Abstract
Background
This study aimed to construct a novel nomogram based on the number of positive lymph nodes to predict the overall survival of patients with pancreatic head cancer after radical surgery.
Materials and methods
2271 and 973 patients in the SEER Database were included in the development set and validation set, respectively. The primary clinical endpoint was OS (overall survival). Univariate and multivariate Cox regression analyses were used to screen independent risk factors of OS, and then independent risk factors were used to construct a novel nomogram. The C-index, calibration curves, and decision analysis curves were used to evaluate the predictive power of the nomogram in the development and validation sets.
Results
After multivariate Cox regression analysis, the independent risk factors for OS included age, tumor extent, chemotherapy, tumor size, LN (lymph nodes) examined, and LN positive. A nomogram was constructed by using independent risk factors for OS. The C-index of the nomogram for OS was 0.652 [(95% confidence interval (CI): 0.639–0.666)] and 0.661 (95%CI: 0.641–0.680) in the development and validation sets, respectively. The calibration curves and decision analysis curves proved that the nomogram had good predictive ability.
Conclusions
The nomogram based on the number of positive LN can effectively predict the overall survival of patients with pancreatic head cancer after surgery.
Keywords: Lymph node, Nomogram, Overall survival (OS), Pancreatic head cancer, SEER database
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a malignant tumor with a poor prognosis, with a 5-year survival rate of less than 10% [1, 2]. And with the increase of incidence, it will rise to the second leading cause of cancer death in the future [3]. Pancreatic cancer has the characteristics of early metastasis and rapid progression, so many patients with pancreatic head cancer are diagnosed at an advanced stage and have no chance of radical surgery [4]. Patients with early pancreatic head cancer have the opportunity of radical surgery, and the surgical method of pancreatic head cancer is pancreaticoduodenectomy (PD) [5]. When patients with pancreatic head cancer undergo radical surgery, lymph node dissection is an important issue, because if some early metastatic LN are not dissected, the prognosis of patients will be affected [6]. Therefore, the extent of intraoperative lymph node dissection and postoperative lymph node positivity will affect the prognosis of patients [7, 8]. To improve the prognosis of patients with pancreatic head cancer, many studies have investigated the extent of lymph node dissection in PD. The Heidelberg Triangle proposed by Heidelberg University has become a hot topic in pancreatic surgery [9, 10].
However, whether the number of positive LN is related to the survival of patients has not been proven. This study aimed to explore the effect of the number of positive LN on the prognosis of patients and to construct a novel nomogram based on the number of positive LN to predict the overall survival of patients with pancreatic head cancer after radical surgery.
Materials and methods
Data source and patient selection
The data of the patients in this study were obtained from the SEER database. The SEER database was a cancer repository established by the National Cancer Institute and contained data on cancer diagnosis, treatment, and survival for approximately 30% of the population in the United States. Inclusion criteria for patients in the study included: (1) was diagnosed with pancreatic head cancer between 2000 and 2015; (2) had undergone radical resection with lymphadenectomy; (3) postoperative pathology showed pancreatic ductal adenocarcinoma. The exclusion criteria for this study were as follows: (1) absence of key variables, such as the number of LN examined and the number of positive LN; (2) incomplete surgical information, including resection of the primary site and dissection of regional LN; (3) incomplete survival data. Included patients were randomly assigned 7:3 to the development and validation sets.
Data description
The clinical baseline data of the patients in this study included age, sex, tumor extent, AJCC (the American Joint Committee on Cancer) stage, AJCC T, AJCC N, AJCC M, LN scope SUR (surgery), chemotherapy, tumor size, LN examined, LN positive, survival status, and OS. The TNM stage included in this study was based on the AJCC sixth edition. Survival data included vital status at the cut-off date of follow-up and survival months. The primary clinical endpoint was OS, defined as the time from diagnosis to death, or from diagnosis to the end of follow-up if the status was still alive.
