Abstract
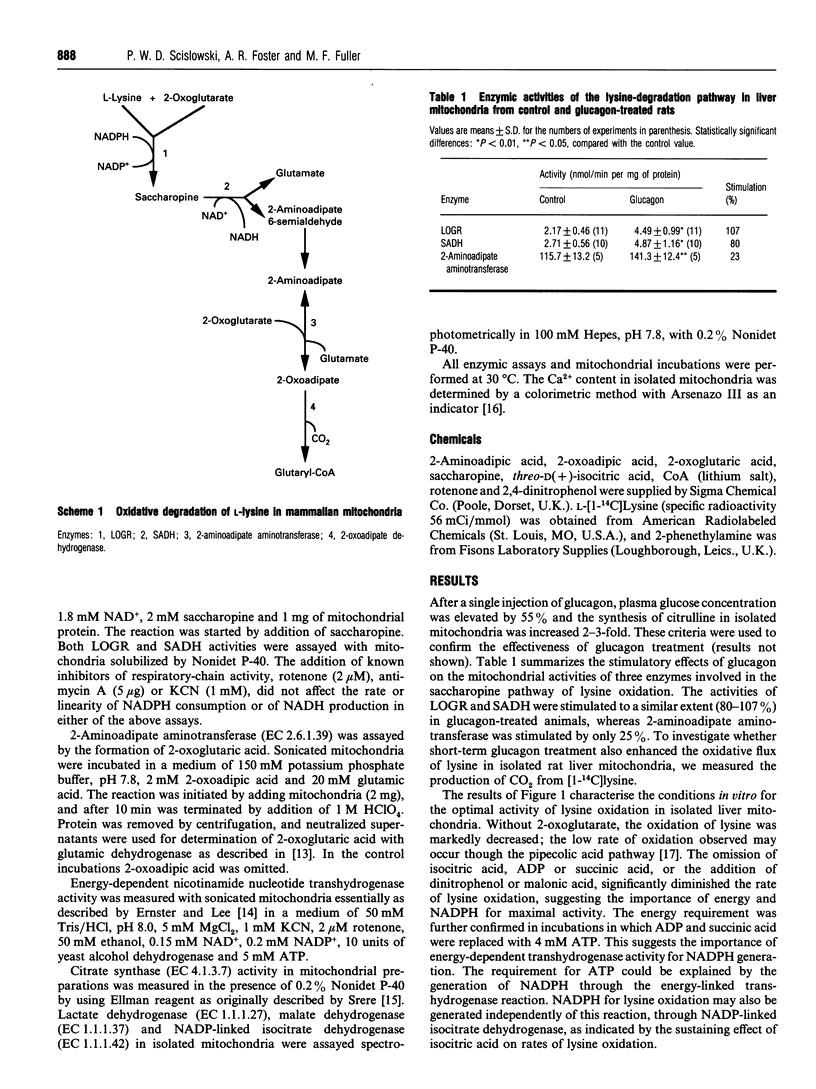
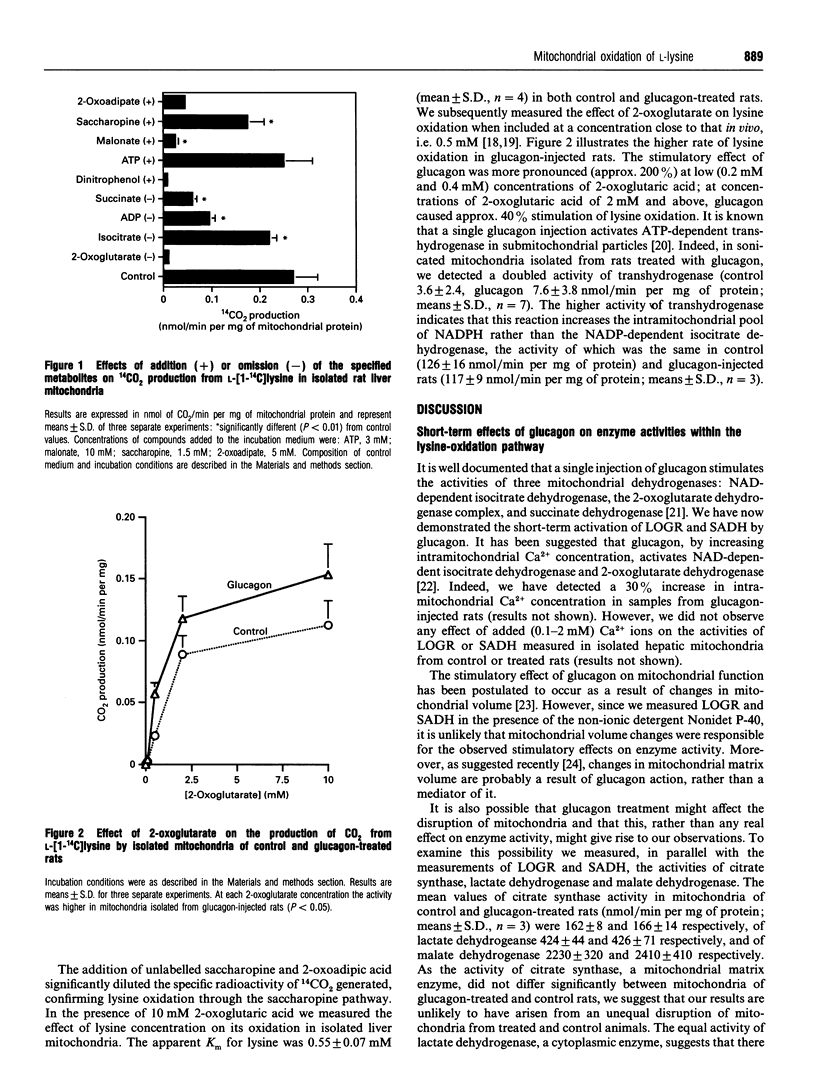
The generation of 14CO2 from [1-14C]lysine by hepatic mitochondria through the saccharopine pathway is controlled by intramitochondrial concentrations of lysine, 2-oxoglutarate and NADPH. Mitochondria, isolated from rats pre-treated with glucagon, exhibited higher activities of L-lysine: 2-oxoglutarate reductase, saccharopine dehydrogenase and 2-aminoadipate aminotransferase. The flux through this pathway is stimulated in liver mitochondria after glucagon treatment. Multiple regulation of lysine oxidation in liver mitochondria confirms a complex mechanism of 'mitochondrial activation' by glucagon.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armston A. E., Halestrap A. P., Scott R. D. The nature of the changes in liver mitochondrial function induced by glucagon treatment of rats. The effects of intramitochondrial volume, aging and benzyl alcohol. Biochim Biophys Acta. 1982 Sep 15;681(3):429–439. doi: 10.1016/0005-2728(82)90185-2. [DOI] [PubMed] [Google Scholar]
- Bauer P. J. Affinity and stoichiometry of calcium binding by arsenazo III. Anal Biochem. 1981 Jan 1;110(1):61–72. doi: 10.1016/0003-2697(81)90112-3. [DOI] [PubMed] [Google Scholar]
