Abstract
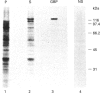
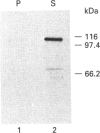
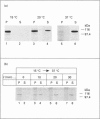
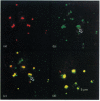
Plasmodium falciparum, a protozoan parasite of the human erythrocyte, causes the most severe form of malaria. During its intraerythrocytic development, the parasite synthesizes proteins which are exported into the host cell. The compartments involved in the secretory pathway of P. falciparum are still poorly characterized. A Golgi apparatus has not been identified, owing to the lack of specific protein markers and Golgi-specific post-translational modifications in the parasite. The fungal metabolite brefeldin A (BFA) is known to inhibit protein secretion in higher eukaryotes by disrupting the integrity of the Golgi apparatus. We have used the parasite-encoded glycophorin-binding protein (GBP), a soluble protein found in the host cell cytoplasm, as a marker to investigate the effects of BFA on protein secretion in the intracellular parasite. In the presence of BFA, GBP was not transported into the erythrocyte, but remained inside the parasite cell. The effect caused by BFA was reversible, and the protein could be chased into the host cell cytoplasm within 30 min. Transport of GBP from the BFA-sensitive site into the host cell did not require protein synthesis. Similar observations were made when infected erythrocytes were incubated at 15 degrees C. Incubation at 20 degrees C resulted in a reduction rather than a complete block of protein export. The relevance of our findings to the identification of compartments involved in protein secretion from the parasite cell is discussed.
Full text
PDF
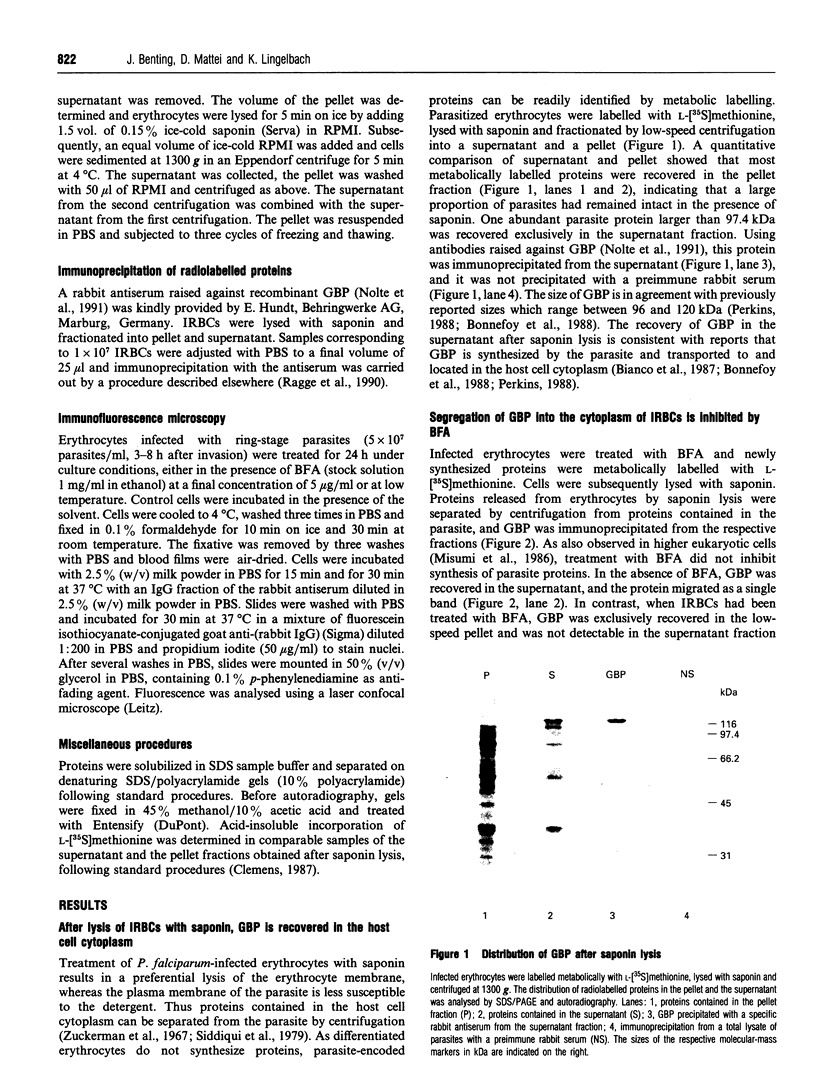
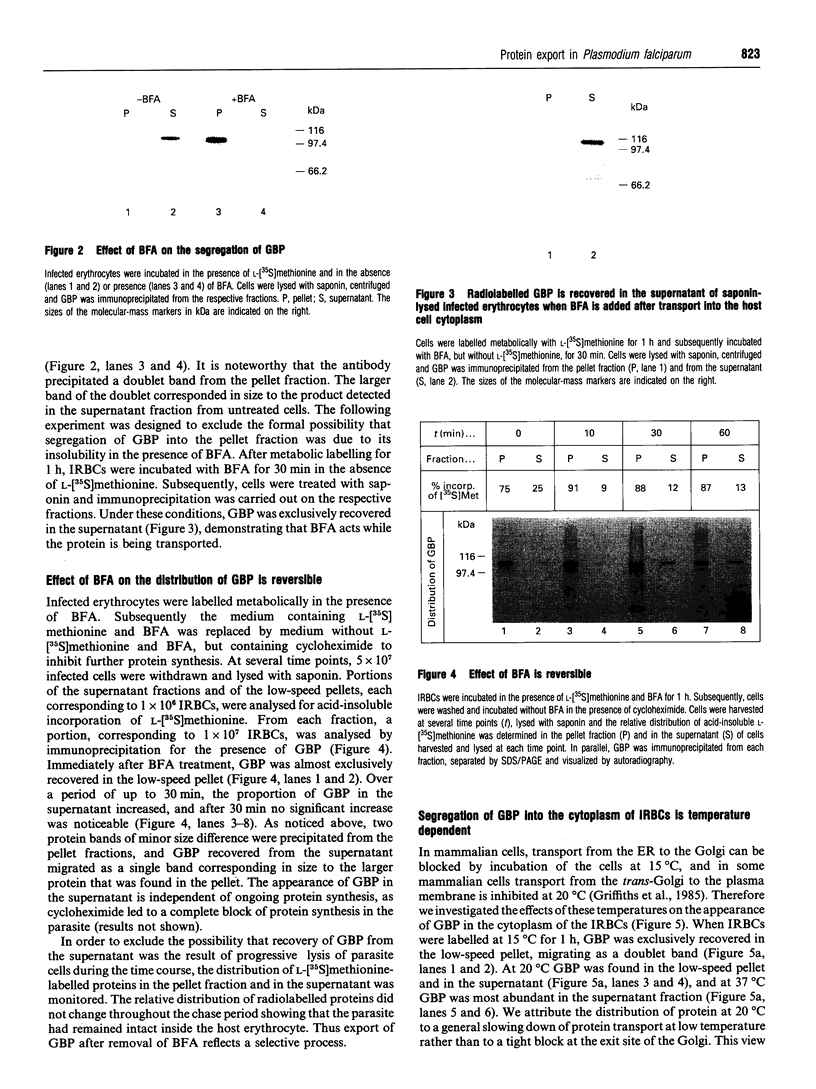



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannister L. H., Dluzewski A. R. The ultrastructure of red cell invasion in malaria infections: a review. Blood Cells. 1990;16(2-3):257–297. [PubMed] [Google Scholar]
- Barnwell J. W. Vesicle-mediated transport of membrane and proteins in malaria-infected erythrocytes. Blood Cells. 1990;16(2-3):379–395. [PubMed] [Google Scholar]
- Bianco A. E., Culvenor J. G., Coppel R. L., Crewther P. E., McIntyre P., Favaloro J. M., Brown G. V., Kemp D. J., Anders R. F. Putative glycophorin-binding protein is secreted from schizonts of Plasmodium falciparum. Mol Biochem Parasitol. 1987 Feb;23(1):91–102. doi: 10.1016/0166-6851(87)90191-5. [DOI] [PubMed] [Google Scholar]
- Bonnefoy S., Mattei D., Dubremetz J. F., Guillotte M., Jouin H., Ozaki L. S., Sibilli L., Mercereau-Puijalon O. Plasmodium falciparum: molecular analysis of a putative protective antigen, the thermostable 96-kDa protein. Exp Parasitol. 1988 Feb;65(1):69–83. doi: 10.1016/0014-4894(88)90108-7. [DOI] [PubMed] [Google Scholar]
