Abstract
Metals are widely studied environmental contaminants for several reasons, including their ubiquity, potential toxicity to aquatic life, and tendency for their aquatic toxicity to vary widely with the chemistry of the surface water in which they occur. These interactions between metal and underlying water chemistry are described as influencing metal bioavailability, an index of the rate and extent to which the metal reaches the site of toxic action within an exposed organism. The implications of metal bioavailability for ecological risk assessment is large, as it can produce differences in toxicity of as much as 100-fold across a range of water chemistries in surface waters. Beginning as early as the 1930s, considerable research effort has been expended toward documenting and understanding metal bioavailability, as a function of total and dissolved metal, water hardness, natural organic matter, pH, and other characteristics of natural waters. The growing understanding of these factors, and improvements in both analytical and computational chemistry, led in turn to a series of modeling approaches intended to describe and predict the relationship between water chemistry and metal toxicity, including the Free Ion Activity Model, the Gill Surface Interaction Model, the Biotic Ligand Model, and additional derivatives and regression models that arose from similar knowledge. The arc of these scientific advances can also be traced through the evolution of U.S. EPA Ambient Water Quality Criteria over the last 50 years, from guidance in the “Green Book” published in 1968, to metal-specific criteria produced in the last decade. Through time, these criteria have incorporated increasingly sophisticated means of addressing metal bioavailability, as has regulatory guidance developed by other jurisdictions across the globe. These actions have shifted the debate toward identifying harmonized approaches for determining when knowledge is adequate to establish bioavailability-based approaches and how best to implement them. This Focus Article presents and discusses the history of scientific understanding of metal bioavailability, and the development and application of models to incorporate this knowledge into regulatory practice.
Keywords: Metal, Bioavailability, Water Quality Models
Introduction
In December of 2017, a technical workshop on Bioavailability-Based Aquatic Toxicity Models for Metals was convened in Pensacola, FL, coordinated by the Society of Environmental Toxicology and Chemistry (SETAC) – North America. Experts in environmental toxicology, chemistry, modeling, and regulatory application discussed the state of the science for the aquatic toxicology of metals, and how predictive models representing this understanding could be best developed and evaluated to support environmental risk assessment and regulation.
Metals were the focus for this effort because their intrinsic characteristics present challenges for environmental risk assessment. Metals are among the most ubiquitous environmental contaminants, as they are widely distributed and frequently elevated in the environment above natural background as a result of many different anthropogenic activities. Many are also toxic to aquatic life at comparatively low environmental concentrations (e.g., μg/L). The combination of ubiquity and high hazard have raised the priority of metals for development of regulatory guidance, such as water quality criteria (WQC) or other environmental guidelines. However, development of robust regulatory guidelines is in turn hampered by another feature of many metals – the tendency for their toxicity in water to vary widely as a result of the physicochemical characteristics of the surface water in which they occur. When expressed as total recoverable, or even dissolved (filterable) metal concentration, many metals may show a variation of 1–2 orders of magnitude in toxicity across waters with differing characteristics. Yet as articulated in the U.S. EPA guidelines for deriving WQC, they “should attempt to provide a reasonable and adequate amount of protection with only a small possibility of considerable overprotection or underprotection” (U.S. EPA 1985a). To provide this balance in the face of large variability in effect concentrations in natural waters demands that the sources of this variability be effectively explained so they can be incorporated into regulatory frameworks.
The terms “water quality criteria,” “metals,” and “bioavailability” are used extensively in this and other papers arising from the workshop, but they do not have universal definitions that neatly apply to the issues of interest here, and their usage herein is typically more conceptual than specific. The U.S. EPA issues guidance explicitly called Water Quality Criteria; though that term is sometimes used in the literature generically, in this series of papers the term “protective values for aquatic life” (PVAL) is used to emphasize a more universal concept, and the terms “criteria” or WQC are only used when referring specifically to WQC produced by the U.S. EPA. “Metals” can be generally defined as those elements classified as such according to the periodic table, but those of most interest to the workshop are the subset for which: 1) aquatic toxicity varies substantially (within a given aquatic species) across waters with differing characteristics; and 2) for which toxicity to aquatic organisms in the environment is driven primarily by waterborne exposure. Notable examples include Ag, Cd, Cu, Ni, Pb, and Zn.
With regard to bioavailability, there are many aspects of water chemistry that have been shown to affect the aquatic toxicity of one or more metals, including hardness (generally, or as the specific ions like Ca and Mg that comprise it), pH, carbonates alkalinity, temperature, sodium, suspended solids, and colloidal/dissolved natural organic matter (NOM). “Bioavailability” (see Box 1) is defined here as an index of the rate and extent to which a toxic substance (metal) reaches the toxic site of action, and this concept lies at the heart of most modeling approaches discussed at the workshop (e.g., Biotic Ligand Model or BLM). For most organisms, the exact site(s) of metal binding to produce toxic action is not fully understood, so the influence of water chemistry on that interaction is inferred rather than known. In the workshop and its proceedings, the term bioavailability was used in a generic way, to include additional aspects not captured within a narrow definition of the term.
Box 1 – What is Bioavailability?
In aquatic toxicology, bioavailability is considered a measure of the rate and extent to which a substance reaches the site of action. Its estimation is one of the essential properties in ecotoxicology when calculating exposure or dosages derived from water or any other exposure routes than the systemic circulation. The extent of bioavailability is commonly a limiting factor in eliciting an organism response. Limitations in bioavailability are typically due to solubility limitations, sorption to various solids or humic substances, or partitioning into inaccessible phases in the environment such as minerals. Other factors affecting bioavailability of metals can include pH of the exposure media or presence of chemical that compete for binding sites (such as calcium, sodium or magnesium), and finally, organism specifics such as the number and types of binding sites at the site of action. These considerations over the decades have led to significant advances in assessing toxicity of metals in natural waters. In this manuscript we trace the history of the development of measures allowing for assessment of bioavailability in natural waters.
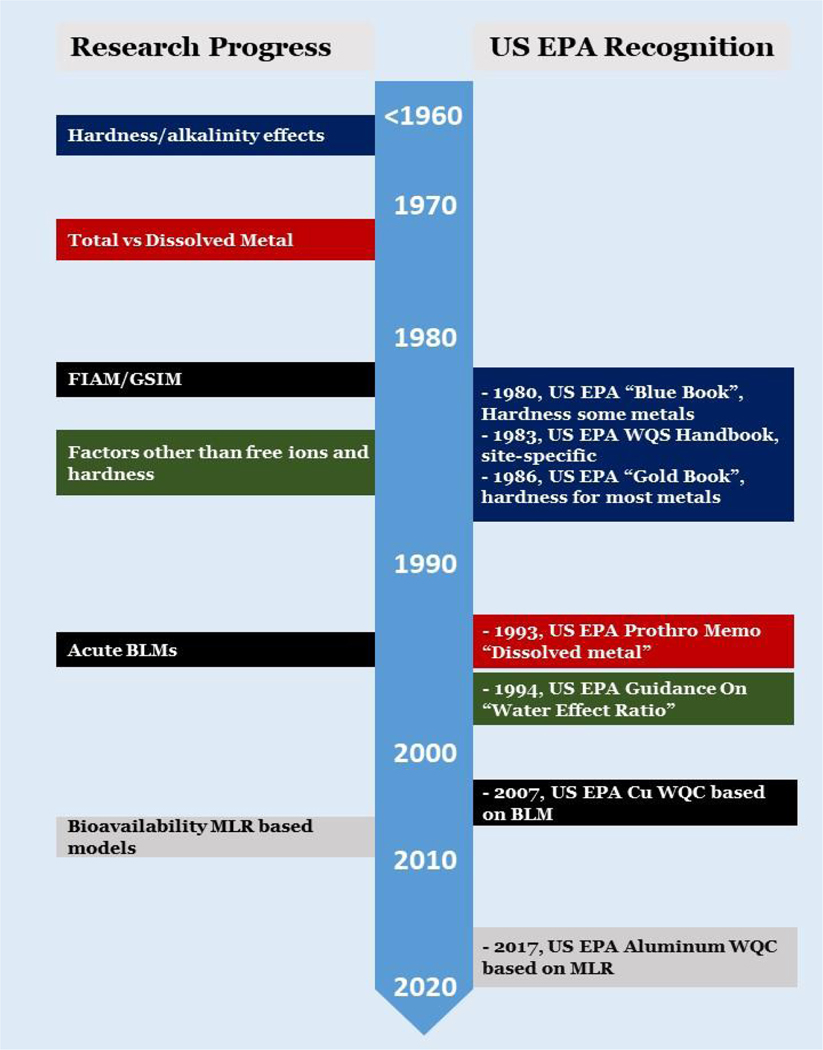
Research to understand the aquatic toxicity of metals spans many decades, from studies in the 1930’s (e.g., Erichsen Jones, 1938) through the present day; within this research history one can trace the growth of our collective understanding, from early empirical observations through complex mechanistic constructions. Alongside the growth of this understanding came the development of conceptual and mathematical models to describe and predict the response of aquatic organisms to metals on the basis of water chemistry. Parallel to this research trajectory, one can also trace the incorporation of the evolving science into PVAL for metals, from some of the earliest single value WQC developed for the U.S. in 1968 (the “Green Book”; FWPCA 1968), to the bioavailability-based models used in current regulatory approaches for some metals (U.S. EPA 2007; Danish Environmental Protection Agency 2008). This parallel history of research and regulatory guidance is summarized in Figure 1 and explained in greater detail in the following section.
