial to understand that the increased risk of peripheral neuropathy associated with bortezomib medication is not present with carfilzomib. It does, however, come with an increased risk of pulmonary and cardiac adverse effects, which usually take the form of ischemic heart disease, hypertension, dyspnea, or heart failure. It is not well established whether carfilzomib-containing induction therapy should be used before salvage ASCT or as maintenance therapy after salvage ASCT [27,28].
Overall 8 literatures were added in this study, consisting of 2018 patients in the treatment group and 1743 patients in the untreated group. Compared with the placebo group, proteasome inhibitor in the treatment of multiple myeloma patients increases the level of survival without progression and Overall survival. Systematic review showed that multiple myeloma patients who received proteasome inhibitor had increased occurrence of Peripheral neuropathy compared with untreated group (OR: 1.98; 95 % Cl: 1.35, 2.92; P < 0.001). Based on the systematic review results of Serious adverse events, compared to the untreated group, showed that the Serious adverse events of the treatment group was remarkably greater (OR:1.60; 95 % Cl:1.19,2.14; P < 0.01). Based on the systematic review results of Rash, compared to the untreated group, showed that the Rash of the treatment group was remarkably greater (OR:2.23; 95 % Cl: 1.62,3.05; P < 0.001). Based on the systematic review results of Vomiting, compared to the untreated group, showed that the Vomiting of the treatment group was remarkably greater (OR:5.12; 95 % Cl: 3.36,7.80; P < 0.001).
5. Limitations
The limitations are: The search was limited to English-language literature; no other language literature was found. Selection bias and insufficient research inclusion may also exist. As a result, you ought to view some of the meta analysis's findings with objectivity. However, our research will further assist clinical doctors in selecting the most favorable option.
6. Conclusion
The study results revealed that inhibitor of proteasomes are effective in the multiple myeloma maintenance treatment compared with the placebo group. Bortezomib has certain advantages in prolonging PFS, followed by ixazomib and carfilzomib in terms of efficacy. Bortezombib may be superior to carfilzombib in extending OS. However, the adverse reactions caused by proteasome inhibitors, such as Peripheral neuropathy, Serious adverse events, Rash, Vomiting, etc., should be paid enough attention.
Data availability
The data could be obtained by contacting corresponding author.
Funding
This work was supported by the Medical and health science and technology plan of Zhejiang province of China (No.2023XY007).
CRediT authorship contribution statement
Xin-xin Yang: Writing – original draft. Guoli Yao: Conceptualization. Yujing Yang: Data curation. Yahui Han: Formal analysis. Lin Yang: Data curation. Yuefeng Zhang: Supervision.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Yuefeng Zhang reports financial support was provided by Medical and health science and technology plan of Zhejiang province of China. Yuefeng Zhang reports a relationship with Medical and health science and technology plan of Zhejiang province of China that includes: employment. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Guoli Yao designed the study; Xin-xin Yang wrote the original draft; Yujing Yang and Lin Yang collected raw data; Yahui Han performed statistical and bioinformatics analyses. Yuefeng Zhang supervised the study. All authors read and approved the final version of the manuscript.
References
- 1.Kyle R.A., Rajkumar S.V. Multiple myeloma. Blood. 2008;111:2962–2972. doi: 10.1182/blood-2007-10-078022. https://doi:10.1182/blood-2007-10-078022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howell D., et al. Multiple myeloma: routes to diagnosis, clinical characteristics and survival - findings from a UK population-based study. Br. J. Haematol. 2017;177:67–71. doi: 10.1111/bjh.14513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Jemal A. Cancer statistics. CA A Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. https://doi:10.3322/caac.21590 2020. [DOI] [PubMed] [Google Scholar]
- 4.Lindqvist E.K., et al. History of autoimmune disease is associated with impaired survival in multiple myeloma and monoclonal gammopathy of undetermined significance: a population-based study. Ann. Hematol. 2017;96:261–269. doi: 10.1007/s00277-016-2859-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowan A.J., et al. Diagnosis and management of multiple myeloma: a review. JAMA. 2022;327:464–477. doi: 10.1001/jama.2022.0003. [DOI] [PubMed] [Google Scholar]
- 6.LeMasters G.K., et al. Cancer risk among firefighters: a review and meta-analysis of 32 studies. J. Occup. Environ. Med. 2006;48:1189–1202. doi: 10.1097/01.jom.0000246229.68697.90. [DOI] [PubMed] [Google Scholar]
