Abstract
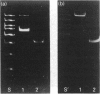
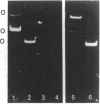
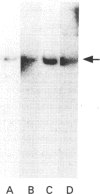
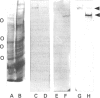
A 230 kDa polypeptide component of the hemidesmosome, an epithelial-cell-connective-tissue attachment device, is thought to be involved in cytoskeleton-cell-surface anchorage. This 230 kDa polypeptide is recognized by bullous pemphigoid auto-antibodies and for this reason is generally termed the bullous pemphigoid antigen (BPA). We have identified two distinct mRNA products of the single BPA gene by RACE (rapid amplification of cDNA ends)/PCR techniques. The first of these mRNAs encodes the 230 kDa protein component of the hemidesmosome. A second mRNA lacks over 1800 bases that encode the C-terminus of the 230 kDa protein. We have raised antibodies against a peptide specific to the predicted protein product of this second mRNA. To our surprise this antibody recognizes a protein that migrates at 280 kDa on SDS/PAGE of extracts of a variety of human epidermal cell lines that also express the 230 kDa BPA. Moreover, we have confirmed the co-expression of the 230 and 280 kDa polypeptides in these cells by immunoblotting analyses using a monoclonal antibody preparation directed against a polypeptide encoded by sequence common to both mRNAs transcribed from the BPA gene. Intriguingly, in one non-epidermal tumour line (a pancreatic cell line termed FG), the 280 kDa polypeptide appears to be the only product of the BPA gene. Furthermore, in FG cells the 280 kDa protein is found in association with the intermediate filament cytoskeleton. We discuss our results in relation to control of BPA gene expression and with regard to potential functions of the domains of the protein products of the BPA gene.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amagai M., Elgart G. W., Klaus-Kovtun V., Stanley J. R. Southern analysis of the 230-kD bullous pemphigoid antigen gene in normal humans, animals, and patients with junctional epidermolysis bullosa. J Invest Dermatol. 1991 Aug;97(2):249–253. doi: 10.1111/1523-1747.ep12480358. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Green K. J., Parry D. A., Steinert P. M., Virata M. L., Wagner R. M., Angst B. D., Nilles L. A. Structure of the human desmoplakins. Implications for function in the desmosomal plaque. J Biol Chem. 1990 Feb 15;265(5):2603–2612. [PubMed] [Google Scholar]
- Green K. J., Virata M. L., Elgart G. W., Stanley J. R., Parry D. A. Comparative structural analysis of desmoplakin, bullous pemphigoid antigen and plectin: members of a new gene family involved in organization of intermediate filaments. Int J Biol Macromol. 1992 Jun;14(3):145–153. doi: 10.1016/s0141-8130(05)80004-2. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Amagai M., Ebihara T., Gamou S., Shimizu N., Tsubata T., Hasegawa A., Miki K., Nishikawa T. Further analyses of epitopes for human monoclonal anti-basement membrane zone antibodies produced by stable human hybridoma cell lines constructed with Epstein-Barr virus transformants. J Invest Dermatol. 1993 Mar;100(3):310–315. doi: 10.1111/1523-1747.ep12469916. [DOI] [PubMed] [Google Scholar]
- Hopkinson S. B., Riddelle K. S., Jones J. C. Cytoplasmic domain of the 180-kD bullous pemphigoid antigen, a hemidesmosomal component: molecular and cell biologic characterization. J Invest Dermatol. 1992 Sep;99(3):264–270. doi: 10.1111/1523-1747.ep12616615. [DOI] [PubMed] [Google Scholar]
- Jones J. C., Green K. J. Intermediate filament-plasma membrane interactions. Curr Opin Cell Biol. 1991 Feb;3(1):127–132. doi: 10.1016/0955-0674(91)90175-x. [DOI] [PubMed] [Google Scholar]
- Jones J. C., Steinman H. K., Goldsmith B. A. Hemidesmosomes, collagen VII, and intermediate filaments in basal cell carcinoma. J Invest Dermatol. 1989 Nov;93(5):662–671. doi: 10.1111/1523-1747.ep12319833. [DOI] [PubMed] [Google Scholar]
- Klatte D. H., Kurpakus M. A., Grelling K. A., Jones J. C. Immunochemical characterization of three components of the hemidesmosome and their expression in cultured epithelial cells. J Cell Biol. 1989 Dec;109(6 Pt 2):3377–3390. doi: 10.1083/jcb.109.6.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- London L., Keene R. G., Landick R. Analysis of premature termination in c-myc during transcription by RNA polymerase II in a HeLa nuclear extract. Mol Cell Biol. 1991 Sep;11(9):4599–4615. doi: 10.1128/mcb.11.9.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maa M. C., Chinsky J. M., Ramamurthy V., Martin B. D., Kellems R. E. Identification of transcription stop sites at the 5' and 3' ends of the murine adenosine deaminase gene. J Biol Chem. 1990 Jul 25;265(21):12513–12519. [PubMed] [Google Scholar]