Figure 1 –
Timeline of research advances for metal bioavailability and incorporation of those approaches into U.S. EPA Water Quality Criteria. FIAM = Free Ion Activity Model; GSIM = Gill Surface Interaction Model; BLM = Biotic Ligand Model; MLR = Multiple Linear Regression.
Early Developments Related to Assessing Bioavailability
As early as the 1930s, researchers recognized that the toxicity of metals to aquatic organisms was variable and sought to explain this variability. Early work described a reduction in metal toxicity associated with increased calcium (Erichsen Jones, 1938; Doudoroff and Katz, 1953) or hardness (Herbert and Vandyke, 1964). While variation in metal toxicity across water hardness was considerable, the influence of other factors also came to light. Work by Sprague (1968) showed that complexing copper with chelating compounds like nitrilotriacetic acid (NTA) or ethylenediaminetetracetic acid (EDTA) greatly reduced metal toxicity, and later work showed a similar effect of humic and fulvic acids, and naturally occurring organic matter (Zitko et al., 1973; Brown et al. 1974). Another focus was parsing the ameliorative-effect of water hardness among the many water quality factors that typically co-vary with hardness in natural waters, such as pH and carbonate alkalinity. Electrochemical measurements and chemical speciation calculations were used to identify the chemical species of metals (particularly copper) that correlated with toxicity (Zitko and Carson 1976; Andrew 1976; Andrew et al., 1977). This work pointed to free metal ion and metal hydroxide complexes as being more toxic than forms such as carbonate complexes or metal associated with particles. Pagenkopf et al. (1974) were also pursuing free metal ion measurements and chemical speciation calculations as a basis for understanding metal toxicity to fish. Parallel work evaluating free copper ion activity in marine waters confirmed the importance of dissolved organic ligands in decreasing toxicity to algal species (Anderson and Morel, 1978; Sunda and Lewis, 1978).
One of the more comprehensive studies of the effects of factors modifying copper toxicity was reported by Nelson et al. (1986) and expanded by Erickson et al. (1996). This work involved a systematic evaluation of copper toxicity to fathead minnows (Pimephales promelas), and involved manipulations of calcium, sodium, clay, pH, humic acid, and alkalinity in the dilution water, and examined toxicity to P. promelas when exposure was expressed as total, dissolved, or free ionic copper. LC50 values varied by more than 60-fold over the range of conditions tested; while the effects of many toxicity modifying factors could be related to the effects on free copper ion concentration, other responses could not, implying that metal toxicity was caused by more copper species than only the free metal ion.
Advances in analytical chemistry were also key to advancing the understanding of metal toxicity. By the early 1970s the development of new technological solutions to measure metals in natural waters, such as, inductively-coupled argon plasma using optical emission detectors (ICP-AES) or detecting metals using mass spectrometry (ICP-MS), which allowed the measurements of metals concentrations in the range present in natural water (μg/L), and the use of clean chemistry techniques allowed a precise determination of metals in complex aquatic systems. The development of electrodes to measure the thermodynamic activity of ions in natural water samples, stimulated the progress in the studies of metal speciation and its effect on aquatic systems.
In parallel, the increases in computational power of computers allowed complex chemical equilibrium problems to be solved. Applications such as MINEQL (Westall et al., 1976, Schecher and McAvoy, 1992) and MINTEQA2 (Brown and Allison, 1987) became available to the study of metals toxicity. In the mid-1990s, the complexity of the interaction of natural organic matter with metals was mechanistically described by Tipping (1994) in the Windermere Humic Aqueous Model (WHAM).
Free Ion Activity, Gill Surface Interaction, and Biotic Ligand Models
In the 1980s, the combination of advancing toxicological and chemical understanding gave rise to two important models attempting to use growing mechanistic understanding to predict metal toxicity. The Free Ion Activity Model (FIAM) was proposed by Morel (1983), which described interaction of the organism with the free metal ion in a rather general equilibrium formulation; the free ion and its complexes interact with cellular binding sites, where the metal may exert its toxic action and the competing interactions of other major cations (hardness) for the metal binding site. However, the FIAM did not address the complexing effect of organic ligands such as the components of NOM. In parallel, the Gill Surface Interaction Model (GSIM), was proposed by Pagenkopf (1983). This model was similar to the FIAM, with the distinction that it was able to explain the experimental toxicity results for individual metals and, indirectly, the significance of the complexation of free metal ions by NOM.
The concept of metal binding to critical sites on receptor organisms (e.g., gills of fish) leading to toxic effects received important support through the work of Playle, who demonstrated experimentally that metal accumulated in the gills, and disrupted the sodium entry and the activity of the Na-K ATPase present in this tissue (Playle et al, 1992, 1993a, 1993b; Janes and Playle, 1995). Also, in this period Campbell (1995) published an important critical review of the FIAM approach with a focus on its applicability to algae.
Building from the foundation laid by the FIAM and GSIM approaches, a SETAC Pellston Workshop was held in 1996 that proposed the development of a mechanistically-based BLM for metal toxicity, with the explicit goal of producing a model that could be used as a regulatory tool (Bergman and Dorward-King, 1997). The initial development and application of the BLM was focused on predicting the acute toxicity of copper to freshwater organisms (Di Toro et al, 2001; Santore et al, 2001). Similar to the GSIM, the BLM is based on the principle that toxicity occurs by accumulation of the metal bound to a biotic ligand site located on the surface of the organism (for a comprehensive historical review see Paquin et al. 2002a). The mechanistic basis of the model was substantiated by the work of Playle et al. (1992, 1993a,b), and the relationship of metal accumulation to toxicity was further affirmed by MacRae et al. (1999) who measured copper accumulation on fish gills in acute studies as a function of water chemistry. Advanced chemical speciation modeling and modeling of competition between dissolved ions (metal and non-metal) and complexes for binding to the biotic ligand is used to predict the relationship between water chemistry and metal accumulation/toxicity (see Box 2 for further discussion of the BLM).
Box 2 – The Biotic Ligand Model.
In the BLM, reactions between the metal of interest and cations with various naturally occurring ligands in water are modelled using the strength of ligand affinity. This affinity is defined by an equilibrium constant. One of those ligands, termed the biotic ligand, is the site of action on a respiratory membrane where iono-regulatory processes can be disrupted by metal binding. The model requires input of the concentrations of key water chemistry parameters for a given metal of interest. For any given configuration of metal concentrations and water quality conditions, the model predicts the concentration of the various metal-ligand complexes as well as that of the free metal ion. When the concentration of the metal on the biotic ligand reaches the LA50 (lethal accumulation), 50% effect is predicted. The latter value can be confirmed by measuring the metal bound to gill tissue (using radio isotopic techniques) at the concentration of metal in standard bioassays that causes a 50% effect (i.e., mortality).
Metal toxicity can be modified by both inorganic and organic water components. In both cases the formation of complexes with ligands other than the biotic ligand will render the metal less available for interaction with the biotic ligand itself. Generally, only the free metal ion, and, for some metals (e.g., copper) hydroxyl-metal complexes, will be available for interaction with the biotic site. Also, the interaction of other cations (calcium, magnesium, etc.) with biotic ligand sites can render those cations less available for interaction with the metal, thus providing a protective effect, i.e., higher metal concentrations will be required to push the metal-biotic ligand complex concentration beyond the toxicity level. Therefore, the performance of the model will depend on the parameters that determine the set of speciation reactions as well as on those that define the critical concentration of metal-biotic ligand complex at which 50% toxicity occurs.
As originally configured, the BLM was an equilibrium model, and assumed that the kinetics of exposure are not critical to predicting toxicity. Therefore, BLM models for acute or chronic toxicity are representative of equilibrium at different time periods. An extension of the BLM concept to consider the physiological state of the organism over time was proposed by Paquin et al. (2002b). This approach relates metal concentration on the biotic ligand to the loss of iono-regulatory capacity, with death occurring not because metal accumulation reaches a particular concentration, but because the accumulated effects of metal binding create lethal iono-regulatory disturbance.
In the last 10 years, the research on the development of the BLM as a predictive toxicity tool for metals has expanded to other metals, such as Ag, Cd, Pb, and Zn among others, and for several species (see Adams and Chapman, 2007). The model also has been extended to predict chronic toxicity for Al, Co, Cu, Ni, Pb, and Zn (De Schamphelaere and Janssen, 2004a, 2004b, Heijerick et al., 2005, Nys et al., 2014, Schlekat et al., 2010 and Santore el al., 2017).