Statistical analysis
Clinical data were extracted by using the client-server model of SEER*Stat 8.4.3. All statistical analyses in this study were performed by using SPSS 26.0 software and R software version 4.2.3. Continuous numerical variables in baseline data that did not follow a normal distribution were described with the use of median and IQR (interquartile range). Categorical variables were described by using counts and percentages. Continuous numerical variables between the two sets were compared by using the Mann-Whitney U test. Categorical variables between the two sets were compared by using the chi-square test. Univariate and multivariate Cox regression analyses were used to screen independent risk factors for OS. Independent risk factors were then used to construct a novel nomogram. The predictive performance of the nomogram was evaluated by calculating the C-index. Calibration curves for 1 -, 3 -, and 5-year OS were generated to observe the difference between the predicted and actual survival rates in the development and validation sets. The clinical benefit of the model was observed by comparing the decision analysis curves between the nomogram and conventional AJCC TNM stage. During the statistical analysis, P-value < 0.05 was considered statistically significant.
Results
Clinical characteristics of the included patients
A total of 3244 eligible patients were included in this study. According to randomization in a 7:3 ratio, 2271 and 973 patients were included in the development and validation sets, respectively. Clinical baseline data for patients in the development and validation sets are shown in Table 1. There were statistical differences in age, tumor size, and LN examined between the two sets. Most patients in the development (94.5%) and validation (95.2%) sets had 4 or more lymph node dissection during surgery. The median number of LN examined and LN positive in the development set was 14 (9–21) and 1 (0–4), respectively. And the median number of LN examined and LN positive in the validation set was 16 (11–21) and 2 (0–4), respectively. The median OS of patients in the development set was 21 (11–45) months, and the median OS of patients in the validation set was 22 (11–45) months. There was no statistical difference between the two sets for comparing the median OS ((p-value = 0.165).
Table 1.
General characteristics of development set and validation set
| Variables | Development set(n = 2271) | Validation set (n = 973) |
P value | |
|---|---|---|---|---|
| Age | <65 | 894(39.4%) | 442(45.4%) | 0.001 |
| ≥ 65 | 1377(60.6%) | 531(54.6%) | ||
| Sex | Male | 1183(52.1%) | 498(51.2%) | 0.635 |
| Female | 1088(47.9%) | 475(48.8%) | ||
|
Tumor Extent |
Local | 195(8.6%) | 83(8.5%) | 0.361 |
| Region | 1915(84.3%) | 807(82.9%) | ||
| Distant | 161(7.1%) | 83(8.5%) | ||
| AJCC Stage | I | 195(8.6%) | 83(8.5%) | 0.064 |
| II | 1922(84.6%) | 799(82.1%) | ||
| III | 76(3.3%) | 50(5.1%) | ||