- McNutt N. S. Ultrastructural comparison of the interface between epithelium and stroma in basal cell carcinoma and control human skin. Lab Invest. 1976 Aug;35(2):132–142. [PubMed] [Google Scholar]
- Mechti N., Piechaczyk M., Blanchard J. M., Jeanteur P., Lebleu B. Sequence requirements for premature transcription arrest within the first intron of the mouse c-fos gene. Mol Cell Biol. 1991 May;11(5):2832–2841. doi: 10.1128/mcb.11.5.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaranta V., Jones J. C. The internal affairs of an integrin. Trends Cell Biol. 1991 Jul;1(1):2–4. doi: 10.1016/0962-8924(91)90046-c. [DOI] [PubMed] [Google Scholar]
- Riddelle K. S., Green K. J., Jones J. C. Formation of hemidesmosomes in vitro by a transformed rat bladder cell line. J Cell Biol. 1991 Jan;112(1):159–168. doi: 10.1083/jcb.112.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamura D., Li K., Chu M. L., Uitto J. Human bullous pemphigoid antigen (BPAG1). Amino acid sequences deduced from cloned cDNAs predict biologically important peptide segments and protein domains. J Biol Chem. 1991 Sep 25;266(27):17784–17790. [PubMed] [Google Scholar]
- Sawamura D., Nomura K., Sugita Y., Mattei M. G., Chu M. L., Knowlton R., Uitto J. Bullous pemphigoid antigen (BPAG1): cDNA cloning and mapping of the gene to the short arm of human chromosome 6. Genomics. 1990 Dec;8(4):722–726. doi: 10.1016/0888-7543(90)90261-r. [DOI] [PubMed] [Google Scholar]
- Staehelin L. A. Structure and function of intercellular junctions. Int Rev Cytol. 1974;39:191–283. doi: 10.1016/s0074-7696(08)60940-7. [DOI] [PubMed] [Google Scholar]
- Stanley J. R., Hawley-Nelson P., Yuspa S. H., Shevach E. M., Katz S. I. Characterization of bullous pemphigoid antigen: a unique basement membrane protein of stratified squamous epithelia. Cell. 1981 Jun;24(3):897–903. doi: 10.1016/0092-8674(81)90115-x. [DOI] [PubMed] [Google Scholar]
- Stanley J. R., Tanaka T., Mueller S., Klaus-Kovtun V., Roop D. Isolation of complementary DNA for bullous pemphigoid antigen by use of patients' autoantibodies. J Clin Invest. 1988 Dec;82(6):1864–1870. doi: 10.1172/JCI113803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura R. N., Rozzo C., Starr L., Chambers J., Reichardt L. F., Cooper H. M., Quaranta V. Epithelial integrin alpha 6 beta 4: complete primary structure of alpha 6 and variant forms of beta 4. J Cell Biol. 1990 Oct;111(4):1593–1604. doi: 10.1083/jcb.111.4.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Parry D. A., Klaus-Kovtun V., Steinert P. M., Stanley J. R. Comparison of molecularly cloned bullous pemphigoid antigen to desmoplakin I confirms that they define a new family of cell adhesion junction plaque proteins. J Biol Chem. 1991 Jul 5;266(19):12555–12559. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virata M. L., Wagner R. M., Parry D. A., Green K. J. Molecular structure of the human desmoplakin I and II amino terminus. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):544–548. doi: 10.1073/pnas.89.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. A., Michael A., Yuan D. Role of transcriptional termination in the regulation of mu mRNA expression in B lymphocytes. J Immunol. 1989 Aug 1;143(3):1046–1052. [PubMed] [Google Scholar]
- Weiss E. A., Tucker P. W., Finkelman F. D., Yuan D. Analysis of immunoglobulin heavy chain delta transcription termination in the production of delta S or delta M mRNA. Mol Immunol. 1991 Jul;28(7):687–695. doi: 10.1016/0161-5890(91)90110-6. [DOI] [PubMed] [Google Scholar]
- Wiche G., Becker B., Luber K., Weitzer G., Castañon M. J., Hauptmann R., Stratowa C., Stewart M. Cloning and sequencing of rat plectin indicates a 466-kD polypeptide chain with a three-domain structure based on a central alpha-helical coiled coil. J Cell Biol. 1991 Jul;114(1):83–99. doi: 10.1083/jcb.114.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zackroff R. V., Goldman A. E., Jones J. C., Steinert P. M., Goldman R. D. Isolation and characterization of keratin-like proteins from cultured cells with fibroblastic morphology. J Cell Biol. 1984 Apr;98(4):1231–1237. doi: 10.1083/jcb.98.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]