Abstract
Background
High-density lipoprotein cholesterol (HDL-C) is widely recognized for its protective effects against cognitive decline. However, recent studies have presented conflicting results, with some suggesting no significant cognitive benefits or even an increased risk of dementia associated with high HDL-C levels. For those who suffer from depression, the cognitive benefits of HDL-C may be diminished or reversed. The purpose of this study is to investigate the associations between HDL-C, cognitive ability, and depressive symptoms in middle-aged and older Chinese adults.
Methods
The datasets utilized were sourced from the China Health and Retirement Longitudinal Study (CHARLS) for the years 2011 and 2015, comprising 4,302 participants. Cross-lagged models were employed to explore the temporal sequence between cognitive performance and HDL-C levels, and to examine the interplay among depression, cognition, and HDL-C. Confounding factors such as sociodemographic characteristics, sleep conditions, and history of chronic diseases were controlled for.
Results
The analysis revealed unidirectional effects of baseline impaired cognition and greater severity of depression on increased HDL-C levels at follow-up (β = − 0.036 and β = 0.028, respectively, P < 0.05). However, higher baseline HDL-C levels did not significantly predict cognitive performance or depression 4 years later (β = − 0.008 and β = 0.023, respectively, P > 0.05). Depressive symptoms and cognition were found to have a significant bidirectional association (β = − 0.026 and β = − 0.053, respectively, P < 0.05).
Conclusions
Cognitive impairment and depression are associated with higher HDL-C levels, whereas higher HDL-C levels do not appear to protect against cognitive decline or depressive symptoms. These findings underscore the importance of preserving cognitive and mental health, which may lower the likelihood of cardiovascular disease and dementia. Future studies should validate these findings and develop targeted interventions tailored to specific populations.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-024-02285-9.
Keywords: High-density lipoprotein cholesterol, Cognition, Depression, Cross-lagged panel analysis, Middle-aged and older Chinese adults
Background
High-density lipoprotein cholesterol (HDL-C) is widely regarded as a beneficial lipoprotein, particularly for its protective effects on cognitive function in older adults [1–3]. HDL-C exerts multiple protective actions, including atheroprotection, endothelial protection, immune regulation, oxidative stress inhibition, anti-inflammatory effects, and antithrombotic effects [4]. These functions are crucial in preventing cerebrovascular and cardiovascular diseases, which are major risk factors for cognitive deterioration in older adults [5]. Furthermore, HDL-C is crucial in reducing dementia risk by preventing amyloid β-protein accumulation in brain vessels, improving nitric oxide production, slowing amyloid β-protein fibrillization, and preserving memory and cortical complexity in specific brain areas such as the insular and frontal opercular regions [6, 7]. Evidence suggests that HDL-C levels under 40 mg/dL are linked to various cognitive impairments in older adults, affecting areas such as working memory, executive function, and immediate and delayed recall [8].
However, not all studies support HDL-C’s potential to protect cognition. For instance, two longitudinal studies conducted among the elderly population in China discovered no significant impact of HDL-C on cognitive status [9, 10]. Additionally, an increased risk of dementia development was linked to HDL-C levels above 80 mg/dL in initially healthy individuals aged 75 and older, and this association was independent of traditional dementia risk factors, for example, physical activity, education, smoking status, alcohol consumption, and diabetes [11].
Depression may be a contributing factor to these inconsistencies. A longitudinal study reported that HDL-C positively impacts cognition in individuals without depression, but offers no cognitive protection for those with depressive symptoms, and might even lead to cognitive decline [12]. Path analysis indicated that more severe depressive symptoms are positively correlated with higher HDL-C levels in middle-aged men [13]. Additionally, two studies in people with diabetes reported a similar positive correlation between depression or vital exhaustion and HDL-C, which was unexpected [14, 15].
Currently, there is a lack of nationally representative data exploring the relationships among HDL-C, cognitive performance, and depression in middle-aged and older Chinese adults. Therefore, this study used a comprehensive longitudinal database in China to investigate the temporal sequence between cognition and HDL-C, as well as the impact of depressive symptoms on this relationship. It was hypothesized that HDL-C and cognition are causally related, with depressive symptoms affecting this association.
Methods
Data and sample
Data were acquired from the China Health and Retirement Longitudinal Study (CHARLS). The subjects were chosen from 150 counties (districts) and 450 villages (urban communities) nationwide using a multi-stage stratified probability sampling technique, ensuring the data’s representativeness and a high quality for population aged 45 years or above [16]. Ethical approval was granted by the Institutional Review Board at Peking University, with permission numbers IRB00001052-11015 and IRB00001052-11014 for the primary household survey and biomarker collection, respectively. All participants provided written informed consent. Further details can be found on the official website of CHARLS.
Data from two independent waves of the CHARLS study were analyzed in this research. Wave 1 was the national baseline survey conducted between 2011 and 2012 involving 17,708 residents, with 11,847 (67%) completing blood tests. Wave 3, conducted from 2015 to 2016, included 21,100 individuals, with 13,420 providing blood samples. Among them, 10,384 participants underwent a follow-up interview, and 7,648 (74%) completed the blood test. Exclusions were made for 226 individuals under the age of 45 years, 2,468 individuals with missing cognitive data, 100 participants without HDL-C information, and 552 individuals lacking covariables such as depression symptoms, sleep data, and body mass index (BMI). Ultimately, 4,302 participants were included in the analysis. The detailed exclusion process is shown in Fig. 1.
Fig. 1.
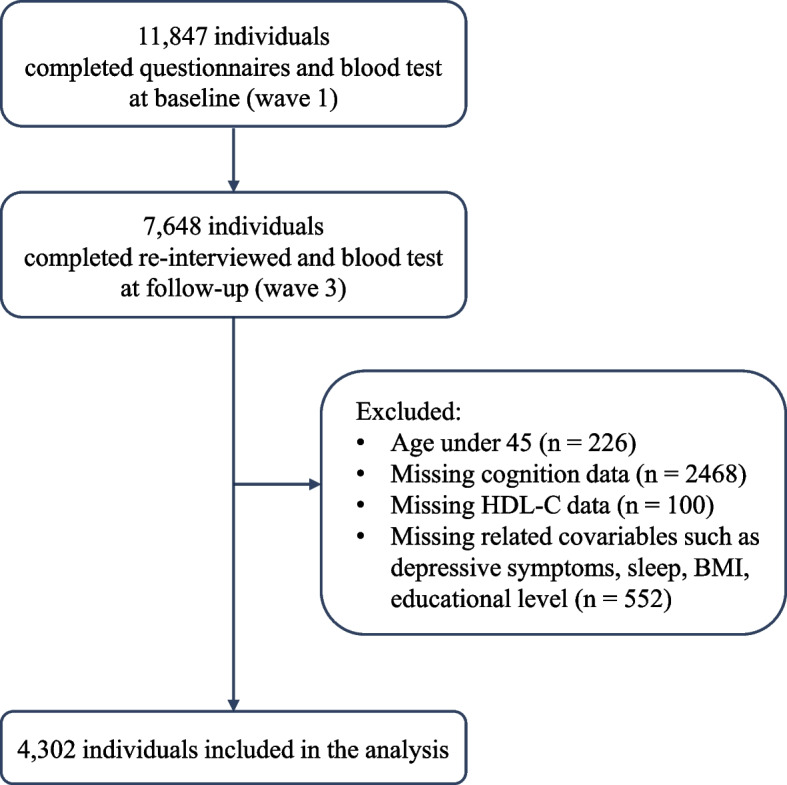
Participant selection schematic
Study variables
Cognitive function was assessed using several phone-based measures across two main domains: episodic memory and mental intactness [17, 18]. Immediate and delayed word recall tests were used to evaluate episodic memory, in which participants were required to recollect 10 words immediately and again after a few minutes, with a final score of 0–20. Mental intactness was assessed using three components, resulting in a total score of 0–11: arithmetic ability (subtracting 7 from 100 in a sequence, five times), time orientation (recognizing the year, month, day, day of the week, and season), and visual and spatial ability (drawing two overlapping pentagons). The global cognitive score ranging from 0 to 31 was formed by summing these individual scores. The higher the total score, the better the cognitive function. These neuropsychological tests have shown good validity in the Chinese adult population [19].