- Chege N. W., Pfeffer S. R. Compartmentation of the Golgi complex: brefeldin-A distinguishes trans-Golgi cisternae from the trans-Golgi network. J Cell Biol. 1990 Sep;111(3):893–899. doi: 10.1083/jcb.111.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crary J. L., Haldar K. Brefeldin A inhibits protein secretion and parasite maturation in the ring stage of Plasmodium falciparum. Mol Biochem Parasitol. 1992 Jul;53(1-2):185–192. doi: 10.1016/0166-6851(92)90020-k. [DOI] [PubMed] [Google Scholar]
- Dieckmann-Schuppert A., Bender S., Odenthal-Schnittler M., Bause E., Schwarz R. T. Apparent lack of N-glycosylation in the asexual intraerythrocytic stage of Plasmodium falciparum. Eur J Biochem. 1992 Apr 15;205(2):815–825. doi: 10.1111/j.1432-1033.1992.tb16846.x. [DOI] [PubMed] [Google Scholar]
- Doms R. W., Russ G., Yewdell J. W. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J Cell Biol. 1989 Jul;109(1):61–72. doi: 10.1083/jcb.109.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson J. G., Finazzi D., Klausner R. D. Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature. 1992 Nov 26;360(6402):350–352. doi: 10.1038/360350a0. [DOI] [PubMed] [Google Scholar]
- Donaldson J. G., Lippincott-Schwartz J., Bloom G. S., Kreis T. E., Klausner R. D. Dissociation of a 110-kD peripheral membrane protein from the Golgi apparatus is an early event in brefeldin A action. J Cell Biol. 1990 Dec;111(6 Pt 1):2295–2306. doi: 10.1083/jcb.111.6.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmendorf H. G., Haldar K. Identification and localization of ERD2 in the malaria parasite Plasmodium falciparum: separation from sites of sphingomyelin synthesis and implications for organization of the Golgi. EMBO J. 1993 Dec;12(12):4763–4773. doi: 10.1002/j.1460-2075.1993.tb06165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmendorf H. G., Haldar K. Secretory transport in Plasmodium. Parasitol Today. 1993 Mar;9(3):98–102. doi: 10.1016/0169-4758(93)90216-3. [DOI] [PubMed] [Google Scholar]
- Feder R., Blobel G. In vitro biosynthesis and core glycosylation of the histidine-rich protein of Plasmodium lophurae. Mol Biochem Parasitol. 1983 Dec;9(4):351–362. doi: 10.1016/0166-6851(83)90091-9. [DOI] [PubMed] [Google Scholar]
- Futerman A. H., Stieger B., Hubbard A. L., Pagano R. E. Sphingomyelin synthesis in rat liver occurs predominantly at the cis and medial cisternae of the Golgi apparatus. J Biol Chem. 1990 May 25;265(15):8650–8657. [PubMed] [Google Scholar]
- Griffiths G., Pfeiffer S., Simons K., Matlin K. Exit of newly synthesized membrane proteins from the trans cisterna of the Golgi complex to the plasma membrane. J Cell Biol. 1985 Sep;101(3):949–964. doi: 10.1083/jcb.101.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther K., Tümmler M., Arnold H. H., Ridley R., Goman M., Scaife J. G., Lingelbach K. An exported protein of Plasmodium falciparum is synthesized as an integral membrane protein. Mol Biochem Parasitol. 1991 May;46(1):149–157. doi: 10.1016/0166-6851(91)90208-n. [DOI] [PubMed] [Google Scholar]