Multiple Linear Regression (MLR) Model Development
The use of a multiple-linear regression (MLR) model approach has emerged as a statistical means of incorporating a several key variables into a model that evaluates the bioavailability of metals and predicts toxicity to aquatic organisms via water exposure. The approach used is similar in concept to the existing procedure for pooled analysis (across species) of the relationship between water hardness and toxicity used by U.S. EPA to develop hardness-dependent WQC. While these WQC use a simple linear regression model (based on log-transformed data), MLR extends this approach to fitting response to multiple variables (e.g., pH, dissolved organic carbon and hardness), and models may be developed for a single species or the data may be pooled to provide a single model for multiple species.
Use of MLRs for describing the effects of water chemistry on metal toxicity is not a novel concept, and several examples can be found in the literature (Erickson et al. 1987a,b; Esbaugh et al. 2012). More recently, a MLR was developed for copper using a single equation based on the pooling of data across species (Brix et al. 2017). Species-specific MLRs to derive WQC have recently been used by U.S. EPA for aluminum (DeForest et al. 2018). From a conceptual development perspective, it might seem that MLR models would have been popularized before the development of biotic ligand models (BLM) in the late 1990s. However, its re-emergence as a regulatory approach is in part due to advancing mechanistic understanding, such that it can focus on independent variables known to influence metal bioavailability. This builds confidence in the robustness of the approach despite its seemingly lower complexity. MLR development also relies on extensive toxicity data sets covering wide ranges of water chemistry parameters and ecotoxicity endpoints, some of which are only recently becoming available.
Biodynamic Models
The uptake, accumulation and toxicity of metals in aquatic organisms are dynamic processes in which the concentration at a given target site depends on the kinetics of the different steps and processes involved. The dynamic modeling approach describes the uptake, distribution and elimination of substance over time, and considers the different steps or sequences and accounts for changes in conditions that may occur (Ashauer and Escher, 2010). In contrast, equilibrium-based models such as the BLM assume that equilibrium is reached and that there is no change in reactions rates or conditions over time. This also explains why acute and chronic BLMs, although structurally the same, use different ligand affinities (stability constants) to predict acute or chronic toxicity, as they represent different states of assumed equilibrium.
Within the framework of metal ecotoxicology, biodynamic modelling has been primarily used to describe and understand the high variability in tissue metal concentrations among different species and environments, and, to a certain extent, link this to toxicity (Buchwalter et al., 2007, Khan et al., 2016). This approach requires data on assimilation efficiencies (bioavailability) of metals from the diet. A typical bioaccumulation model depicts an organism as a one or two compartmental model which in the latter case are characterized by different uptake and elimination kinetics. In reality, an organism is much more complex, but on a whole organism level one or two compartments often suffice to describe the bioaccumulation kinetics (Adams et al., 2011).
Metal uptake rate constants for dissolved and dietary exposure routes are developed in laboratory experiments. By defining different uptake pathways, the relative importance of dissolved and dietary exposure routes can be considered. Measurements of elimination rates are also required, and the relative importance of uptake versus elimination can help explain why tissue metal concentrations are higher in some organisms compared with others (Stewart et al. 2004). The effect of chemical speciation on metal uptake or bioavailability can be accounted for by expressing the uptake rate constants on a free ion activity scale. Competitive and non-competitive effects can be considered by defining a maximum rate of transport and metal binding constant to characterize the metal transporters involved in uptake.
Luoma and Rainbow (2005) applied a biodynamic modeling approach to compare and predict the bioaccumulation of a range of metals in different freshwater and marine invertebrates. They modelled the metal accumulation process using a one compartment approach and defining two separate uptake routes (i.e. waterborne and dietary). Uptake from the dietary source was characterized by the food ingestion rate and assimilation efficiency. These results demonstrated the applicability of the approach and explained the large differences in metal tissue concentrations in terms of differences in uptake and eliminations rates and the relative importance of water and diet as sources of metal.
Similar approaches have been used to study the relative importance of water and food to different types of organisms and describe the kinetics of metal bioaccumulation processes over time. The dynamic modelling approach has also been used to model metal transfer from one trophic level to another and predict metal accumulation in food webs (Rainbow et al. 2009; Van Campenhout et al. 2009).
Although the kinetics of uptake and bioaccumulation are key features in metal risk assessment, they are not necessarily predictive of metal toxicity. For instance, the critical body residue concept works for some species but not for others (Adams et al. 2011). This is explained by the fact that once inside the organism a metal enters a completely new environment in which metals bind with different biological ligands and can be stored in more or less inert pools (Gao et al., 2015). Metal toxicity will occur when the binding or accumulation of a metal to one or more critical components of the system reaches a certain threshold. In terms of dynamic modelling this can be accounted for by defining an internal compartment in which metals are not contributing to toxicity (i.e., inactive or detoxified pool). Using tissue and subcellular fractionation techniques attempts have been made to discriminate between metals in biologically active or metabolically available pools and inactive or detoxified pools (Wallace and Lopez 1997; Liao et al., 2011). This kind of results provide information on the accumulation of metals in sensitive and less sensitive pools and can be incorporated in biodynamic models.
Overall, biodynamic modelling is a powerful approach to understand and predict the uptake, accumulation and toxicity of metals in biological systems. Compared to BLM type models and its alternatives, it requires more detailed information for its parameterization. Especially the coupling of metal uptake and accumulation to toxicity requires further research to fully exploit the power of the dynamic modelling approach. This approach is not directly linked to toxicity and hence, has not been used in regulatory guidelines for PVAL.
Application of Bioavailability in a Regulatory Context to Date
For more than 50 years the regulatory framework to protect the aquatic environment from the effect of anthropogenic sources of metals has been based on setting Water Quality Criteria in U.S. or Environmental Quality Standards (EQS) for Europe; other jurisdictions have closely followed and adapted these approaches. Prior to 1980, WQC in the U.S. were developed from expert workgroups that reviewed available information on the toxicity of individual pollutants and developed recommendations for water quality based primarily on professional judgement, with a minimum of standardized evaluation processes (the “Green”, “Blue”, and “Red” Books; (FWPCA 1968; U.S. EPA 1973, 1976). Beginning in the late 1970’s, U.S. EPA began to formulate more prescriptive procedures for deriving PVAL, culminating in the 1985 “Guidelines for Deriving Numerical National Water Quality Criteria for the Protection of Aquatic Organisms and Their Uses” (U.S. EPA 1985a). Among many procedural advances, these guidelines established a minimum of toxicity information (8 families) that must be available for a pollutant in order to derive a water quality criterion, advanced new procedures for selecting a level of protection, and formalized procedures to adjust WQC as a function of important water quality characteristics such as pH or hardness. While the 1985 Guidelines remain the core guidance used by U.S. EPA today, there have been some modifications made to account for characteristics of individual pollutants. For metals in particular, tracing the history of WQC development reflects the gradually increasing consideration of bioavailability within regulatory guidance.
Hardness-Based Equations
As early as 1968, narrative in the “Green Book” (FWPCA 1968) discussing WQC for metals recognized that the toxicity of metals such as copper and zinc were depending on the characteristics of the receiving water, but the quantitative understanding of the underlying relationships was still limited. For the Green Book, and the “Blue Book” and “Red Book” that followed (U.S. EPA 1973, 1976), the WQC guidance was not single values, but instead expressed acceptable exposures relative to the acute toxicity of the metal to “the most sensitive important species in the locality” when tested in the actual receiving water. For example, the 1973 guidance was 0.1*(96-h LC50) for copper, 0.05*(96-h LC50) for nickel, and 0.005*(96-h LC50) for zinc, with the application factors being derived based on studies from which the relationship between acute and chronic effect concentrations might be inferred. One could argue that this guidance was hardness-based, as hardness was one characteristic to which differences in toxicity might be related, but the WQC were not quantitatively normalized to hardness.
An explicit normalization of metal WQC to hardness first appeared in 1980, in freshwater WQC for copper and later for cadmium, lead, nickel, and zinc (U.S. EPA 1980a-e). The first official guidance recommending hardness equations as a general approach was in the U.S. EPA Water Quality Standards Handbook from 1983 (U.S. EPA 1983), and hardness-based equations were also used in revised WQC for copper, cadmium, lead published in 1985 (U.S. EPA 1985b-d). The WQC guidelines published that same year contained specific methods for evaluating hardness-dependence of toxicity, and for generating WQC normalized to hardness (or other water quality characteristics) (U.S. EPA 1985a).