- 7.Birmann B.M., et al. Young adult and usual adult body mass index and multiple myeloma risk: a pooled analysis in the International Multiple Myeloma Consortium (IMMC) Cancer Epidemiol. Biomarkers Prev. 2017;26:876–885. doi: 10.1158/1055-9965.EPI-16-0762-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lynch H.T., et al. Phenotypic heterogeneity in multiple myeloma families. J. Clin. Oncol. 2005;23:685–693. doi: 10.1200/JCO.2005.10.126. [DOI] [PubMed] [Google Scholar]
- 9.Sawyer J.R., et al. Hyperhaploidy is a novel high-risk cytogenetic subgroup in multiple myeloma. Leukemia. 2017;31:637–644. doi: 10.1038/leu.2016.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jang J., et al. Decursin and doxorubicin are in synergy for the induction of apoptosis via STAT3 and/or mTOR pathways in human multiple myeloma cells. Evid Based Complement Alternat Med. 2013 doi: 10.1155/2013/506324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.AKyle R., Rajkumar S.V. Multiple myeloma. Blood. 2008;111:2962–2972. doi: 10.1182/blood-2007-10-078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Attal M., et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N. Engl. J. Med. 2003;349:2495–2502. doi: 10.1056/NEJMoa032290. [DOI] [PubMed] [Google Scholar]
- 13.Maloney D.G., et al. Allografting with nonmyeloablative conditioning following cytoreductive autografts for the treatment of patients with multiple myeloma. Blood. 2003;102:3447–3454. doi: 10.1182/blood-2002-09-2955. [DOI] [PubMed] [Google Scholar]
- 14.Goldschmidt H., et al. Bortezomib before and after high-dose therapy in myeloma: long-term results from the phase III HOVON-65/GMMG-HD4 trial. Leukemia. 2018;32:383–390. doi: 10.1038/leu.2017.211. [DOI] [PubMed] [Google Scholar]
- 15.Dimopoulos M.A., et al. Oral ixazomib maintenance following autologous stem cell transplantation (TOURMALINE-MM3): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2019;93:253–264. doi: 10.1016/S0140-6736(18)33003-4. [DOI] [PubMed] [Google Scholar]
- 16.Dimopoulos M.A., et al. Ixazomib as postinduction maintenance for patients with newly diagnosed multiple myeloma not undergoing autologous stem cell transplantation: the phase III TOURMALINE-MM4 trial. J. Clin. Oncol. 2020;38:4030–4041. doi: 10.1200/JCO.20.02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregersen H., et al. Carfilzomib and dexamethasone maintenance following salvage ASCT in multiple myeloma: a randomised phase 2 trial by the Nordic Myeloma Study Group. Eur. J. Haematol. 2022;108:34–44. doi: 10.1111/ejh.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yong K.L., et al. Carfilzomib or bortezomib in combination with cyclophosphamide and dexamethasone followed by carfilzomib maintenance for patients with multiple myeloma after one prior therapy: results from a multicenter, phase II, randomized, controlled trial (MUKfive) Haematologica. 2021;106:2694–2706. doi: 10.3324/haematol.2021.278399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosiñol L., et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood. 2012;120:1589–1596. doi: 10.1182/blood-2012-02-408922. [DOI] [PubMed] [Google Scholar]
- 20.Rosiñol L., et al. Bortezomib and thalidomide maintenance after stem cell transplantation for multiple myeloma: a PETHEMA/GEM trial. Leukemia. 2017;31:1922–1927. doi: 10.1038/leu.2017.35. [DOI] [PubMed] [Google Scholar]
- 21.Sonneveld P., et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/GMMG-HD4 trial. J. Clin. Oncol. 2012;30:2946–2955. doi: 10.1200/JCO.2011.39.6820. [DOI] [PubMed] [Google Scholar]
- 22.Gerecke C., et al. The diagnosis and treatment of multiple myeloma. Dtsch Arztebl Int. 2016;113:470–476. doi: 10.3238/arztebl.2016.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dimopoulos M.A., et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021;32:309–322. doi: 10.1016/j.annonc.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Hideshima T., et al. Bortezomib induces canonical nuclear factor-kappaB activation in multiple myeloma cells. Blood. 2009;114:1046–1052. doi: 10.1182/blood-2009-01-199604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhn D.J., et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood. 2007;110:3281–3290. doi: 10.1182/blood-2007-01-065888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ziogas D.C., et al. An overview of the role of carfilzomib in the treatment of multiple myeloma. Expet Opin. Pharmacother. 2017;18:1883–1897. doi: 10.1080/14656566.2017.1404575. [DOI] [PubMed] [Google Scholar]
- 27.Dimopoulos M.A., et al. Carfilzomib-lenalidomide-dexamethasone vs lenalidomide-dexamethasone in relapsed multiple myeloma by previous treatment. Blood Cancer J. 2017;7:554. doi: 10.1038/bcj.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fakhri B., et al. Measuring cardiopulmonary complications of carfilzomib treatment and associated risk factors using the SEER-Medicare database. Cancer. 2020;126:808–813. doi: 10.1002/cncr.32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data could be obtained by contacting corresponding author.