- Haldar K., Uyetake L., Ghori N., Elmendorf H. G., Li W. L. The accumulation and metabolism of a fluorescent ceramide derivative in Plasmodium falciparum-infected erythrocytes. Mol Biochem Parasitol. 1991 Nov;49(1):143–156. doi: 10.1016/0166-6851(91)90137-u. [DOI] [PubMed] [Google Scholar]
- Helms J. B., Rothman J. E. Inhibition by brefeldin A of a Golgi membrane enzyme that catalyses exchange of guanine nucleotide bound to ARF. Nature. 1992 Nov 26;360(6402):352–354. doi: 10.1038/360352a0. [DOI] [PubMed] [Google Scholar]
- Jeckel D., Karrenbauer A., Birk R., Schmidt R. R., Wieland F. Sphingomyelin is synthesized in the cis Golgi. FEBS Lett. 1990 Feb 12;261(1):155–157. doi: 10.1016/0014-5793(90)80659-7. [DOI] [PubMed] [Google Scholar]
- Kochan J., Perkins M., Ravetch J. V. A tandemly repeated sequence determines the binding domain for an erythrocyte receptor binding protein of P. falciparum. Cell. 1986 Mar 14;44(5):689–696. doi: 10.1016/0092-8674(86)90834-2. [DOI] [PubMed] [Google Scholar]
- Lingappa V. R., Lingappa J. R., Blobel G. Chicken ovalbumin contains an internal signal sequence. Nature. 1979 Sep 13;281(5727):117–121. doi: 10.1038/281117a0. [DOI] [PubMed] [Google Scholar]
- Lingelbach K. R. Plasmodium falciparum: a molecular view of protein transport from the parasite into the host erythrocyte. Exp Parasitol. 1993 May;76(3):318–327. doi: 10.1006/expr.1993.1039. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Donaldson J. G., Schweizer A., Berger E. G., Hauri H. P., Yuan L. C., Klausner R. D. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990 Mar 9;60(5):821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L. C., Bonifacino J. S., Klausner R. D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989 Mar 10;56(5):801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L., Tipper C., Amherdt M., Orci L., Klausner R. D. Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 1991 Nov 1;67(3):601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- Matlin K. S., Simons K. Reduced temperature prevents transfer of a membrane glycoprotein to the cell surface but does not prevent terminal glycosylation. Cell. 1983 Aug;34(1):233–243. doi: 10.1016/0092-8674(83)90154-x. [DOI] [PubMed] [Google Scholar]
- Meek R. L., Walsh K. A., Palmiter R. D. The signal sequence of ovalbumin is located near the NH2 terminus. J Biol Chem. 1982 Oct 25;257(20):12245–12251. [PubMed] [Google Scholar]
- Misumi Y., Misumi Y., Miki K., Takatsuki A., Tamura G., Ikehara Y. Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J Biol Chem. 1986 Aug 25;261(24):11398–11403. [PubMed] [Google Scholar]
- Nolte D., Hundt E., Langsley G., Knapp B. A Plasmodium falciparum blood stage antigen highly homologous to the glycophorin binding protein GBP. Mol Biochem Parasitol. 1991 Dec;49(2):253–264. doi: 10.1016/0166-6851(91)90069-i. [DOI] [PubMed] [Google Scholar]