| IV | 78(3.4%) | 41(4.2%) | ||
| AJCC T | 1 | 119(5.2%) | 61(6.3%) | 0.077 |
| 2 | 267(11.8%) | 110(11.3%) | ||
| 3 | 1803(79.4%) | 750(77.1%) | ||
| 4 | 82(3.6%) | 52(5.3%) | ||
| AJCC N | 0 | 716(31.5%) | 281(28.9%) | 0.134 |
| 1 | 1555(68.5%) | 692(71.1%) | ||
| AJCC M | 0 | 2193(96.6%) | 932(95.8%) | 0.279 |
| 1 | 78(3.4%) | 41(4.2%) | ||
| LN scope SUR | 1 to 3 | 126(5.5%) | 47(4.8%) | 0.404 |
| 4 or more | 2145(94.5%) | 926(95.2%) | ||
| Chemotherapy | No | 636(28.0%) | 248(25.5%) | 0.140 |
| Yes | 1635(72.0%) | 725(74.5%) | ||
| Tumor Size (mm) | 30(25–40) | 30(23–40) | 0.039 | |
| LN examined | 14(9–21) | 16(11–21) | <0.001 | |
| LN positive | 1(0–4) | 2(0–4) | 0.434 | |
| Status | Alive | 257(11.3%) | 131(13.5%) | 0.084 |
| Dead | 2014(88.7%) | 842(86.5%) | ||
| OS (months) | 21(11–45) | 22(11–45) | 0.165 | |
AJCC, the American Joint Committee on Cancer; LN, lymph nodes; SUR, surgery; OS, overall survival
The construction of the nomogram
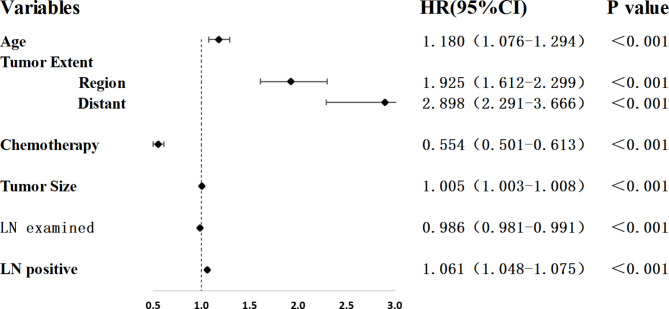
The AJCC TNM stage was variable for comparison in this study, so Cox regression analysis was not performed for the AJCC TNM stage. As shown in Fig. 1 and Table 2, after univariate and multivariate Cox regression analyses in the development set, independent risk factors for OS included age (HR = 1.180; 95%CI: 1.076, 1.294; p < 0.001), tumor extent [Region (HR = 1.925; 95%CI: 1.612, 2.299); Distant (HR = 2.898; 95%CI: 2.291, 3.666); p < 0.001], chemotherapy (HR = 0.554; 95%CI: 0.501, 0.613; p < 0.001), tumor size (HR = 1.005; 95%CI: 1.003, 1.008; p < 0.001), LN examined (HR = 0.986; 95%CI: 0.981, 0.991; p < 0.001), and LN positive (HR = 1.061; 95%CI: 1.048, 1.075; p < 0.001). These independent risk factors were used to construct a novel nomogram to predict 1-year OS, 3-year OS, and 5-year OS. The novel nomogram is shown in Fig. 2.
Fig. 1.
Multivariate Cox regression analysis of LN positive and other clinical characteristics in the Development set. HR, hazard ratio; CI, confidence interval; LN, lymph nodes
Table 2.
Univariate and multivariate analysis of os in development set
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | ||
| Age (≥ 65) |
1.272 (1.162,1.392) |
<0.001 |
1.180 (1.076,1.294) |
<0.001 | |
| Sex(Male) |
1.068 (0.978,1.165) |
0.142 | |||
| Tumor Extent | Region |
1.801 (1.519,2.135) |
<0.001 |
1.925 (1.612,2.299) |
<0.001 |
| Distant |
2.804 (2.230,3.525) |
2.898 (2.291,3.666) |
|||
|
LN scope SUR (4 or more) |
0.878 (0.728,1.059) |
0.173 | |||
| Chemotherapy (Yes) |
0.610 (0.554,0.672) |
<0.001 |
0.554 (0.501,0.613) |
<0.001 | |
| Tumor Size (mm) |
1.008 (1.005,1.010) |
<0.001 |
1.005 (1.003,1.008) |
<0.001 | |
| LN examined |
0.993 (0.989,0.998) |
0.004 |
0.986 (0.981,0.991) |
<0.001 | |
| LN positive |
1.056 (1.044,1.068) |
<0.001 |
1.061 (1.048,1.075) |
<0.001 | |
OS, overall survival; HR, hazard ratio; CI, confidence interval; LN, lymph nodes; SUR, surgery
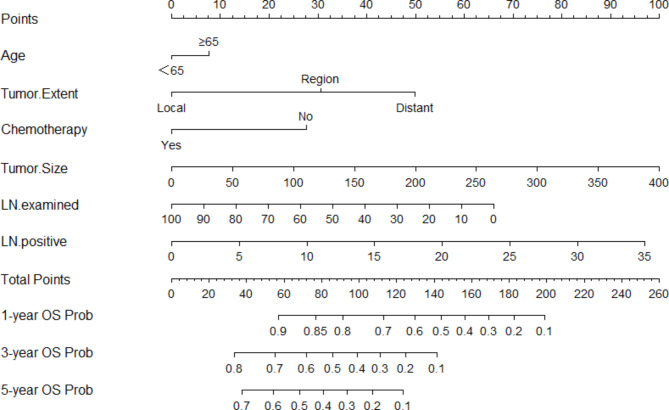
Fig. 2.