Total Recoverable vs Dissolved Metal
In the U.S. through 1980 (e.g., USEPA 1980a-e), compliance with WQC was based on total recoverable metal. This involves a fairly rigorous acid digestion and captures many forms of metals that were associated with solids and had little or no toxicological effect. While there was a growing realization that total recoverable metal measures could exaggerate the likely toxicity of many metals, this measure was retained in part because of an absence of an alternative to reliably distinguish non-available forms from those that were potentially available. WQC for copper, cadmium, and lead released in 1985 (U.S. EPA 1985b-d) changed from total recoverable metal to “acid-soluble metal,” defined as metal that would pass a 0.45 μm filter after acidification to pH between 1.5 and 2. The text of these documents recognizes that while acid-soluble metal measurements should be an improvement over total recoverable metal, it still could over-represent the potency of metals in natural waters.
The advancing scientific knowledge on metal toxicity continued to demonstrate that total recoverable or acid-soluble metal were poorly correlated with toxicity in natural waters and likely overprotective when used as a basis for regulation. Accordingly, an EPA-sponsored scientific workshop (U.S. EPA 1992, 1993) recommended, that WQC/standards to be based on the concentration of dissolved metals in water (operationally defined as metals passing through a 0.45 μm filter). The U.S. EPA soon afterward revised its policy and recommended that the dissolved metal fraction be used both to set, and to measure compliance with WQC for aquatic life (Prothro 1993).
Despite the recommendations for using dissolved metals in assessing compliance with water quality WQC, discharge limits were still set using total recoverable metal, because effluents often have chemistry very different from ambient waters, and because particulates in effluents could release dissolved metals to ambient waters. To address this issue, U.S. EPA reviewed paired total recoverable and dissolved measurements in a range of ambient waters (Brungs et al. 1992) to recommend options for setting discharge limits at total recoverable measurements that were likely to comply with criteria and standards based on the dissolved fraction (Prothro 1993). Subsequently, the U.S. EPA issued “Metals Translator” Guidance (U.S. EPA 1996), which provided tables of conversion factors that gave State agencies and permit writers the ability to establish permit limits that were coherent with the expected dissolved metal concentration and the WQC based on those concentrations.
Water Effects Ratio Development
While the policy shift to setting aquatic life criteria based on dissolved metal was viewed as a significant advance, it was still recognized that hardness-adjusted WQC based on dissolved metal left significant influences on the bioavailability and toxicity of metals (e.g., dissolved organic carbon, pH) unaccounted for. Yet at the time, no reliable analytical methods or models were available to more accurately assess or predict toxicity for the water chemistry of a particular site. In parallel efforts, the Water Effect Ratio (WER; U.S. EPA 1983, 1984) was developed as an empirical tool to address this gap. The WER approach uses laboratory tests to compare the toxicities of a metal in both standard laboratory water and in ambient water(s) of interest; the concept is to use the ratio of effect concentration (e.g., LC50) in ambient water to the effect concentration in standard water as factor to adjust national WQC to reflect the site-specific bioavailability of metal.
While scientifically logical, the early WER guidance lacked detailed practical guidance, and the WER method was infrequently used. In 1993, an expert panel recommended the development and use of more explicit operational guidelines for application of WERs, which led to the development and testing of more detailed WER guidance in 1994 (U.S. EPA 1994). Pilot site studies using copper, zinc, cadmium, lead, nickel and chromium (Brungs et al. 1992; Hall and Raider 1993) demonstrated the robustness of the WER method and provided the underpinnings of the 1994 guidance. The wide range of WERs derived from these studies (Table 1-1) underscores the impact of more completely accounting for the influence of local water chemistries in deriving site-specific WQC for metals.
Table 1–1:
Water Effect Ratios from early studies:
| Metal | Water bodies | Number of Studies | Water Effect Ratios (range) |
|---|---|---|---|
| Copper | 4 Eastern & Midwestern Rivers1 | 8 | 1.1 – 15.3 |
| Zinc | 4 Eastern & Midwestern Rivers1 | 9 | 1.2 – 2.9 |
| Cadmium | 4 Eastern & Midwestern Rivers1 | 20 | 1.0 – 10.8 |
| Chromium | 2 Midwestern Rivers1 | 2 | 1.2 – 2.7 |
| Lead | 1 Midwestern River1 | 4 | 1.9 – 5.7 |
| Nickel | 1 Gulf estuary1 | 1 | 1.6 |
| Copper | 3 Eastern Rivers2 | 3 | 26 – 64 |
| Zinc | 1 Eastern River2 | 1 | 2.4 |
| Cadmium | 1 Eastern River2 | 1 | 38 |
additional studies reported by Hall and Raider, 1993.
Despite these additional studies and refined guidance, many U.S. States were initially reluctant to allow the use of WER-adjusted national WQC as site-specific WQC, and the expense of meeting the testing requirements in the guidance seemed onerous to regulated facilities. A group of municipal wastewater agencies in southeastern Pennsylvania petitioned the Pennsylvania Department of Environmental Protection to allow a streamlined WER study to set a site-specific copper standard applicable to all their receiving streams. Data from these studies, and other studies by U.S. EPA and others, provided a large body of chemical data and associated toxicity, which then led to the issuance of a streamlined WER approach for copper (U.S. EPA 2001) that was much less expensive and could be more readily implemented by individual dischargers.
Biotic Ligand Model (BLM)
The WER approach was developed to address the complicated relationship between water chemistry and metal toxicity in the absence of a robust chemical measure or model to assess that relationship mechanistically. As described previously, a 1996 Pellston workshop set in motion the development of the BLM, designed to fill that void and provide a tool that could be used to develop national WQC that were intrinsically adaptive to site-specific water chemistry (Bergman and Dorward-King, 1997). In 2000, the U.S. EPA Science Advisory Board (SAB) reviewed proposals to advance BLM-based criteria for both copper and silver (U.S. EPA 2000). The SAB concluded the BLM was a practical tool for site specific water quality regulations and assessment and supported its application to developing context-sensitive national WQC for copper. For silver, the SAB recommended further research on the scientific foundations of the model, particularly to better understand the mechanistic interaction of silver and the biological target.
The original formulation of the BLM was ultimately applied in the 2007 WQC for copper (U.S. EPA 2007), which calculated WQC values based on the water chemistry of individual water bodies or sites. The U.S. 2007 copper WQC is based on a BLM for acute toxicity that is then adjusted to account for chronic toxicity. The chemical speciation computations to are handled by the CHESS Model (Santore and Driscoll, 1995) coupled with an organic-metal complexation model (WHAM V model; Tipping, 1994). While BLM models have since been developed for metals beyond copper, copper remains the only metal for which U.S. EPA has issued BLM-based WQC to date.
Multiple Linear Regression (MLR)
In 2017, the U.S. EPA released draft WQC for aluminum, which are based on MLR models addressing relationships of aluminum toxicity with pH, hardness, and dissolved organic carbon. The selection of MLR models over a BLM approach was driven in large part by the greater complexity of aluminum chemistry and the evidence that aluminum may act through more than one toxic mechanism, not all of which are represented well by the biotic ligand concept. In addition, implementation of the BLM-based copper WQC in the U.S. has been slowed by resistance stemming from the complexity of the BLM model (perceived by some as decreased transparency), and by resistance to measuring (or estimating) the multiple water chemistry parameters required as BLM inputs. The MLR model for aluminum eliminates most of these concerns since it only requires measurement of pH, DOC and hardness.
Developments Outside the U.S.
Regulatory guidance for water quality is the result not only of the science, but also policy, which establishes the intended level of protection, margin of safety and level of certainty. These policy frameworks vary among jurisdictions. The Australian and New Zealand guidelines for aquatic ecosystem protection (ANZECC/ARMCANZ, 2000) have a framework that allows for different levels of species protection, depending on the state of the ecosystem under consideration. Typically, this is 95% but ranges from 99% to 80% depending principally on the level of ecosystem disturbance. Canada’s stated goal is to protect all forms of aquatic life and all aspects of the aquatic life cycles, including the most sensitive life stage of the most sensitive species over the long term (CCME 2007). In the EU Environmental Quality Standards derived under the Water Framework Directive protect the most sensitive receptor (EC 2011). International jurisdictions give equal weight to fish, invertebrates and aquatic plants/algae; the U.S. EPA 1985 guidelines gave less weight to plants/algae unless they were the most sensitive, because at the time, interpreting the results of those tests were not as well developed. The result of these policy decisions is that regulatory values do still vary among jurisdictions despite the fact that many jurisdictions are moving toward better accounting for bioavailability in the development of PVAL for metals.
In Canada, water quality guidelines (WQG) are applied on a voluntary basis to inform water management decisions but may also be incorporated into legislation. The intent is to protect all aquatic life stages during indefinite exposure. CCREM (1987) adopted U.S. EPA hardness-based equations but implemented the use of 4 discrete hardness bands for cadmium, copper, lead and nickel. For aluminum two different WQG were established above and below pH 6.5. Under a revised protocol (CCME 2007), Canada is currently developing WQG using MLR or BLM approaches to better incorporate hardness, pH and DOC. Recently Canada has moved toward expressing WQG in terms of dissolved metal. Nevertheless, much of the surface water data collected continue to be unfiltered total metals, creating the potential for false positive exceedances of WQG, since there is no Canadian equivalent to the U.S. EPA’s Metals Translator (U.S. EPA 1996).