- Orci L., Tagaya M., Amherdt M., Perrelet A., Donaldson J. G., Lippincott-Schwartz J., Klausner R. D., Rothman J. E. Brefeldin A, a drug that blocks secretion, prevents the assembly of non-clathrin-coated buds on Golgi cisternae. Cell. 1991 Mar 22;64(6):1183–1195. doi: 10.1016/0092-8674(91)90273-2. [DOI] [PubMed] [Google Scholar]
- Pasvol G., Wilson R. J., Smalley M. E., Brown J. Separation of viable schizont-infected red cells of Plasmodium falciparum from human blood. Ann Trop Med Parasitol. 1978 Feb;72(1):87–88. doi: 10.1080/00034983.1978.11719283. [DOI] [PubMed] [Google Scholar]
- Perkins M. Stage-dependent processing and localization of a Plasmodium falciparum protein of 130,000 molecular weight. Exp Parasitol. 1988 Feb;65(1):61–68. doi: 10.1016/0014-4894(88)90107-5. [DOI] [PubMed] [Google Scholar]
- Ragge K., Arnold H. H., Tümmler M., Knapp B., Hundt E., Lingelbach K. In vitro biosynthesis and membrane translocation of the serine rich protein of Plasmodium falciparum. Mol Biochem Parasitol. 1990 Aug;42(1):93–100. doi: 10.1016/0166-6851(90)90116-4. [DOI] [PubMed] [Google Scholar]
- Randazzo P. A., Yang Y. C., Rulka C., Kahn R. A. Activation of ADP-ribosylation factor by Golgi membranes. Evidence for a brefeldin A- and protease-sensitive activating factor on Golgi membranes. J Biol Chem. 1993 May 5;268(13):9555–9563. [PubMed] [Google Scholar]
- Reaves B., Banting G. Perturbation of the morphology of the trans-Golgi network following Brefeldin A treatment: redistribution of a TGN-specific integral membrane protein, TGN38. J Cell Biol. 1992 Jan;116(1):85–94. doi: 10.1083/jcb.116.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J., Kuismanen E. Pre- and post-Golgi vacuoles operate in the transport of Semliki Forest virus membrane glycoproteins to the cell surface. Cell. 1984 Sep;38(2):535–549. doi: 10.1016/0092-8674(84)90508-7. [DOI] [PubMed] [Google Scholar]
- Siddiqui W. A., Kan S. C., Kramer K., Richmond-Crum S. M. In vitro production and partial purification of Plasmodium falciparum antigen. Bull World Health Organ. 1979;57 (Suppl 1):75–82. [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Tsai S. C., Adamik R., Haun R. S., Moss J., Vaughan M. Effects of brefeldin A and accessory proteins on association of ADP-ribosylation factors 1, 3, and 5 with Golgi. J Biol Chem. 1993 May 25;268(15):10820–10825. [PubMed] [Google Scholar]
- Wood S. A., Park J. E., Brown W. J. Brefeldin A causes a microtubule-mediated fusion of the trans-Golgi network and early endosomes. Cell. 1991 Nov 1;67(3):591–600. doi: 10.1016/0092-8674(91)90533-5. [DOI] [PubMed] [Google Scholar]
- Zuckerman A., Spira D., Hamburger J. A procedure for the harvesting of mammalian plasmodia. Bull World Health Organ. 1967;37(3):431–436. [PMC free article] [PubMed] [Google Scholar]