The HDL-C levels were measured through an enzymatic colorimetric test, which had a detection range of 3–120 mg/dL. The normal range for HDL-C in adult males is 45–55 mg/dL, whereas in females it is 50–60 mg/dL, slightly higher. Further details regarding the blood sample procedures can be found in the CHARLS 2011–2012 National Baseline Blood Data User Guide.
Covariables
The current study identified several sociodemographic characteristics as potential confounders in the connection between HDL-C levels and cognition. These include age, sex, BMI, residence (rural or urban), educational level, and marital status. Other factors were depressive symptoms, sleep conditions, history of chronic diseases, alcohol usage, and smoking status.
Depressive symptoms were measured through the 10-item Center for Epidemiological Studies Depression Scale (CESD-10), which has good internal validity in Chinese middle-aged and above individuals [20–22]. A 4-point Likert scale was used to score, with 12 points as the cutoff point. Higher total scores indicate more severe depressive symptoms [22, 23]. Educational attainment was categorized into four groups: no formal education, primary school, middle and high school, and college or above. Marital status was divided into two categories: married/partnered and separated/divorced/widowed. Sleep conditions were assessed through self-reported data on nightly sleep duration and nap duration, defined as the average number of hours slept per night and the number of minutes napped after lunch in the past month, respectively. Histories of four chronic diseases—cancer, hypertension, diabetes, and stroke—were coded as “yes” or “no.” Smoking and drinking status were classified into three categories: never, former, and current.
Statistical analysis
Continuous variables were presented as means (standard deviations), whereas categorical variables were reported as numbers (proportions). A paired t-test was conducted to compare the differences in HDL-C levels and cognitive function scores between wave 1 and wave 3. The cross-lagged panel model (CLPM) was applied as a path analysis technique in this longitudinal study to explore the temporal relationships between variables measured at multiple time points [24, 25].
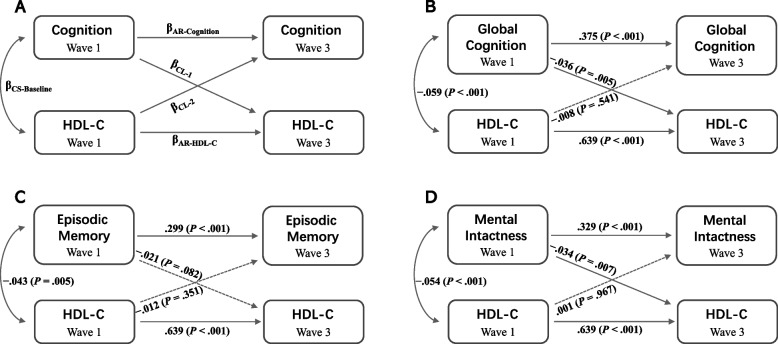
The cross-lagged analysis evaluated three types of relationships between cognition and HDL-C by testing the following five paths: one baseline correlation path, two autoregressive effects, and two cross-lagged effects. The modeling strategy is presented in Fig. 2. Furthermore, a cross-lagged panel analysis of the relationships between depressive symptoms, cognition, and HDL-C was conducted. The parameters in the CLPM were assessed using the robust maximum likelihood method. Several indices were employed to evaluate model fit. Normed chi-square (χ2/df) < 3, goodness-of-fit index (GFI), incremental fit index (IFI), and normed fit index (NFI) > 0.90, standardized root mean square residual (SRMR) < 0.05, and root mean square error of approximation (RMSEA) < 0.08 are considered acceptable ranges, suggesting a good fit for the model.
Fig. 2.
Cross-lagged panel models of HDL-C levels and cognition in middle-aged and older Chinese adults. A The modeling framework. B CLPM of global cognition and HDL-C levels. C CLPM of episodic memory and HDL-C levels. D CLPM of mental intactness and HDL-C levels. Standardized structural regression coefficients are presented. CS: cross sectional; AR: autoregressive; CL: cross-lagged
Data were analyzed using IBM SPSS version 26.0 (IBM Corp., Armonk, NY) and SPSS AMOS version 28.0 software. Statistical significance was established at a two-tailed P value of less than 0.05.
Results
Demographic data
Table 1 presents descriptive information for a total of 4,302 individuals at baseline. The mean age of the study sample was 57.80 (SD 8.19) years, with a slightly higher proportion of males (2,225/4,302, 51.7%). Educational attainment tended toward the lower end, with 62.9% holding a primary school diploma or lower qualification. In 2011, the average level of HDL-C was 50.66 mg/dL (SD 15.38), with 28% of individuals (n = 1,203) having elevated HDL-C levels (16.4% of males and 11.6% of females), and by 2015, the mean HDL-C level increased to 51.17 mg/dL (SD 11.97). There was a significant difference between these HDL-C levels (t = − 2.86, P = 0.004). The average global cognitive function score decreased from 15.74 (SD 4.53) at baseline to 14.96 (SD 4.92) at follow-up, with a statistically significant difference (t = 11.03, P < 0.001). Episodic memory scored lower (7.56 out of 20) compared to mental intactness (8.18 out of 11). In terms of depressive symptoms, the average prevalence in 2011 was 24.8%, with 40.7% (n = 434) of men and 59.3% (n = 633) of women exhibiting symptoms.
Table 1.
Baseline demographic data of the population (N = 4,302)
| Variables | Values |
|---|---|
| Age (years), mean (SD) | 57.80 (8.19) |
| Female, n (%) | 2077 (48.3) |
| BMI (kg/m2), mean (SD) | 24.67 (38.69) |
| Rural Village, n (%) | 2687 (62.5) |
| Married/partnered, n (%) | 3914 (91) |
| Educational level, n (%) | |
| No formal education | 718 (16.7) |
| Primary school | 1988 (46.2) |
| Middle and high school | 1545 (35.9) |
| College or above | 51 (1.2) |
| History of chronic diseases, n (%) | |
| Hypertension | 1149 (26.7) |
| Diabetes | 273 (6.3) |
| Cancer | 35 (0.8) |
| Stroke | 95 (2.2) |
| Current smoker, n (%) | 1401 (32.6) |
| Current drinker, n (%) | 1269 (34.1) |
| Cognition, mean (SD) | |
| Global cognition | 15.74 (4.53) |
| Episodic memory | 7.56 (3.21) |
| Mental intactness | 8.18 (2.45) |
| CESD-10 scores, mean (SD) | 7.87 (6.10) |
| Nightly sleep duration (hours), mean (SD) | 6.43 (1.76) |
| Daytime nap duration (minutes), mean (SD) | 32.78 (42.94) |
| HDL-C (mg/dL), mean (SD) | 50.66 (15.38) |
Spearman correlations between HDL-C levels and cognition in waves 1 and 3 are shown in Table 2. All variables showed significant negative relationships. The correlation between HDL-C levels in 2011 and mental intactness in 2015 deviated from the outcomes of the cross-lagged analysis, whereas the remaining variables were consistent with the CLPM results.