Australia/New Zealand initially cited Canadian approaches (Hart et al. 1993) which in turn only recognized hardness and pH as bioavailability-modifying factors. With the release of the Australian and New Zealand Guidelines for Fresh and Marine Water Quality (ANZECC/ARMCANZ 2000) they introduced innovative approaches including chronic WQG at different protection levels using species sensitivity distributions and “reliability triggers” based on data quality and quantity. Toxicity modifying factors for metals were still limited to hardness. Where used, 30 mg/L CaCO3 was chosen, with other values calculated after U.S. EPA. Currently Australia/New Zealand recognize the full suite of toxicity modifying factors including, organic carbon, pH, temperature, alkalinity, hardness and inorganic ligands. They are evaluating MLR and BLM procedures (Warne et al. 2015, rev 2017).
In Europe, the Water Framework Directive (EC 2000) and the Priority Substances Daughter Directive (EC 2013) are the basis for setting Environmental Quality Standards (EQS) for chemical substances that focus on aquatic life protection goals. Metals are classified as either Priority Substances (Ni and Pb), meaning that all Member States have to comply with the same EQS, or as Specific Pollutants (e.g., Cu, Mn, Zn), for which Member States and regional authorities have jurisdiction. Guidance for determining EQS’s for metals has identified approaches for incorporating bioavailability (EC 2011). This guidance was based on comprehensive risk assessment guidance developed for the Existing Substances Directive, which resulted in the application of bioavailability correction for Cd, Cu, Ni, and Zn (EQS TGD). Guidance for a subsequent legislation called Registration Evaluation and Authorization of Chemicals (REACH) continued consideration of bioavailability normalization for metals in water, sediment, and soil.
In terms of standards set to protect aquatic life, the European Commission established bioavailability-based EQS’s for Ni and Pb in 2013 (2013/39/EU). A number of EU Member States established bioavailability-based EQS’s for metals classified as Specific Pollutants 2005 and 2017, including Cu, Mn, and Zn. For Priority Substances like Ni and Pb, all Member States must comply with a single numerical EQS. To manage this requirement while also incorporating bioavailability, a tiered approach is used where the first tier involves comparing dissolved metal concentrations with a reference EQS. The reference EQS is based on water chemistry parameters representing a worst case in terms of bioavailability for each metal. If the ambient dissolved concentrations exceed the reference EQS, then site- or region-specific water chemistry is used in conjunction with appropriate bioavailability correction approaches (Merrington et al. 2016).
Abbreviated BLM Models
The complexity of BLMs can limit their practical application; as a result, “user friendly” bioavailability tools/models have been developed for metals that can be used for regulatory implementation of bioavailability-based environmental water quality standards. To date, user-friendly models have been developed exclusively to determine compliance with bioavailability-based Environmental Quality Standards under the European Union’s Water Framework Directive These tools incorporate site-specific metal bioavailability using fewer input parameters than BLMs, require less training and allow for rapid assessment of large data sets. Any loss in the precision of bioavailability estimates relative to BLMs is considered to be balanced against decreased costs and time associated with greater sample throughput and reduced monitoring burden relative to the use of a full BLM. Examples of user friendly models used in Europe include Bio-met, PNEC-pro and MBAT as listed below:
Bio-met bioavailability tool v. 2.3 – a “lookup table1” based tool in both MS Excel spreadsheet and online formats developed collaboratively by wca environment and ARCHE (www.bio-met.net);
PNECPro v.5 – an “algorithm” based tool in MS Excel format developed by Deltares in the Netherlands (www.pnec-pro.com);
M-BAT v.31 – an “algorithm” based tool in MS Excel developed by wca environment (based on bio-met database).
An algorithm-based tool (like M-BAT) does not contain a database of predictions from BLMs but is based on a mathematical relationship that attempts to simulate BLM calculations but uses a restricted set of input parameters. Algorithm based approaches are derived from training datasets of the results of bioavailability calculations from BLMs. The eventual choice of tool for regulatory purposes will be made by individual regulatory jurisdictions, based on their own particular circumstances and requirements, however each of the bioavailability tools strive to meet a minimum standard of performance in terms of precision and applicability relative to the full BLMs they report to mimic, in order to provide outputs that are scientifically defensible. These tools have principally been used in Europe and reflect a progression in the effort to develop models that require less data and less time to run than BLM models.
Peters et al. (2016) compared the performance of the Bio-met (v. 5), PNECPro (v. 5), and M-BAT, and showed that the models reported widely different bioavailability-based EQS’s for Ni. Differences were pronounced in high pH waters and demonstrates the need for developing a common set of performance criteria for tools where the primary use is to determine compliance with regulatory standards.
Remaining Challenges
Despite the extensive research that has advanced our understanding of metal bioavailability and the ability to develop predictive models, experiences using bioavailability models in the derivation of PVALs has exposed a number of challenging issues. One is the proliferation of models themselves, and the variety of methods of validation; several different modeling approaches have been developed and published, each with different approximations, structure, assumptions, and strengths/weaknesses. This leads regulatory authorities to grapple with evaluating the appropriateness of different models, not only on a purely technical basis, but also in terms of how the characteristics of different models intersect with the policy considerations that underlie regulatory values used by different authorities. Other issues relate to the practicality of implementing models in regulatory programs; professionals charged with developing discharge permits or other applications of regulatory PVAL may not have the background necessary to fully evaluate the details of the models they could be asked to use, and the complexity of some models inhibits straightforward communication of their inner workings. Finally, some modeling approaches require a variety of water chemistry parameters as input variables, requiring either collection of additional data on water quality characteristics, or some means to estimate values appropriate to individual surface waters. These and other issues have hampered the process of incorporating the most current understanding of metal bioavailability into regulatory PVAL.
Considering the current availability of several scientifically defensible approaches, the SETAC Technical workshop was held to take stock of the current state of the science of metals bioavailability models, to evaluate the performance of the models, and to identify best practices in the use of these models in the determination and application of bioavailability-based effects concentrations for metals that are intended to protect aquatic life (e.g., criteria and standards).
This publication series summarizes the discussions and recommendations from four Workgroups, which include the following publications:
Mebane et al. (2018): “Metal bioavailability models: current status, lessons learned, considerations for regulatory use, and model development path forward”
Brix et al. (2018): Guidance on the development of empirical bioavailability models for deriving Water Quality Criteria for metals.
Garman et al. (2018): Validation of Bioavailability-based Freshwater Toxicity Models for Metals
Van Genderen et al. (2018): Application of Bioavailability-based Metals Freshwater Toxicity Models for Criteria Derivation Using Acute and Chronic Species Sensitivity Distributions
The workshop resulted in several high-level recommendations regarding the development of metals bioavailability models and their use. First, all bioavailability models should be informed by mechanistic understanding of metal toxicity and of metal speciation. Second, the development of simplified tools is feasible, as long as the tools have mechanistic links. Third, all models should undergo qualitative and quantitative validation, and be applied within appropriate application ranges of water chemistry. Finally, different models can be used for different situations; the critical aspect is that the choice of the most appropriate model needs to be transparently communicated.
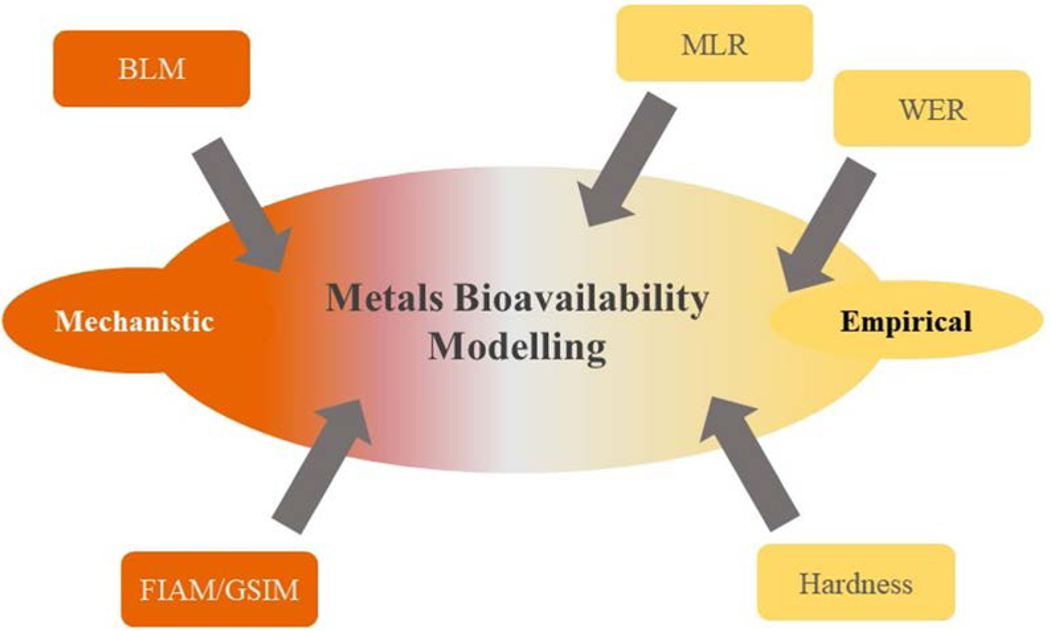
Box 3 – The Continuum Between Empirical Mechanistic Approaches.