A novel nomogram was constructed combining LN positive with other independent risk factors of patients with pancreatic head cancer undergoing radical surgery to predict OS. LN, lymph nodes; OS, overall survival
Assessment of the predictive performance
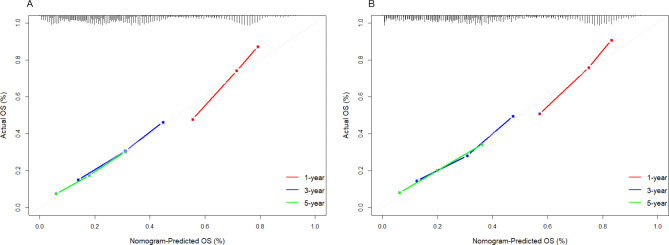
The C-index of this nomogram for OS in the development set and validation set is 0.652 [95% confidence interval (CI): 0.639–0.666)] and 0.661 (95%CI: 0.641–0.680), respectively. The C-index indicates that the nomogram has good predictive ability. As shown in Fig. 3, the calibration curves for 1-year OS, 3-year OS, and 5-year OS in the development and validation sets are close to the reference line. This means that the predicted survival rates of the nomogram are close to the actual survival rates.
Fig. 3.
Calibration curves for the nomogram predicting 1-year, 3-year, and 5-year survival of patients in the Development set (A) and the Validation set (B). OS, overall survival
To assess the clinical utility of the nomogram, decision analysis curves are used to compare the net benefit and clinical utility of the nomogram with the conventional AJCC TNM stage. As shown in Fig. 4, the decision analysis curves for 1-year OS, 3-year OS, and 5-year OS in both the development and validation sets show that the constructed nomogram has a larger area than the conventional AJCC TNM stage. This indicates that the nomogram constructed in this study can provide more clinical benefit than the conventional AJCC TNM stage.
Fig. 4.
The decision curves evaluate the 1-, 3-, and 5-year clinical benefit of the nomogram and compare the clinical benefit between the nomogram and conventional AJCC TNM stage in the Development (A-C) and Validation (D-F) set. LN, lymph nodes; AJCC, the American Joint Committee on Cancer
Discussion
LN is an important prognostic factor for patients with pancreatic head cancer, which affects postoperative pathological stage and adjuvant therapy [11]. Positive postoperative lymph node is an independent risk factor for the prognosis of patients with pancreatic head cancer [12, 13]. In the AJCC TNM stage, lymph node positivity determines the N stage. In the eighth edition of the AJCC TNM stage, the number of LN has further become the criterion for reference to the N stage [7, 14]. However, due to the anatomy of patients, the experience and technology of operators, neoadjuvant chemotherapy, and the extent of lymph node dissection, the number of LN examined after radical surgery is small in some patients, so the number of positive LN may be inaccurate or even negative. The ratio of the number of positive LN to the number of examined LN in this case does not represent the true level of the lymph node condition of patients with pancreatic head cancer. In this study, the number of examined LN and the number of positive LN were respectively used as variables to explore their relationship with the overall survival of patients with pancreatic head cancer after surgery and a novel nomogram was constructed based on the number of positive LN to predict the overall survival of patients with pancreatic head cancer after radical surgery.