Table 2.
Spearman correlations between HDL-C and cognition at baseline and follow-up
| Baseline HDL-C | Follow-up HDL-C | |
|---|---|---|
| Baseline Cognition scores | ||
| Global cognition | − .060*** | − .086*** |
| Episodic memory | − .041** | − .047** |
| Mental intactness | − .060*** | − .098*** |
| Follow-up cognition scores | ||
| Global cognition | − .059*** | − .061*** |
| Episodic memory | − .049** | − .039* |
| Mental intactness | − .052** | − .063*** |
*P < 0.05
** P < 0.01
*** P < 0.001
Cross-lagged panel analyses of HDL-C and cognition
Table 3 and Fig. 2 present the findings from the cross-lagged path analysis of the relationship between HDL-C levels and cognition. HDL-C levels in wave 1 were negatively associated with global cognitive scores and episodic memory scores in wave 3 in both directions, without controlling for covariables (βCL-1 = − 0.048, P < 0.001; βCL-2 = − 0.030, P < 0.05 in Model 1; and βCL-1 = − 0.026, P < 0.05; βCL-2 = − 0.035, P < 0.05 in Model 3). However, after adjusting for covariables (i.e., age, sex, BMI, education, marital status, residence, depression symptoms, night sleep duration, nap duration, and history of four chronic diseases), unidirectional cross-lagged effects were observed for impaired cognitive function and increased HDL-C levels 4 years later (βCL-1 = − 0.036, P < 0.01, and βCL-2 = − 0.008, P > 0.05 in Model 2), whereas the association between episodic memory and HDL-C was not statistically significant (βCL-1 = − 0.021, P > 0.05, and βCL-2 = − 0.012, P > 0.05 in Model 4). Mental intactness scores in 2011 exhibited a one-way negative association with HDL-C in 2015, regardless of whether covariables were controlled (βCL-1 = − 0.055, P < 0.001; βCL-2 = − 0.021, P > 0.05 in Model 5; and βCL-1 = − 0.034, P < 0.01; βCL-2 = 0.001, P > 0.05 in Model 6). All CLPM fit indices demonstrated good alignment with the observed data, meeting the criteria within the reference range (Table 3).
Table 3.
Cross-lagged panel model results for HDL-C and cognition scores (N = 4,302)
| HDL-C and cognition scores | Autoregressive | Correlations | Cross sectional | Model fit indicesa | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| βAR-Cognition | βAR-HDL-C | βCL-1 | βCL-2 | βCS-Baseline | χ2/df | GFI | IFI | NFI | SRMR | RMSEA | |
| Global cognitive | |||||||||||
| Model 1b | .525*** | .625*** | − .048*** | − .030* | − .059*** | 0.5 | 1.000 | 1.000 | 1.000 | 0.002 | 0.000 |
| Model 2c | .375*** | .639*** | − .036** | − .008 | − .059*** | 1.4 | 1.000 | 1.000 | 1.000 | 0.001 | 0.009 |
| Episodic memory | |||||||||||
| Model 3b | .417*** | .654*** | − .026* | − .035* | − .043** | 0.5 | 1.000 | 1.000 | 1.000 | 0.002 | 0.000 |
| Model 4c | .299*** | .639*** | − .021 | − .012 | − .043** | 1.9 | 1.000 | 1.000 | 1.000 | 0.001 | 0.014 |
| Mental intactness | |||||||||||
| Model 5b | .455*** | .652*** | − .055*** | − .021 | − .054*** | 1.4 | 1.000 | 1.000 | 1.000 | 0.004 | 0.010 |
| Model 6c | .329*** | .639*** | − .034** | .001 | − .054*** | 0.4 | 1.000 | 1.000 | 1.000 | 0.001 | 0.000 |
| Referent range | < 3 | > 0.90 | > 0.90 | > 0.90 | < 0.05 | < 0.08 | |||||
*P < 0.05
** P < 0.01
*** P < 0.001
aAbbreviation of model fit indices: χ2/df: normed chi-square; GFI: goodness-of-fit index; NFI: normed fit index; IFI: incremental fit index; SRMR: standardized root mean square residual; RMSEA: root mean square error of approximation
bModel 1, 3, and 5 were crude models without covariables adjusted
cModel 2, 4, and 6 were adjusted for covariables including age, sex, BMI, educational level, marital status, residence, depression symptom, nightly sleep duration and nap duration, history of four chronic diseases (i.e., cancer, hypertension, diabetes, and stroke)
Based on sex and age, subgroup analyses are shown in Supplementary Materials Fig. 1. In the female subgroup and the 45 to under 60 age subgroup, baseline cognition were significantly negatively associated with subsequent HDL-C levels (β = − 0.048, P = 0.013 and β = − 0.045, P = 0.007, respectively). No significant association was observed between baseline HDL-C levels and follow-up cognitive function in any subgroup, in line with the overall sample analysis.
Further analysis
Figure 3 depicts the associations among depressive symptoms, cognition, and HDL-C. Higher CESD-10 scores and lower global cognition scores at baseline were significantly associated with higher HDL-C levels (β = 0.049, P = 0.001 and β = − 0.059, P < 0.001, respectively). A marked bidirectional relationship was observed between CESD-10 scores and global cognition scores, indicating that more severe depression at baseline predicts worse cognitive function at follow-up (β = − 0.026, P = 0.046), and vice versa (β = − 0.053, P < 0.001).
Fig. 3.
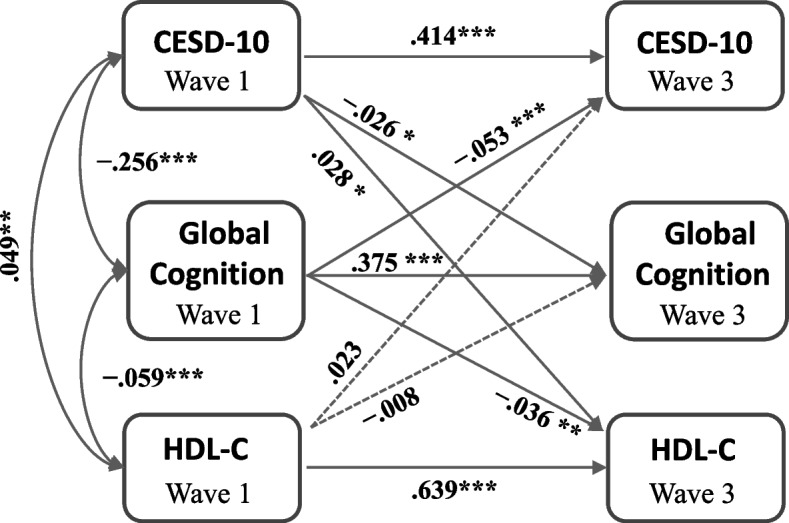
Cross-lagged panel model of the associations among depressive symptoms, cognition, and HDL-C. Standardized structural regression coefficients are provided. * P < 0.05, ** P < 0.01, *** P < 0.001
Notably, the severity of depression in wave 1 was positively associated with HDL-C levels in wave 3 (β = 0.028, P = 0.024), whereas global cognition in wave 1 was negatively associated with HDL-C in wave 3 (β = − 0.036, P < 0.001). However, the cross-sectional pathway from HDL-C in 2011 to depression in 2015 and from HDL-C in 2011 to cognition in 2015 did not provide statistically significant results (β = 0.023, P = 0.090 and β = − 0.008, P = 0.541, respectively).