Models designed to evaluate bioavailability of metals in water fall in a continuum between models that are strictly empirical (WER) and those that are largely mechanistic (BLM), as depicted below. While BLMs have as their basis the mechanism of metals creating bonds with a biological ligand with subsequent toxic effects, MLR models, while less mechanistic, are predicating on observations showing that key water chemistry parameters (DOC, pH, Ca) are both mechanistically and statistically related to the toxic effect. See text for discussion of the specifics of the different approaches.
Acknowledgements
The authors acknowledge the financial support of the SETAC Technical Workshop, including the US Environmental Protection Agency, the Metals Environmental Research Associations, Dow Chemical Company, Newmont Mining Company, Rio Tinto, Umicore, and Windward Environmental. We would also like to thank the SETAC Staff for their support in organizing the workshop. WJ Berry provided helpful comments on a previous version of this manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency.
Footnotes
Algorithm: Set of calculation rules
References
- Adams WJ, Chapman PM. 2007. Assessing the hazard of metals and inorganic metal substances in aquatic and terrestrial systems: Summary of a SETAC Pellston Workshop. Society of Environmental Toxicology and Chemistry (SETAC): Pensacola, FL, USA. [Google Scholar]
- Adams WJ, Blust R, Borgmann U, Brix KV, DeForest DK, Green AS, Meyer JS, McGeer JC, Paquin PR, Rainbow PS, Wood C. 2011. Utility of tissue residues for predicting effects of metals on aquatic organisms. Integ Environ Assess Manag 7(1): 75–98. [DOI] [PubMed] [Google Scholar]
- Anderson DM, Morel FMM. 1978. Copper sensitivity of Gonyaulax tamarensis,” Limnol Oceanog 23(2):283–295. [Google Scholar]
- Andrew RW. 1976. Toxicity relationships to copper forms in natural waters. In: Toxicity to Biota of Metal Forms in Natural Waters. Andrew RW, Hodson PV, Konasewich DE Eds. Great Lakes Research Advisory Board, Ontario, pp. 127–143 [Google Scholar]
- Andrew RW, Biesinger KE, Glass GE. 1977. Effects of inorganic complexing on the toxicity of copper to Daphnia magna. Water Res 11:309–315. [Google Scholar]
- ANZECC/ARMCANZ. 2000. Australian and New Zealand guidelines for fresh and marine water quality. Vol 1 – 7. Australian and New Zealand Environment and Conservation Council / Agriculture and Resource Management Council of Australia and New Zealand. [Google Scholar]
- Arche/wca. 2014. A systematic comparison of user friendly tools used to estimate the bioavailability of copper, nickel and zinc in the aquatic environment of Europe. A report prepared for NiPERA, ECI, IZA. Arche Consulting, Gent, Belgium. [Google Scholar]
- Ashauer R, Escher BI, 2010. Advantages of toxicokinetic and toxicodynamic modelling in aquatic ecotoxicology and risk assessment. J Environ Monitor 12: 2056–2061. [DOI] [PubMed] [Google Scholar]
- Bergman HL, Dorward-King EJ. 1997. Reassessment of Metals Criteria for Aquatic Life protections. Proceedings of a Society of Environmental Toxicology and Chemistry (SETAC) Pellston Workshop (1996), SETAC Press, Pensacola, FL, USA. [Google Scholar]
- Brix KV, DeForest DK, Tear L, Grosell M, Adams WJ. 2017. Use of multiple linear regression models for setting water quality criteria for copper: A complementary approach to the biotic ligand model. Environ Sci Technol 51:5182–5192. [DOI] [PubMed] [Google Scholar]
- Brix KV, DeForest DK, Tear L, Peijnenburg W, Peters A, Traudt E, Erickson RJ. 2018. Guidance on the development of empirical bioavailability models for deriving water quality criteria for metals. Environ Toxicol Chem (in review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VM, Shaw TL, Shurben DG 1974. Aspects of water quality and the toxicity of copper to rainbow trout. Water Res 8(10):797–803. [Google Scholar]
- Brown DS, Allison J. 1987. MINTEQAI, An Equilibrium Metal Speciation Model: User’s Manual. EPA/600/23–87/012. United States Environmental Protection Agency, Athens, GA. [Google Scholar]
- Brungs W, Holderman T, Southerland M. 1992. Synopsis of Water-Effect Ratios for Heavy Metals as Derived for Site-Specific Water Quality Criteria. EPA Contract No. 68-C0–0700. [Google Scholar]
- Buchwalter DB, Cain DJ, Clements WH, Luoma SN. 2007. Using biodynamic models to reconcile differences between laboratory toxicity tests and field biomonitoring with aquatic insects. Environ Sci Technol 41:4821–4828. [DOI] [PubMed] [Google Scholar]
- Campbell PGC. 1995. Interactions between trace metals and aquatic organisms: a critique of the free-ion activity model. In: Tessier A, Turner DR (Eds.), Metal Speciation and Bioavailability in Aquatic Systems. Wiley, Chichester, pp.45–102. [Google Scholar]
- Canadian Council of Resource and Environment Ministers (CCREM). 1987. Canadian Water Quality Guidelines. Prepared by the Task Force on Water Quality Guidelines. [Google Scholar]
- Canadian Council of Ministers of the Environment (CCME). 2007. A protocol for the derivation of water quality guidelines for the protection of aquatic life 2007. In: Canadian environmental quality guidelines, 1999, Canadian Council of Ministers of the Environment, 1999, Winnipeg, MB, Canada. [Google Scholar]
- Danish Environmental Protection Agency 2008. European Union risk assessment report on nickel, nickel sulphate, nickel carbonate, nickel chloride, nickel dinitrate. Danish Environmental Protection Agency on behalf of the European Union, Copenhagen, Denmark. [Google Scholar]
- DeForest DK, Brix KV, Tear LM, Adams WJ. 2018. Multiple Linear Regression (MLR) Models for Predicting Chronic Aluminum Toxicity to Freshwater Aquatic Organisms and Developing Water Quality Guidelines. Environ Toxicol Chem 37(1):80–90. [DOI] [PubMed] [Google Scholar]
- De Schamphelaere KAC, Janssen CR. 2004a. Bioavailability and chronic toxicity of zinc to juvenile rainbow trout (Oncorhynchus mykiss): comparison with other fish species and development of a biotic ligand model. Environ Sci Technol 38(23):6201–6209. [DOI] [PubMed] [Google Scholar]
- De Schamphelaere KAC, Janssen CR. 2004b. Development and field validation of a biotic ligand model predicting chronic copper toxicity to Daphnia magna. Environ Toxicol Chem 23(6):1365–75. [DOI] [PubMed] [Google Scholar]
- Di Toro DM, Allen HE, Bergman HL, Meyer JS, Paquin PR and Santore RC. 2001. Biotic ligand model of the acute toxicity of metals. 1. Technical basis. Environ Toxicol Chem 20:2383–2396. [PubMed] [Google Scholar]
- Doudoroff P, Katz M. 1953. Critical review of literature on the toxicity of industrial wastes and their components to fish. II. The metals, as salts. Sewage Ind Waste 1953: 802–839. [Google Scholar]
- European Commission (EC). 2000. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. OJ. L; 327. p. 1–72. [Google Scholar]
- European Commission (EC). 2011. Guidance Document No. 27 Technical Guidance for Deriving Environmental Quality Standards. Common Implementation Strategy for the Water Framework Directive (2000/60/EC) Technical Report (Revised 2016). [Google Scholar]
- European Commission (EC). 2013. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. [Google Scholar]
- Jones Erichsen JR. 1938. The relative toxicity of salts of lead, zinc and copper to the stickleback, and the effects of calcium on the toxicity of lead and zinc salts. J Exp Biol 15:394–407. [Google Scholar]
- Erickson RJ, Benoit DA, Mattson VR. 1987a. Effects of physical and chemical parameters of test water on acute toxicity of copper to larval fathead minnows; presented before Division of Environmental Chemistry, American Chemical Society, New Orleans, LA, August 30-September 4, 1987. [Google Scholar]
- Erickson RJ, Benoit DA, Mattson VR 1987b. A prototype toxicity factors model for site-specific water quality criteria; U.S Environmental Protection Agency: Duluth, MN, pp 1–40. [Google Scholar]
- Erickson RJ, Benoit DA, Mattson VR, Nelson HP Jr., Leonard EN. 1996. “The Effects of Water Chemistry on the Toxicity of Copper to Fathead Minnows,” Environ Toxicol Chem 15(2): 181–193. [Google Scholar]
- Esbaugh AJ, Brix KV, Mager EM, De Schamphelaere KAC, Grosell M. 2012. Multi-linear regression analysis, preliminary biotic ligand modeling, and cross species comparison of the effects of water chemistry on chronic lead toxicity in invertebrates. Comp Biochem Physiol, Part C: Toxicol Pharmacol 155, 423–431. [DOI] [PubMed] [Google Scholar]
- Federal Register. 1992. Water Quality Standards; Establishment of Numeric Criteria for Priority Toxic of Numeric Criteria for Priority Toxic Pollutants; States’ Compliance; Final Rule. 40 CFR Part 131 December 22, 1992, Part 2.