Factors found to be associated with prognosis in this study included age, tumor extent, chemotherapy, tumor size, LN examined, and LN positive. Age is not a contraindication for surgery in patients with pancreatic head cancer [15, 16]. However, elderly patients may have more underlying diseases and poor nutritional status, which may lead to fatal complications and affect the survival of patients [17]. Early pancreatic head cancer is resectable, with a high R0 resection rate, no regional lymph node metastasis and distant metastasis, and a good prognosis [18, 19]. Vascular invasion, regional lymph node metastasis, and even distant lymph node or organ metastasis in pancreatic head cancer indicate that the tumor is in the borderline resectable or advanced stage, which may indicate a high degree of malignancy, strong invasion, and poor prognosis [20]. For pancreatic cancer, a malignant tumor with poor biological behavior, the role of systemic chemotherapy is very important. The role of chemotherapy includes reducing tumor volume, reducing tumor burden, eliminating micrometastases, and thereby reducing postoperative recurrence and metastasis. Many studies have shown that both preoperative neoadjuvant therapy and postoperative adjuvant therapy have a great impact on the prognosis of patients with pancreatic cancer [21–23]. Tumor size represents the tumor burden, which can determine the tumor stage, and even affect the treatment and surgical method of patients with pancreatic head cancer. A larger tumor size may indicate a poor prognosis [24, 25]. Studies have found that even small tumor size is associated with poor prognosis once positive LN are present [26]. This study found that the number of examined LN was also an independent risk factor for the prognosis of patients, and more examined LN were associated with a good prognosis. More LN removed means that more potential metastases are eliminated, and the chance of achieving micro-level radical resection is greater, which can be the reason for the better prognosis [27, 28]. Positive LN determines the advanced stage in patients with pancreatic head cancer. As in existing studies, the presence of positive LN indicates a poor prognosis for the patient [12, 14, 29].
In this study, a novel nomogram was constructed based on the number of positive LN combined with other independent risk factors to predict the overall survival of patients with pancreatic head cancer after radical surgery. Age, tumor extent, chemotherapy, tumor size, LN examined, and LN positive are variables that are readily available in the clinic. This indicates that the nomogram constructed in this study is simple and can be promoted in other institutions. Through the verification of the C-index and calibration curves, it can be proved that the nomogram has good predictive ability. To further evaluate its performance, the decision analysis curves of the nomogram and conventional AJCC TNM stage were compared in this study. Figure 4 shows that this nomogram has greater clinical utility. This indicates that the nomogram is effective and practical.
However, this study has some limitations. First of all, this study is a retrospective database study, and there is a lack of multicenter, prospective studies to verify the results. Secondly, the data of this study came from a database, and some important prognostic variables were difficult to obtain, such as liver function, nutritional status, chemotherapy regimen, and other information about patients. They could not be included in the model and could not further improve the predictive power of the model.
Conclusion
In conclusion, this study demonstrated that the number of LN examined and positive LN after radical surgery was closely related to OS in patients with pancreatic head cancer. The nomogram based on the number of positive LN can effectively predict the OS of patients with pancreatic head cancer after radical surgery.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Ke You, Kai Lei, Xingxing Wang, Run Hu, Huizhi Zhang, Jie Xu, and Zuojin Liu. The first draft of the manuscript was written by Ke You and Kai Lei, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No.82070678).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics statement
According to the Office for Human Research Protection, the study involved data from non-human subjects, and because of its openness and non-identifying nature, they were investigated through the US Department of Health and Human Services. Written informed consent from the patients/participants was not required to participate in this study. This study was reviewed and approved by the Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University. This study complied with the Declaration of Helsinki.