Regarding model fit, the value of χ2/df was excessively high at 24.6. The SRMR was 0.0061, the GFI was 0.0098, the NFI and CFI were 0.0095, and the RMSEA was 0.074.
Subgroup analyses based on sex and age are presented in Supplementary Materials Fig. 2. The direction and positive or negative association of each path were consistent with the overall sample analysis.
Discussion
This study marks a pioneering effort in examining the temporal interplay among cognitive function, depression, and HDL-C levels in middle-aged and older Chinese adults, leveraging a nationwide longitudinal database. The present cross-lagged path analysis model not only elucidated a unidirectional cross-lagged effect between cognitive status and HDL-C but also uncovered a bidirectional temporal relationship between depression and cognition, where depression was discovered to elevate subsequent HDL-C levels.
A negative association was found between baseline HDL-C levels and cognitive function. Interestingly, baseline cognitive impairment was associated with an increase in subsequent HDL-C levels, but initially higher HDL-C levels did not appear to protect against future cognitive decline. This finding challenges the notion of HDL-C as solely “good” cholesterol. Previous research suggests that HDL-C affects long-term cognitive function, including both protecting cognitive function [1–3, 26, 27] and contributing to cognitive decline [11]. This study conducted a four-year follow-up of a nationwide East Asian population and found that HDL-C was not associated with the risk of cognitive impairment, which is consistent with findings from studies on elderly individuals in Chinese longevity regions, middle-aged and older adults in developed Chinese cities, and a meta-analysis of cohort studies [9, 10, 28]. The variation in results may be explained by the different follow-up periods used in each study, with longer studies reporting a substantial association and shorter researches not finding such a link [29].
This clinical phenomenon may be related to the following mechanisms. First, the increased HDL-C linked to cognitive impairment may not represent normal functional HDL, but rather HDL that has been modified by oxidation. Cholesteryl ester transfer protein (CETP) serves a vital function in mediating the reverse transport of cholesterol, and its inhibitors can lead to elevated plasma HDL-C levels [30]. Although theoretically they possess anti-atherosclerotic properties and could reduce cardiovascular risks [31, 32], clinical trials have demonstrated that CETP inhibitors can elevate the levels of oxidation-modified HDL, which is dysfunctional in reducing cholesterol clearance and antioxidation [33, 34]. This dysfunctional HDL may contribute to higher incidences of stroke, cardiovascular events, mortality, and cognitive damage [35]. A study from the China Kadoorie Biobank have identified a functional variation, CETP-rs2303790, unique to the East Asian population, which affects CETP protein activity [36]. A 10-year follow-up revealed that this variation did not reduce the risk of cardiovascular or cerebrovascular diseases, thereby indirectly suggesting that HDL does not have cognitive protective effects [36]. In patients with Alzheimer's disease (AD), elevated HDL levels, especially HDL-4, may indicate increased lipid crossing of the blood–brain barrier, potentially promoting the formation of amyloid plaques [37]. Dysfunctional HDL in patients with AD, characterized by a greater proportion of the H5 subfraction rich in apoCIII, is associated with impaired cholesterol efflux and increased proinflammatory components, potentially contributing to cognitive impairment [38]. Second, the association among sleep quality, HDL-C levels, and cognitive function is a complex interplay that has significant implications for overall health and cognitive well-being [21, 39, 40]. Another study from CHARLS suggests a two-way interaction between sleep duration and HDL-C levels, revealing that shorter sleep duration correlates with higher HDL-C levels, which could exacerbate insomnia and potentially impact cognitive function negatively [21]. A Midlife in the United States study revealed that negative emotions and physical symptoms peaked when individuals slept less than 6 h for 3 consecutive days [41]. Studies among East Asian and British populations have shown that both sleep deficiency (< 7 h) and oversleeping (> 7 h) are linked to increased mortality rates, compromised cognitive function, and mental health issues [42, 43]. Even individuals without cognitive impairment who sleep less than 7 h may show increased AD biomarkers in cerebrospinal fluid, indicating the importance of adequate sleep for overall brain health [44]. The overall mean sleep duration in the present work was 6.43 h (SD 1.76), which falls short of the optimal 7 h. Based on the abovementioned findings, it was assumed that cognitive impairment symptoms—such as diminished time orientation, numeracy, and visual-spatial abilities (collectively referred to as mental intactness)—may manifest earlier than cholesterol metabolism disorders in case of sleep deprivation. Additionally, cognitive impairment is considered a marker of disrupted neuroplasticity and brain integrity and an early sign of covert vascular lesions [45]. Notably, longitudinal studies have revealed that the decline in global cognitive function in older individuals (aged > 85 years) occurs earlier than changes in vascular risk factors like blood pressure, total cholesterol, and HDL-C levels, which supports the findings of the current study [46]. Cognitive function, lipid metabolism, and blood pressure regulation share common brain regions (such as the hypothalamus and amygdala), which may be jointly affected by neurodegenerative lesions. Cognitive decline can occur in mild brain disease, whereas metabolic disorders tend to manifest in more severe conditions. This underscores the importance of early detection and intervention for cognitive impairment. Early cognitive assessments are crucial for identifying individuals at risk, enabling timely interventions that may mitigate the progression of cognitive loss and reduce the associated possibility of dementia and other related conditions. Such proactive measures are essential for maintaining cognitive health and preventing dementia in later years.
Besides exploring the direct relationship between cognition and HDL-C, the role of depressive symptoms was further investigated in this context. The present study observed a bidirectional relationship between cognition and depression, suggesting that depression may be linked to later cognitive impairment and vice versa. Moreover, it was found that severe depression may be associated with subsequent increases in HDL cholesterol levels.
The discovery that depressive symptoms can precipitate cognitive difficulties, and conversely, cognitive impairment can exacerbate depressive symptoms, supplements some prior studies suggesting a one-way association [47, 48], and aligns with recent researches that also supported a bidirectional relationship [49–51]. The mechanisms of cognitive decline in patients with depression may include elevated oxidative stress, diminished antioxidant levels, inflammatory responses, reduced neurogenesis, hypothalamic–pituitary–adrenal axis imbalance, increased cell apoptosis, and decreased monoamine activity [52, 53]. In elderly patients with depression, cognitive impairment may also have pathological bases such as cerebral ischemia and amyloid-beta deposition. Genetic studies have identified a group of single nucleotide polymorphisms that significantly interact between major depressive disorder and cognitive domains, such as REST, TNFRSF21, and ARFGEF1, which are involved in neuronal development, oligodendrocyte maturation, and myelination, respectively [54]. In addition, a comprehensive model of neuroplasticity illuminates various changes, such as neuronal atrophy, synaptic loss, impaired neuroplasticity, and weakened neural circuit connectivity, in common brain regions associated with emotional regulation and cognition (e.g., medial prefrontal cortex, amygdala, and hippocampus) [55]. These alterations give rise to depressive moods and cognitive deficits, offering a plausible explanation for this bidirectional relationship.