- FWPCA (Federal Water Pollution Control Administration). 1968. Water Quality Criteria (the “Green Book”), Report of the National Technical Advisory Committee to the Secretary of the Interior. U.S. Department of the Interior. Washington, DC. [Google Scholar]
- Gao Y, Feng J, Zhu, L. 2015. Prediction of acute toxicity of cadmium and lead to zebrafish larvae by using a refined toxicokinetic-toxicodynamic model. Aquatic Toxicol. 169: 37–45. [DOI] [PubMed] [Google Scholar]
- Garman ER, Meyer JS, Bergeron CM, Blewett TA, Clements WH, Elias MC, Farley KJ, Gissi F, Ryan AC. 2018. Validation of bioavailability-based toxicity models for metals. Environ Toxicol Chem (in review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JC, Raider RL. 1993. A Reflection on metals criteria. Water Environ Tech 5:63–66. [Google Scholar]
- Hart BT, Campbell IC, Angehrn-Bettinazzi C, Jones MJ. 1993. Australian water quality guidelines: a new approach for protecting ecosystem health. J Aquat Ecosystem Health 2:151–163. [Google Scholar]
- Heijerick DG, De Schamphelaere KAC, Van Sprang PA, Janssen CR, 2005. “Development of a chronic zinc biotic ligand model for Daphnia magna”. Ecotoxicol Environ Saf 62(1):1–10. [DOI] [PubMed] [Google Scholar]
- Herbert DWM, Vandyke JM. 1964. The toxicity to fish of mixtures of poisons. II. Copper-ammonia and zinc-phenol mixtures. Ann Appl Biol 53:415–421. [Google Scholar]
- Janes N, Playle JC. 1995. Modeling Silver Binding to Gills of Rainbow Trout (Oncorhynchus mykiss). Environ Toxicol Chem 14(11):1847–1858. [Google Scholar]
- Khan FR, Paul KB, Dybowska AD, Valsami-Jones E, Lead JR, Stone V, Fernandes TF. 2015. Accumulation dynamics and acute toxicity of silver nanoparticles to Daphnia magna and Lumbriculus variegatus: Implications for metal modeling approaches. Environ Sci Tech 49:4389–4397. [DOI] [PubMed] [Google Scholar]
- Liao CM, Yun R., Chen WY. 2011. Subcellular portioning links BLM-based toxicokinetics for assessing cadmium toxicity to rainbow trout. Environ Contam Toxicol 26: 600–609. [DOI] [PubMed] [Google Scholar]
- Luoma SN., Rainbow PS. 2005. Why is metal bioaccumulation so variable? Biodynamics as a unifying concept. Environ Sci Technol 39: 1921–1931. [DOI] [PubMed] [Google Scholar]
- MacRae RK, Smith DE, Swoboda-Colberg N, Meyer JS, Bergman HL. 1999. Copper binding affinity of rainbow trout (Oncorhynchus mykiss) and brook trout (Salvelinus fontinalis) gills, Environ Toxicol Chem 18:1180–1189. [Google Scholar]
- Mebane CA, Chowdhury MJ, Lofts S, Paquin PR, Santore RC, de Schamphelaere KAC, Wood CM. 2018. Metal bioavailability models: Current status, lessons learned, considerations for regulatory use, and the path forward. Environ Toxicol Chem (in review). [DOI] [PubMed] [Google Scholar]
- Merrington G, Peters A, Schlekat CE. 2016. Accounting for metal bioavailability in assessing water quality: A step change? Environ Toxicol Chem, 35:257–265. [DOI] [PubMed] [Google Scholar]
- Morel FMM, 1983. “Complexation: Trace metals and microorganisms,” in Chapter 6 of Principles of Aquatic Chemistry, Wiley Interscience, New York, pp. 301–308. [Google Scholar]
- National Academy of Science. 1972. Water Quality Criteria (The “Blue Book”), a Report of the Committee on Water Quality Criteria. National Academy of Science and National Academy of Engineering, Washington, DC. NTIS-PB 236199. USGPO #5501–00520. [Google Scholar]
- Nelson H, Benoit D, Erickson R, Mattson V, Lindberg J. 1986. The effect of variable hardness, pH and alkalinity suspended clay and humics on chemical speciation. EPA-600/3–86/023, U.S. Environmental Protection Agency, Office of Research and Development, Duluth, MN USA. [Google Scholar]
- Nys C, Janssen CR, Mager EM, Esbaugh AJ, Brix KV, Grosell M., Stubblefield WA, Holtze K, De Schamphelaere KAC. 2014. Development and validation of a biotic ligand model for predicting chronic toxicity of lead to Ceriodaphnia dubia. Environ Toxicol Chem 33:394–403 [DOI] [PubMed] [Google Scholar]
- Pagenkopf GK. 1983. Gill surface interaction model for trace-metal toxicity to fishes: Role of complexation, pH, and water hardness. Environ Sci Technol 17: 342–347. [Google Scholar]
- Pagenkopf GK, Russo RC, Thurston RV. 1974. Effect of complexation on toxicity of copper to fishes. J Fish Res Bd Can 31:462–465. [Google Scholar]
- Paquin PR, Gorsuch JW, Apte S, Batley GE, Bowles KC, Campbell PGC, Delos CG, Di Toro DM, Dwyer RL, Galvez F, Gensemer RW, Goss GG, Hogstrand C, Janssen CR, McGeer JC, Naddy RB, Playle RC, Santore RC, Schneider U, Stubblefield WA, Wood CM, Wu KB. 2002a. The biotic ligand model: a historical overview. Comp Biochem Physiol C Toxicol Pharmacol 133 (1–2):3–35. [DOI] [PubMed] [Google Scholar]
- Paquin PR, Zoltay V, Winfield RP, Wu KB, Mathew R, Santore RC, Di Toro DM. 2002b. Extension of the biotic ligand model of acute toxicity to a physiologically-based model of the survival time of rainbow trout (Oncorhynchus mykiss) exposed to silver. Comp Biochem Physiol C Toxicol Pharmacol 133(1):305–343. [DOI] [PubMed] [Google Scholar]
- Peters A, Merrington G, Delbeke K, de Schamphelaere KAC. 2011. Regulatory consideration of bioavailability for metals: Simplification of input parameters for the chronic copper Biotic Ligand Model. Int Environ Assess and Mgmt 7:437–444. [DOI] [PubMed] [Google Scholar]
- Peters A, Schlekat C, Merrington G. 2016. Does the scientific underpinning of regulatory tools to estimate bioavailability of nickel in freshwaters matter? The European-wide Environmental Quality Standard for nickel. Environ Toxicol Chem 35:2397–2404. [DOI] [PubMed] [Google Scholar]
- Playle RC, Gensemer RW, Dixon DG. 1992. Copper Accumulation on Gills of Fathead Minnows: Influence of Water Hardness, Complexation and pH on the Gill Micro-environment. Environ Toxicol Chem 11:381–391. [Google Scholar]
- Playle RC, Dixon DG, Burnison KD. 1993a. Copper and cadmium binding to fish gills: Modification by dissolved organic carbon and synthetic ligands. Can J Fish Aquat Sci 50:2667–2677. [Google Scholar]
- Playle RC, Dixon DG, Burnison KD. 1993b. Copper and cadmium binding to fish gills: Estimates of metal-gill stability constants and modeling of metal accumulation, Can J Fish Aquat Sci 50: 2678–2687. [Google Scholar]
- Prothro MG. 1993. Office of Water Policy and Technical Guidance on Interpretation and Implementation of Aquatic Life Metals Criteria, Memorandum from U.S. EPA Acting Assistant Administrator for Water to Water Management Division Directors and Environmental Services Division Directors, Regions I-X, dated October 1, 1993. [Google Scholar]
- Rainbow PS, Smith BD, Luoma SN, 2009. Biodynamic modelling and the prediction of Ag, Cd and Zn accumulation from solution and sediment by the polychaete Nereis diversicolor. Mar Ecol Prog Series 390:145–155. [Google Scholar]
- Santore RC, Ryan AC, Kroglund F, Rodriquez P, Stubblefield W, Cardwell A, Adams W, Nordheim E, 2018. Development and application of a biotic ligand model for predicting the chronic toxicity of dissolved and precipitated aluminum to aquatic organisms. Environ Toxicol Chem 37(1):70–79. [DOI] [PubMed] [Google Scholar]
- Santore RC, Driscoll CT. 1995. “The CHESS Model for Calculating Chemical Equilibria in Soils and Solutions,” Chemical Equilibrium and Reaction Models, SSSA Special Publication 42, The Soil Society of America, American Society of Agronomy. [Google Scholar]