Conflict of interest
All authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Statistics C et al. 2021. CA Cancer J Clin, 2021. 71(1): pp. 7–33.10.3322/caac.21654
- 2.Rahib L, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–21. 10.1158/0008-5472.Can-14-0155. 10.1158/0008-5472.CAN-14-0155 [DOI] [PubMed] [Google Scholar]
- 3.Rahib L, et al. Estimated projection of US Cancer incidence and death to 2040. JAMA Netw Open. 2021;4(4):e. 10.1001/jamanetworkopen.2021.4708. 10.1001/jamanetworkopen.2021.4708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mizrahi JD, et al. Pancreatic cancer. Lancet. 2020;395(10242):2008–20. 10.1016/s0140-6736(20)30974-0. 10.1016/S0140-6736(20)30974-0 [DOI] [PubMed] [Google Scholar]
- 5.Schneider M, et al. Technical advances in surgery for pancreatic cancer. Br J Surg. 2021;108(7):777–85. 10.1093/bjs/znab133. 10.1093/bjs/znab133 [DOI] [PubMed] [Google Scholar]
- 6.Tanaka K, et al. Appropriate Lymph Node Dissection sites for Cancer in the body and tail of the pancreas: a Multicenter Retrospective Study. Cancers (Basel). 2022;14(18). 10.3390/cancers14184409. [DOI] [PMC free article] [PubMed]
- 7.Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC Cancer staging Manual: pancreas and hepatobiliary cancers. Ann Surg Oncol. 2018;25(4):845–7. 10.1245/s10434-017-6025-x. 10.1245/s10434-017-6025-x [DOI] [PubMed] [Google Scholar]
- 8.Warschkow R, et al. Role of lymphadenectomy, adjuvant chemotherapy, and treatment at high-volume centers in patients with resected pancreatic cancer-a distinct view on lymph node yield. Langenbecks Arch Surg. 2020;405(1):43–54. 10.1007/s00423-020-01859-2. 10.1007/s00423-020-01859-2 [DOI] [PubMed] [Google Scholar]
- 9.Klotz R, et al. The TRIANGLE operation for pancreatic head and body cancers: early postoperative outcomes. HPB (Oxford). 2022;24(3):332–41. 10.1016/j.hpb.2021.06.432. 10.1016/j.hpb.2021.06.432 [DOI] [PubMed] [Google Scholar]
- 10.Lin Q, et al. Standard pancreatoduodenectomy versus extended pancreatoduodenectomy with modified retroperitoneal nerve resection in patients with pancreatic head cancer: a multicenter randomized controlled trial. Cancer Commun (Lond). 2023;43(2):257–75. 10.1002/cac2.12399. 10.1002/cac2.12399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukuda Y, et al. Prognostic impact of nodal statuses in patients with pancreatic ductal adenocarcinoma. Pancreatology. 2017;17(2):279–84. 10.1016/j.pan.2017.01.003. 10.1016/j.pan.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 12.Liu ZQ, et al. Effect of the number of positive LN and lymph node ratio on prognosis of patients after resection of pancreatic adenocarcinoma. Hepatobiliary Pancreat Dis Int. 2014;13(6):634–41. 10.1016/s1499-3872(14)60264-2. 10.1016/S1499-3872(14)60264-2 [DOI] [PubMed] [Google Scholar]
- 13.Fischer LK, et al. The number and ratio of positive LN affect pancreatic cancer patient survival after neoadjuvant therapy and pancreaticoduodenectomy. Histopathology. 2016;68(2):210–20. 10.1111/his.12732. 10.1111/his.12732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarantino I, et al. Staging of pancreatic cancer based on the number of positive LN. Br J Surg. 2017;104(5):608–18. 10.1002/bjs.10472. 10.1002/bjs.10472 [DOI] [PubMed] [Google Scholar]
- 15.van der Heijde N, et al. The role of older age and obesity in minimally invasive and open pancreatic surgery: a systematic review and meta-analysis. Pancreatology. 2020;20(6):1234–42. 10.1016/j.pan.2020.06.013. 10.1016/j.pan.2020.06.013 [DOI] [PubMed] [Google Scholar]