Moreover, the baseline depression results in elevated HDL-C levels at follow-up were presented, which contradicts most previous studies but is consistent with findings comparing adults or adolescents with severe depression to those with mild depression or healthy controls [56, 57]. A meta-analysis found that this positive association was significant only in women [58]. These studies attributed dyslipidemia in patients with depression to common unhealthy lifestyles, such as lack of exercise, malnutrition or excessive fat intake, and sleep disturbances [13, 59, 60]. Animal-based studies have shown that mouse brains spontaneously generate transient and localized hypoxic regions during wakefulness, occurring with considerable frequency [61]. Physical activities such as running can effectively reduce the hypoxic burden, providing compelling evidence of how lifestyle influences the nervous system. Besides, genetic studies have identified potential relationships between the neurotransmitter 5-hydroxytryptamine (5-HT) system, which is closely linked to depressive disorders, and HDL-C levels. This involves the 5-HT2A receptor gene T102C polymorphism and the serotonin transporter gene promoter region polymorphism (5-HTTLPR) [62–64]. Furthermore, it is suggests that inflammatory markers are increased in elderly individuals with depression symptoms [65], and HDL can exert anti-inflammatory effects by negatively regulating lymphocyte activation [66]. However, there is currently insufficient evidence to support whether elevated HDL is related to delayed immune compensation. Studies involving other ethnic groups have yielded conclusions that diverge from those of the present study [67, 68]. Disparities in dietary habits, lifestyle, and life expectancy among different countries and various regions of China may contribute to variations in the average levels and distribution of blood lipids, which could serve as confounding factors contributing to the inconsistent conclusions.
In addition to the impact of depression, age is another factor that can affect blood lipid levels. Studies involving male adults in their middle and advanced years have shown that as age increases, total cholesterol levels decrease and HDL-C levels increase [69]. These findings align with the present study results. However, there is a perspective that HDL-C may not be a causal marker because many of the functions attributed to HDL are actually carried out by particles or specific components in addition to cholesterol [35]. Therefore, the current evidence is insufficient to fully explain the reasons for the increase in HDL-C caused by cognitive impairment, and this finding may change after a longer follow-up, possibly indicating a bidirectional or no temporal relationship.
In summary, the present study highlights that cognitive deficit is linked to increased HDL-C levels and that depression and cognitive impairment mutually aggravate each other. Depression may also be associated with subsequent HDL-C levels. These findings could be related to dysfunctional HDL-C, neurodegenerative lesions in brain regulatory centers, and changes in neuroplasticity, as well as insufficient sleep and unhealthy lifestyles.
Strengths and limitations
This study exhibits several strengths and limitations worth considering. The national representativeness of the sample and its wide geographic coverage bolster the generalizability of the present findings among middle-aged and older Chinese adults. Cross-lagged analysis not only delineated the association between HDL-C and cognition but also elucidated their temporal sequence, enhancing the depth of this investigation. The standardized process for blood sample collection, processing, transportation, laboratory testing, and quality control further bolsters the reliability of the present results.
However, certain limitations warrant acknowledgment. First, the response rate for individuals who completed both blood tests and cognitive assessments was 74%, potentially influencing the accuracy of the present results. Second, the HDL levels in CHARLS represent HDL-C rather than the proteins and enzymes, the principal functional components of HDL. Future research should delve into functional alterations in HDL, encompassing variations in HDL subtypes, lipid composition, protein conformation, and enzyme activity, rather than focusing solely on quantitative changes. Despite rigorous control for covariables, certain unmeasured factors, such as dietary intake and physical activity levels were not accounted for. Alcohol consumption, although divided into three general categories (i.e., never drinking, previous drinking, and present drinking), lacked detailed information on alcohol use, such as concentration, daily consumption, frequency, and years since quitting, potentially limiting the interpretation of HDL changes. Lastly, the current dataset may not account for familial genetic variations or mutations that could contribute to elevated HDL levels. Long-term follow-up studies, ideally involving diverse ethnicities or age groups, are imperative to more comprehensively evaluate these associations.
Conclusions
In summary, this study emphasizes that elevated HDL-C levels do not confer protection against cognitive decline and depression symptoms, and in fact, cognitive impairment and depression may contribute to increased HDL-C levels among Chinese individuals in midlife and beyond. This underscores the importance of holistic patient care, incorporating early cognitive assessments, routine monitoring, and prompt interventions to manage lipid levels and mitigate risks of cardiovascular disease and dementia. Clinicians should reevaluate the emphasis on achieving high HDL-C levels in patients with cognitive impairment, especially for women and individuals aged 45–60. Future long-term longitudinal research is necessary to confirm these findings, elucidate the underlying mechanisms, and devise more targeted interventions.
Supplementary Information
Acknowledgements
We express our appreciation to the research and field teams of CHARLS for their efforts in gathering valuable and accessible data, as well as to all the respondents who took part in the study.
Abbreviations
- HDL-C
High-density lipoprotein cholesterol
- CHARLS
The China Health and Retirement Longitudinal Study
- BMI
Body mass index
- CESD-10
The 10-item Center for Epidemiological Studies Depression Scale
- CLPM
The cross-lagged panel model
- SD
Standard deviations
- χ2/df
Normed chi-square
- CS
Cross sectional
- AR
Autoregressive
- CL
Cross-lagged
- CETP
Cholesteryl ester transfer protein
- GFI
Goodness-of-fit index
- IFI
Incremental fit index
- NFI
Normed fit index
- SRMR
Standardized root mean square residual
- RMSEA
Root mean square error of approximation
- AD
Alzheimer's disease
- 5-HT
5-Hydroxytryptamine
- 5-HTTLPR
Serotonin transporter gene promoter region polymorphism
Authors’ contributions
Yi-Hui Liu and Rui Zhou contributed to the conception of the article; Mu-Tong Chen offered important inspiration for the study. Yi-Hui Liu and Yong-Yi He directly accessed, verified, and analyzed the data, prepared tables and figures, as well as interpretated the results. Ming Chen, Jia-Rong Liang, and Quan Huang contributed to the interpretation of the results. Yi-Hui Liu drafted the manuscript. Rui Zhou, Fu-Jun Jia, and Cai-Lan Hou thoroughly reviewed the manuscript and gave approval for the final version. Cai-Lan Hou is the guarantor of this work, responsible for ensuring the accuracy of data analysis.
Funding
This research received no funding.
Availability of data and materials
All data used in this study were obtained from the open-access CHARLS database, available through the CHARLS website (http://charls.pku.edu.cn/).
Declarations
Ethics approval and consent to participate
The Institutional Review Board at Peking University provided ethical permission for the CHARLS. The permission number for the primary household survey and collection of biomarkers were IRB00001052-11015 and IRB00001052-11014, respectively. Written informed permission was provided by all subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rui Zhou, Email: 773501312@qq.com.
Cai-Lan Hou, Email: houcl1975@163.com.