- Santore RC, Di Toro DM, Paquin PR, Allen HE, Meyer JS. 2001. Biotic ligand model of the acute toxicity of metals. 2. Application to acute copper toxicity in freshwater fish and Daphnia. Environ Toxicol Chem 20:2397–2402. [PubMed] [Google Scholar]
- Schecher WD, McAvoy DC. 1992. MINEQL+: A software environment for chemical equilibrium modeling. Computers Environ Urban Syst 16:65–76. [Google Scholar]
- Schlekat CE, Van Genderen E, De Schamphelaere KAC, Antunes PM, Rogevich EC, Stubblefield WA, 2010. Cross-species extrapolation of chronic nickel Biotic Ligand Models. Sci Total Environ 408(24):6148–57. [DOI] [PubMed] [Google Scholar]
- Sprague JB. 1968. Promising anti-pollutant: Chelating agent NTA protects fish from copper and zinc. Nature 220:1345–1346. [DOI] [PubMed] [Google Scholar]
- Stewart AR, Luoma SN, Schlekat SC, Doblin MA, Hieb KA. 2004. Food web pathway determines selenium biomagnification in aquatic organisms. Environ Sci Technol 38:4519–4526. [DOI] [PubMed] [Google Scholar]
- Sunda WG, Lewis JAM. 1978. Effect of complexation by natural organic ligands on the toxicity of copper to a unicellular alga, Monochrysis lutheri. Limnol and Oceanog 23:870–876. [Google Scholar]
- Tipping E. 1994. WHAMC - A chemical equilibrium model and computer code for waters, sediments, and soils incorporating a discrete site/electrostatic model of ion-binding by humic substances. Comput Geosci 20:973–1023. [Google Scholar]
- U.S. EPA. 1973. Quality Criteria for Water 1972” (the “Blue Book”) EPA-R3–73-033. U.S. Environmental Protection Agency, Washington, DC. [Google Scholar]
- U.S. EPA. 1976. Quality Criteria for Water (the “Red Book”). EPA 440/9–76-023. U.S. Environmental Protection Agency, Washington, DC. [Google Scholar]
- U.S. EPA 1980a. Ambient Water Quality Criteria for Copper. EPA 440/5–80-036. Office of Water Regulations and Standards, Washington, DC. [Google Scholar]
- U.S. EPA 1980b. Ambient Water Quality Criteria for Cadmium. EPA 440/5–80-025. Office of Water Regulations and Standards, Washington, DC. [Google Scholar]
- U.S. EPA 1980c. Ambient Water Quality Criteria for Lead. EPA 440/5–80-057. Office of Water Regulations and Standards, Washington, DC. [Google Scholar]
- U.S. EPA 1980d. Ambient Water Quality Criteria for Nickel. EPA 440/5–80-060. Office of Water Regulations and Standards, Washington, DC. [Google Scholar]
- U.S. EPA 1980e. Ambient Water Quality Criteria for Zinc. EPA 440/5–80-079. Office of Water Regulations and Standards, Washington, DC. [Google Scholar]
- U.S. EPA. 1983. Water Quality Standards Handbook. Office of Water Regulations and Standards, Washington, D: C. (1st Ed., Out of Print.) [Google Scholar]
- U.S. EPA. 1984. Guidelines for Deriving Numerical Aquatic Site-Specific Water Quality Criteria by Modifying National Criteria. Office of Research and Development. Duluth, MN. (Out of print; accessible at: https://cfpub.epa.gov/si/si_public_record_Report.cfm?dirEntryID=47806) [Google Scholar]
- U.S. EPA. 1985a. Guidelines for Deriving Numerical National Water Quality Criteria for the Protection of Aquatic Organisms and Their Uses. PB85–227049. [Google Scholar]
- U.S. EPA. 1985b. Ambient Water Quality Criteria for Copper – 1984. EPA440/5–84/031. Office of Water Regulations and Standards, Washington, DC. [Google Scholar]
- U.S. EPA 1985c. Ambient Water Quality Criteria for Cadmium – 1984. EPA 440/5–84-032. Office of Water Regulations and Standards, Washington, DC. [Google Scholar]
- U.S. EPA 1985d. Ambient Water Quality Criteria for Lead – 1984. EPA 440/5–84-027. Office of Water Regulations and Standards, Washington, DC. [Google Scholar]
- U.S. EPA. 1986. Quality Criteria for Water (the “Gold Book”). EPA 440/5–86-001. U.S. Environmental Protection Agency, Washington, DC. [Google Scholar]
- U.S. EPA. 1991. Methods for the Determination of Metals in Environmental Samples. EPA/600/4–91/010 (NTIS PB91231498). U.S. Environmental Protection Agency, Washington, DC. [Google Scholar]
- U.S. EPA 1992. Interim Guidance on Interpretation and Implementation of Aquatic Life Criteria for Metals. EPA 821/R-92–009. May 1992. Office of Science and Technology, Washington, DC. U.S. EPA 1992. [Google Scholar]
- U.S. EPA 1993. Workshop on aquatic life criteria for metals – short-term recommendations. Jan. 25–29, 1993. Annapolis MD. Federal Register, July 9, 1993. [Google Scholar]
- U.S. EPA, 1994. Water Quality Standards Handbook: Second Edition, Appendix L, Interim Guidance on Determination and Use of Water Effect Ratios for Metals. EPA-823-B-94–005a. U.S. EPA Office of Water, Washington, DC. [Google Scholar]
- U.S. EPA 1996. The Metals Translator: Guidance for Calculating a Total Recoverable Permit Limit. From A Dissolved Criterion. U.S. EPA Office of Water. EPA 823-B-96–007. [Google Scholar]
- U.S. EPA. 2000. An SAB Report: Review of the Biotic Ligand Model of acute toxicity to metals EPA-SAB-EPEC-00–006. U.S. EPA Office of Water, Washington, DC. [Google Scholar]
- U.S. EPA. 2001. Streamlined Water Effect Ratio Procedure for Discharges of Copper. EPA 822-R-01–005. U.S. EPA Office of Water, Washington, DC. [Google Scholar]
- U.S. EPA 2003. Ambient Water Quality Criteria for Copper. – Draft Update. EPA 822-R-03–026. U.S. EPA Office of Water, Washington, DC. [Google Scholar]
- U.S. EPA, 2007. Aquatic Life Ambient Freshwater Quality Criteria – Copper. EPA-822-R-07–001. U.S. EPA Office of Water, Washington, DC. [Google Scholar]
- Van Campenhout K, Bervoets L, Redeker ES, Blust R. 2009. A kinetic model for the relative contribution of waterborne and dietary cadmium and zinc in the common carp (Cyprinus carpio). Environ Toxicol Chem 28:209–219. [DOI] [PubMed] [Google Scholar]
- Van Genderen EJ, Stauber JL, Delos C, Eignor D, Gensemer RW, McGeer J, Merrington G, Whitehouse P. 2018. Derivation and application of water quality criteria for metals using bioavailability-based approaches. Environ Toxicol Chem (in review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace WG, Lopez GR. 1997. Bioavailability of biologically sequestered cadmium and the implications of metal detoxification. Mar Ecology Progress Series 147:149–157. [Google Scholar]
- Warne M, Batley GE, van Dam RA, Chapman JC, Fox DR, Hickey CW, Stauber JL. 2015. Revised Method for Deriving Australian and New Zealand Water Quality Guideline Values for Toxicants. Updated October 2017. Prepared for the Council of Australian Government’s Standing Council on Environment and Water (SCEW). Department of Science, Information Technology and Innovation, Brisbane, Queensland. 48 pp. [Google Scholar]
- Westall J, Zachary JL, Morel FMM. 1976. MINEQL: A computer program for the calculation of chemical equilibrium composition of aqueous systems. Technical Note 18, Massachusetts Institute of Technology, Cambridge, MA. [Google Scholar]
- Zitko P, Carson WV, Carson WG. 1973. Prediction of incipient lethal levels of copper to juvenile Atlantic salmon in the presence of humic acid by cupric electrode. Environ Contam Toxicol 10:265. [DOI] [PubMed] [Google Scholar]
- Zitko V, Carson WG. 1976. A mechanism of the effects of water hardness on the lethality of heavy metals to fish. Chemosphere 5 (5):299–303. [Google Scholar]