- 16.Ikenaga N, et al. Risks and benefits of pancreaticoduodenectomy in patients aged 80 years and over. Langenbecks Arch Surg. 2023;408(1):108. 10.1007/s00423-023-02843-2. 10.1007/s00423-023-02843-2 [DOI] [PubMed] [Google Scholar]
- 17.Maeda S, et al. Impact of resection margin status on survival in pancreatic cancer patients after neoadjuvant treatment and pancreatoduodenectomy. Surgery. 2020;167(5):803–11. 10.1016/j.surg.2019.12.008. 10.1016/j.surg.2019.12.008 [DOI] [PubMed] [Google Scholar]
- 18.Zhang XP, et al. A novel online calculator to predict early recurrence and long-term survival of patients with resectable pancreatic ductal adenocarcinoma after pancreaticoduodenectomy: a multicenter study. Int J Surg. 2022;106:106891. 10.1016/j.ijsu.2022.106891. 10.1016/j.ijsu.2022.106891 [DOI] [PubMed] [Google Scholar]
- 19.Watson MD, et al. The treatment sequence may matter in patients undergoing pancreatoduodenectomy for early stage pancreatic cancer in the era of modern chemotherapy. Am J Surg. 2021;222(1):159–66. 10.1016/j.amjsurg.2020.10.030. 10.1016/j.amjsurg.2020.10.030 [DOI] [PubMed] [Google Scholar]
- 20.Cai J, et al. Advances in the epidemiology of pancreatic cancer: Trends, risk factors, screening, and prognosis. Cancer Lett. 2021;520:1–11. 10.1016/j.canlet.2021.06.027. 10.1016/j.canlet.2021.06.027 [DOI] [PubMed] [Google Scholar]
- 21.Versteijne E, et al. Preoperative Chemoradiotherapy Versus Immediate surgery for Resectable and Borderline Resectable Pancreatic Cancer: results of the Dutch Randomized Phase III PREOPANC Trial. J Clin Oncol. 2020;38(16):1763–73. 10.1200/jco.19.02274. 10.1200/jco.19.02274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnes CA, et al. Survival of patients with borderline resectable pancreatic cancer who received neoadjuvant therapy and surgery. Surgery. 2019;166(3):277–85. 10.1016/j.surg.2019.05.010. 10.1016/j.surg.2019.05.010 [DOI] [PubMed] [Google Scholar]
- 23.Fogel EL, et al. A Multidisciplinary Approach to Pancreas Cancer in 2016: a review. Am J Gastroenterol. 2017;112(4):537–54. 10.1038/ajg.2016.610. 10.1038/ajg.2016.610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei D, et al. Clinicopathological correlation of radiologic measurement of post-therapy tumor size and tumor volume for pancreatic ductal adenocarcinoma. Pancreatology. 2021;21(1):200–7. 10.1016/j.pan.2020.11.003. 10.1016/j.pan.2020.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchegiani G, et al. Does size Matter in Pancreatic Cancer? Reappraisal of Tumour Dimension as a predictor of Outcome beyond the TNM. Ann Surg. 2017;266(1):142–8. 10.1097/sla.0000000000001837. 10.1097/SLA.0000000000001837 [DOI] [PubMed] [Google Scholar]
- 26.Muralidhar V, et al. Association between very small Tumor size and decreased overall survival in node-positive pancreatic Cancer. Ann Surg Oncol. 2018;25(13):4027–34. 10.1245/s10434-018-6832-8. 10.1245/s10434-018-6832-8 [DOI] [PubMed] [Google Scholar]
- 27.Valsangkar NP, et al. N0/N1, PNL, or LNR? The effect of lymph node number on accurate survival prediction in pancreatic ductal adenocarcinoma. J Gastrointest Surg. 2013;17(2):257–66. 10.1007/s11605-012-1974-7. 10.1007/s11605-012-1974-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Komo T, et al. Prognostic impact of Para-aortic Lymph Node Micrometastasis in Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol. 2016;23(6):2019–27. 10.1245/s10434-016-5120-8. 10.1245/s10434-016-5120-8 [DOI] [PubMed] [Google Scholar]
- 29.Paiella S, et al. The prognostic impact of para-aortic lymph node metastasis in pancreatic cancer: a systematic review and meta-analysis. Eur J Surg Oncol. 2016;42(5):616–24. 10.1016/j.ejso.2016.02.003. 10.1016/j.ejso.2016.02.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.