References
- 1.Lee J, Lee S, Min JY, Min KB. Association between serum lipid parameters and cognitive performance in older adults. J Clin Med. 2021;10(22):10.3390/jcm10225405. 10.3390/jcm10225405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vitali C, Wellington CL, Calabresi L. HDL and cholesterol handling in the brain. Cardiovasc Res. 2014;103(3):405–13. 10.1093/cvr/cvu148. 10.1093/cvr/cvu148 [DOI] [PubMed] [Google Scholar]
- 3.Choi H, Kim HC, Stefani KM, Lee JM, Yoon YM, Lee EY, Kim KM, Rhee Y, Youm Y, Kim CO. Serum high-density lipoprotein cholesterol concentration and functional state: the Korean Urban Rural Elderly (KURE) study. Arch Gerontol Geriatr. 2017;71:115–21. 10.1016/j.archger.2017.04.002. 10.1016/j.archger.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 4.Navab M, Reddy ST, Van Lenten BJ, Fogelman AM. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat Rev Cardiol. 2011;8(4):222–32. 10.1038/nrcardio.2010.222. 10.1038/nrcardio.2010.222 [DOI] [PubMed] [Google Scholar]
- 5.Zheng L, Matthews FE, Anstey KJ. Cognitive health expectancies of cardiovascular risk factors for cognitive decline and dementia. Age Ageing. 2021;50(1):169–75. 10.1093/ageing/afaa111. 10.1093/ageing/afaa111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu H, Zou L, Zhou R, Zhang M, Gu S, Zheng J, Hukportie DN, Wu K, Huang Z, Yuan Z, et al. Long-term increase in cholesterol is associated with better cognitive function: evidence from a longitudinal study. Front Aging Neurosci. 2021;13:691423. 10.3389/fnagi.2021.691423. 10.3389/fnagi.2021.691423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinno R, Mori Y, Kubota S, Nomoto S, Futamura A, Shiromaru A, Kuroda T, Yano S, Ishigaki S, Murakami H et al: High serum high-density lipoprotein-cholesterol is associated with memory function and gyrification of insular and frontal opercular cortex in an elderly memory-clinic population. Neuroimage Clin 2019, 22:101746.10.1016/j.nicl.2019.101746. [DOI] [PMC free article] [PubMed]
- 8.Ihle A, Gouveia ÉR, Gouveia BR, Freitas DL, Jurema J, Tinôco MA, Kliegel M. High-density lipoprotein cholesterol level relates to working memory, immediate and delayed cued recall in brazilian older adults: the role of cognitive reserve. Dement Geriatr Cogn Disord. 2017;44(1–2):84–91. 10.1159/000477846. 10.1159/000477846 [DOI] [PubMed] [Google Scholar]
- 9.Ma C, Yin Z, Zhu P, Luo J, Shi X, Gao X. Blood cholesterol in late-life and cognitive decline: a longitudinal study of the Chinese elderly. Mol Neurodegener. 2017;12(1):24. 10.1186/s13024-017-0167-y. 10.1186/s13024-017-0167-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F, Deng W, Ding D, Zhao Q, Liang X, Wang F, Luo J, Zheng L, Guo Q, Hong Z. High low-density lipoprotein cholesterol inversely relates to dementia in community-dwelling older adults: the shanghai aging study. Front Neurol. 2018;9:952. 10.3389/fneur.2018.00952. 10.3389/fneur.2018.00952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussain SM, Robb C, Tonkin AM, Lacaze P, Chong TT, Beilin LJ, Yu C, Watts GF, Ryan J, Ernst ME et al: Association of plasma high-density lipoprotein cholesterol level with risk of incident dementia: a cohort study of healthy older adults. Lancet Reg Health West Pac 2024, 43:100963.10.1016/j.lanwpc.2023.100963. [DOI] [PMC free article] [PubMed]
- 12.Mehdi SMA, Costa AP, Svob C, Pan L, Dartora WJ, Talati A, Gameroff MJ, Wickramaratne PJ, Weissman MM, McIntire LBJ. Depression and cognition are associated with lipid dysregulation in both a multigenerational study of depression and the National Health and Nutrition Examination Survey. Transl Psychiatry. 2024;14(1):142. 10.1038/s41398-024-02847-6. 10.1038/s41398-024-02847-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Igna CV, Julkunen J, Vanhanen H, Keskivaara P, Verkasalo M. Depressive symptoms and serum lipid fractions in middle-aged men: physiologic and health behavior links. Psychosom Med. 2008;70(9):960–6. 10.1097/PSY.0b013e318189a942. 10.1097/PSY.0b013e318189a942 [DOI] [PubMed] [Google Scholar]
- 14.Gary TL, Crum RM, Cooper-Patrick L, Ford D, Brancati FL. Depressive symptoms and metabolic control in African-Americans with type 2 diabetes. Diabetes Care. 2000;23(1):23–9. 10.2337/diacare.23.1.23. 10.2337/diacare.23.1.23 [DOI] [PubMed] [Google Scholar]
- 15.Golden SH, Williams JE, Ford DE, Yeh HC, Paton Sanford C, Nieto FJ, Brancati FL. Depressive symptoms and the risk of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes Care. 2004;27(2):429–35. 10.2337/diacare.27.2.429. 10.2337/diacare.27.2.429 [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol. 2014;43(1):61–8. 10.1093/ije/dys203. 10.1093/ije/dys203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Zhang J, Zou X, Jia X, Zheng D, Guo X, Xie W, Yang Q. The bidirectional association between cognitive function and gait speed in Chinese older adults: longitudinal observational study. JMIR Public Health Surveill. 2023;9:e44274-10.2196/44274. 10.2196/44274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McArdle JJ, Fisher GG, Kadlec KM. Latent variable analyses of age trends of cognition in the health and retirement study, 1992–2004. Psychol Aging. 2007;22(3):525–45. 10.1037/0882-7974.22.3.525. 10.1037/0882-7974.22.3.525 [DOI] [PubMed] [Google Scholar]
- 19.Meng Q, Wang H, Strauss J, Langa KM, Chen X, Wang M, Qu Q, Chen W, Kuang W, Zhang N, et al. Validation of neuropsychological tests for the China health and retirement longitudinal study harmonized cognitive assessment protocol. Int Psychogeriatr. 2019;31(12):1709–19. 10.1017/s1041610219000693. 10.1017/s1041610219000693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qingbo H, Xiaohua W, Gong C. Reliability and Validity of 10-item CES-D among Middle Aged and Older Adults in China. China J Health Psychol. 2015;23(07):1036–41. 10.13342/j.cnki.cjhp.2015.07.023.
- 21.Chen Z, Zhang X, Duan Y, Mo T, Liu W, Ma Y, Yin P. The relationship between sleep duration and blood lipids among Chinese middle-aged and older adults: cross-lagged path analysis from CHARLS. Front Public Health. 2022;10:868059. 10.3389/fpubh.2022.868059. 10.3389/fpubh.2022.868059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng ST, Chan AC. The center for epidemiologic studies depression scale in older Chinese: thresholds for long and short forms. Int J Geriatr Psychiatry. 2005;20(5):465–70. 10.1002/gps.1314. 10.1002/gps.1314 [DOI] [PubMed] [Google Scholar]
- 23.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med. 1994;10(2):77–84. 10.1016/S0749-3797(18)30622-6 [DOI] [PubMed] [Google Scholar]
- 24.Kearney M: Cross-Lagged Panel Analysis. The SAGE Encyclopedia of Communication Research Methods 2016.10.4135/9781483381411.n117.
- 25.Usami S, Todo N, Murayama K: Cross-Lagged Panel Model in Medical Research: A Cautionary Note. Cold Spring Harbor Laboratory 2018.10.1101/486217.
- 26.Kordestani-Moghadam P, Assari S, Nouriyengejeh S, Mohammadipour F, Pourabbasi A. Cognitive Impairments and Associated Structural Brain Changes in Metabolic Syndrome and Implications of Neurocognitive Intervention. J Obes Metab Syndr. 2020;29(3):174–9. 10.7570/jomes20021. 10.7570/jomes20021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han KT, Kim SJ. Are serum cholesterol levels associated with cognitive impairment and depression in elderly individuals without dementia?: A retrospective cohort study in South Korea. Int J Geriatr Psychiatry. 2021;36(1):163–73. 10.1002/gps.5410. 10.1002/gps.5410 [DOI] [PubMed] [Google Scholar]
- 28.Anstey KJ, Ashby-Mitchell K, Peters R. Updating the evidence on the association between serum cholesterol and risk of late-life dementia: review and meta-analysis. J Alzheimers Dis. 2017;56(1):215–28. 10.3233/jad-160826. 10.3233/jad-160826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anusheel, Avula SN, Joseph KN, Onuchukwu CV, Thondamala V, Shrivastava S, Namburi AR, Mohammed L. The Role of high-density lipoprotein in lowering risk of dementia in the elderly: a review. Cureus. 2022;14(4):e24374. 10.7759/cureus.24374. [DOI] [PMC free article] [PubMed]
- 30.Shrestha S, Wu BJ, Guiney L, Barter PJ, Rye KA. Cholesteryl ester transfer protein and its inhibitors. J Lipid Res. 2018;59(5):772–83. 10.1194/jlr.R082735. 10.1194/jlr.R082735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson AJ, Sniderman AD, Ditmarsch M, Dicklin MR, Nicholls SJ, Davidson MH, Kastelein JJP: Cholesteryl Ester Transfer Protein Inhibition Reduces Major Adverse Cardiovascular Events by Lowering Apolipoprotein B Levels. Int J Mol Sci 2022, 23(16). 10.3390/ijms23169417. [DOI] [PMC free article] [PubMed]
- 32.Schmidt AF, Hunt NB, Gordillo-Marañón M, Charoen P, Drenos F, Kivimaki M, Lawlor DA, Giambartolomei C, Papacosta O, Chaturvedi N, et al. Cholesteryl ester transfer protein (CETP) as a drug target for cardiovascular disease. Nat Commun. 2021;12(1):5640. 10.1038/s41467-021-25703-3. 10.1038/s41467-021-25703-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mani P, Rohatgi A. Niacin Therapy, HDL cholesterol, and cardiovascular disease: is the HDL hypothesis defunct? Curr Atheroscler Rep. 2015;17(8):43. 10.1007/s11883-015-0521-x. 10.1007/s11883-015-0521-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feig JE, Hewing B, Smith JD, Hazen SL, Fisher EA. High-density lipoprotein and atherosclerosis regression: evidence from preclinical and clinical studies. Circ Res. 2014;114(1):205–13. 10.1161/circresaha.114.300760. 10.1161/circresaha.114.300760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Eckardstein A, Nordestgaard BG, Remaley AT, Catapano AL. High-density lipoprotein revisited: biological functions and clinical relevance. Eur Heart J. 2023;44(16):1394–407. 10.1093/eurheartj/ehac605. 10.1093/eurheartj/ehac605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Millwood IY, Bennett DA, Holmes MV, Boxall R, Guo Y, Bian Z, Yang L, Sansome S, Chen Y, Du H, et al. Association of CETP gene variants with risk for vascular and nonvascular diseases among Chinese adults. JAMA Cardiol. 2018;3(1):34–43. 10.1001/jamacardio.2017.4177. 10.1001/jamacardio.2017.4177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berezhnoy G, Laske C, Trautwein C. Quantitative NMR-Based Lipoprotein Analysis Identifies Elevated HDL-4 and Triglycerides in the Serum of Alzheimer's Disease Patients. Int J Mol Sci 2022;23(20). 10.3390/ijms232012472. [DOI] [PMC free article] [PubMed]
- 38.Chan HC, Ke LY, Lu HT, Weng SF, Chan HC, Law SH, Lin IL, Chang CF, Lu YH, Chen CH et al.: An Increased plasma level of apociii-rich electronegative high-density lipoprotein may contribute to cognitive impairment in Alzheimer's disease. Biomedicines 2020, 8(12). 10.3390/biomedicines8120542. [DOI] [PMC free article] [PubMed]
- 39.Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13(10):1017–28. 10.1016/s1474-4422(14)70172-3. 10.1016/s1474-4422(14)70172-3 [DOI] [PubMed] [Google Scholar]
- 40.Irwin MR, Vitiello MV. Implications of sleep disturbance and inflammation for Alzheimer’s disease dementia. Lancet Neurol. 2019;18(3):296–306. 10.1016/s1474-4422(18)30450-2. 10.1016/s1474-4422(18)30450-2 [DOI] [PubMed] [Google Scholar]
- 41.Lee S. Naturally occurring consecutive sleep loss and day-to-day trajectories of affective and physical well-being. Ann Behav Med. 2022;56(4):393–404. 10.1093/abm/kaab055. 10.1093/abm/kaab055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Svensson T, Saito E, Svensson AK, Melander O, Orho-Melander M, Mimura M, Rahman S, Sawada N, Koh WP, Shu XO, et al. Association of sleep duration with all- and major-cause mortality among adults in Japan, China, Singapore, and Korea. JAMA Netw Open. 2021;4(9):e2122837. 10.1001/jamanetworkopen.2021.22837. 10.1001/jamanetworkopen.2021.22837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Sahakian BJ, Kang J, Langley C, Zhang W, Xie C, Xiang S, Yu J, Cheng W, Feng J. The brain structure and genetic mechanisms underlying the nonlinear association between sleep duration, cognition and mental health. Nat Aging. 2022;2(5):425–37. 10.1038/s43587-022-00210-2. 10.1038/s43587-022-00210-2 [DOI] [PubMed] [Google Scholar]
- 44.Blackman J, Stankeviciute L, Arenaza-Urquijo EM, Suárez-Calvet M, Sánchez-Benavides G, Vilor-Tejedor N, Iranzo A, Molinuevo JL, Gispert JD, Coulthard E, et al. Cross-sectional and longitudinal association of sleep and Alzheimer biomarkers in cognitively unimpaired adults. Brain Commun. 2022;4(6):fcac257. 10.1093/braincomms/fcac257. 10.1093/braincomms/fcac257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rostamian S, le Cessie S, Marijt KA, Jukema JW, Mooijaart SP, van Buchem MA, van Hall T, Gussekloo J, Trompet S. Association of cognitive function with increased risk of cancer death and all-cause mortality: Longitudinal analysis, systematic review, and meta-analysis of prospective observational studies. PLoS One. 2022;17(1):e0261826. 10.1371/journal.pone.0261826. 10.1371/journal.pone.0261826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Vliet P, Westendorp RG, van Heemst D, de Craen AJ, Oleksik AM. Cognitive decline precedes late-life longitudinal changes in vascular risk factors. J Neurol Neurosurg Psychiatry. 2010;81(9):1028–32. 10.1136/jnnp.2009.182519. 10.1136/jnnp.2009.182519 [DOI] [PubMed] [Google Scholar]
- 47.Wu Z, Zhong X, Peng Q, Chen B, Zhang M, Zhou H, Mai N, Huang X, Ning Y. Longitudinal association between cognition and depression in patients with late-life depression: a cross-lagged design study. Front Psychiatry. 2021;12:577058. 10.3389/fpsyt.2021.577058. 10.3389/fpsyt.2021.577058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matcham F, Simblett SK, Leightley D, Dalby M, Siddi S, Haro JM, Lamers F, Penninx B, Bruce S, Nica R, et al. The association between persistent cognitive difficulties and depression and functional outcomes in people with major depressive disorder. Psychol Med. 2023;53(13):6334–44. 10.1017/s0033291722003671. 10.1017/s0033291722003671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yin J, John A, Cadar D. Bidirectional Associations of depressive symptoms and cognitive function over time. JAMA Netw Open. 2024;7(6):e2416305. 10.1001/jamanetworkopen.2024.16305. 10.1001/jamanetworkopen.2024.16305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo Y, Pai M, Xue B, Lu W. Bidirectional association between depressive symptoms and mild cognitive impairment over 20 years: evidence from the health and retirement study in the United States. J Affect Disord. 2023;338:449–58. 10.1016/j.jad.2023.06.046. 10.1016/j.jad.2023.06.046 [DOI] [PubMed] [Google Scholar]
- 51.Huang W, Zhu W, Chen H, Li F, Huang J, Zhou Y, Sun X, Lan Y. Longitudinal association between depressive symptoms and cognitive decline among middle-aged and elderly population. J Affect Disord. 2022;303:18–23. 10.1016/j.jad.2022.01.107. 10.1016/j.jad.2022.01.107 [DOI] [PubMed] [Google Scholar]
- 52.Dobielska M, Bartosik NK, Zyzik KA, Kowalczyk E, Karbownik MS. Mechanisms of cognitive impairment in depression. May probiotics help? Front Psychiatry. 2022;13:904426. 10.3389/fpsyt.2022.904426. 10.3389/fpsyt.2022.904426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gałecki P, Talarowska M, Anderson G, Berk M, Maes M: Mechanisms underlying neurocognitive dysfunctions in recurrent major depression. Med Sci Monit 2015, 21:1535–1547.10.12659/msm.893176. [DOI] [PMC free article] [PubMed]
- 54.Thalamuthu A, Mills NT, Berger K, Minnerup H, Grotegerd D, Dannlowski U, Meinert S, Opel N, Repple J, Gruber M, et al. Genome-wide interaction study with major depression identifies novel variants associated with cognitive function. Mol Psychiatry. 2022;27(2):1111–9. 10.1038/s41380-021-01379-5. 10.1038/s41380-021-01379-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Price RB, Duman R. Neuroplasticity in cognitive and psychological mechanisms of depression: an integrative model. Mol Psychiatry. 2020;25(3):530–43. 10.1038/s41380-019-0615-x. 10.1038/s41380-019-0615-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jia QF, Yang HX, Zhuang NN, Yin XY, Zhu ZH, Yuan Y, Yin XL, Wang Y, Cheung EFC, Chan RCK, et al. The role of lipoprotein profile in depression and cognitive performance: a network analysis. Sci Rep. 2020;10(1):20704. 10.1038/s41598-020-77782-9. 10.1038/s41598-020-77782-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khalfan AF, Campisi SC, Lo RF, McCrindle BW, Korczak DJ. The association between adolescent depression and dyslipidemia. J Affect Disord. 2023;338:239–45. 10.1016/j.jad.2023.06.017. 10.1016/j.jad.2023.06.017 [DOI] [PubMed] [Google Scholar]
- 58.Shin JY, Suls J, Martin R. Are cholesterol and depression inversely related? A meta-analysis of the association between two cardiac risk factors. Ann Behav Med. 2008;36(1):33–43. 10.1007/s12160-008-9045-8. 10.1007/s12160-008-9045-8 [DOI] [PubMed] [Google Scholar]
- 59.Warriach ZI, Patel S, Khan F, Ferrer GF. Association of depression with cardiovascular diseases. Cureus. 2022;14(6):e26296. 10.7759/cureus.26296. 10.7759/cureus.26296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Urrila AS, Karlsson L, Kiviruusu O, Pelkonen M, Strandholm T, Marttunen M. Sleep complaints among adolescent outpatients with major depressive disorder. Sleep Med. 2012;13(7):816–23. 10.1016/j.sleep.2012.04.012. 10.1016/j.sleep.2012.04.012 [DOI] [PubMed] [Google Scholar]
- 61.Beinlich FRM, Asiminas A, Untiet V, Bojarowska Z, Plá V, Sigurdsson B, Timmel V, Gehrig L, Graber MH, Hirase H, et al. Oxygen imaging of hypoxic pockets in the mouse cerebral cortex. Science. 2024;383(6690):1471–8. 10.1126/science.adn1011. 10.1126/science.adn1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choi JH, Zhang SY, Park KW, Cho YS, Oh BH, Lee MM, Park YB, Kim HS. The association between the T102C polymorphism of the HTR2A serotonin receptor gene and HDL cholesterol level in Koreans. J Biochem Mol Biol. 2005;38(2):238–42. 10.5483/bmbrep.2005.38.2.238. 10.5483/bmbrep.2005.38.2.238 [DOI] [PubMed] [Google Scholar]
- 63.Tomson K, Merenäkk L, Loit HM, Mäestu J, Harro J. The relationship between serotonin transporter gene promoter polymorphism and serum lipid levels at young age in a longitudinal population-representative study. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(8):1857–62. 10.1016/j.pnpbp.2011.08.004. 10.1016/j.pnpbp.2011.08.004 [DOI] [PubMed] [Google Scholar]
- 64.Kim JM, Stewart R, Kim SW, Shin IS, Yang SJ, Yoon JS. Cholesterol and serotonin transporter polymorphism interactions in late-life depression. Neurobiol Aging. 2011;32(2):336–43. 10.1016/j.neurobiolaging.2009.02.017. 10.1016/j.neurobiolaging.2009.02.017 [DOI] [PubMed] [Google Scholar]
- 65.Lo Buglio A, Bellanti F, Carmignano DFP, Serviddio G, Vendemiale G. Association between Controlling Nutritional Status (CONUT) Score and Body Composition, Inflammation and Frailty in Hospitalized Elderly Patients. Nutrients 2024;16(5). 10.3390/nu16050576. [DOI] [PMC free article] [PubMed]
- 66.Tang H, Xiang Z, Li L, Shao X, Zhou Q, You X, Xiong C, Ning J, Chen T, Deng D, et al. Potential role of anti-inflammatory HDL subclasses in metabolic unhealth/obesity. Artif Cells Nanomed Biotechnol. 2021;49(1):565–75. 10.1080/21691401.2021.1961798. 10.1080/21691401.2021.1961798 [DOI] [PubMed] [Google Scholar]
- 67.Han AL. Association between lipid ratio and depression: a cross-sectional study. Sci Rep. 2022;12(1):6190. 10.1038/s41598-022-10350-5. 10.1038/s41598-022-10350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mulchandani R, Lyngdoh T, Nangia R, Singh S, Grover S, Thakur JS. Relationship between serum lipids and depression: a cross sectional survey among adults in Haryana, India. Indian J Psychiatry. 2023;65(1):61–7. 10.4103/indianjpsychiatry.indianjpsychiatry_967_21. 10.4103/indianjpsychiatry.indianjpsychiatry_967_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abbott RD, Sharp DS, Burchfiel CM, Curb JD, Rodriguez BL, Hakim AA, Yano K. Cross-sectional and longitudinal changes in total and high-density-lipoprotein cholesterol levels over a 20-year period in elderly men: the Honolulu Heart Program. Ann Epidemiol. 1997;7(6):417–24. 10.1016/s1047-2797(97)00043-4. 10.1016/s1047-2797(97)00043-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this study were obtained from the open-access CHARLS database, available through the CHARLS website (http://charls.pku.edu.cn/).