- Begum N. A., Datta A. G. Effect of glucagon and some other alpha and beta adrenergic agonists and antagonists on alanine amino transferase of perfused rat liver. Mol Cell Biochem. 1991 Jun 26;105(1):7–13. doi: 10.1007/BF00230369. [DOI] [PubMed] [Google Scholar]
- Beliveau Carey G., Cheung C. W., Cohen N. S., Brusilow S., Raijman L. Regulation of urea and citrulline synthesis under physiological conditions. Biochem J. 1993 May 15;292(Pt 1):241–247. doi: 10.1042/bj2920241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G., Tappy L., Jadali F., Hoeldtke R. D., Rezvani I., Owen O. E. Role of glucagon in disposal of an amino acid load. Am J Physiol. 1990 Aug;259(2 Pt 1):E225–E232. doi: 10.1152/ajpendo.1990.259.2.E225. [DOI] [PubMed] [Google Scholar]
- Bunik V. I., Pavlova O. G. Inactivation of alpha-ketoglutarate dehydrogenase during oxidative decarboxylation of alpha-ketoadipic acid. FEBS Lett. 1993 May 24;323(1-2):166–170. doi: 10.1016/0014-5793(93)81472-c. [DOI] [PubMed] [Google Scholar]
- Christophe J., Winand J., Kutzner R., Hebbelinck M. Amino acid levels in plasma, liver, muscle, and kidney during and after exercise in fasted and fed rats. Am J Physiol. 1971 Aug;221(2):453–457. doi: 10.1152/ajplegacy.1971.221.2.453. [DOI] [PubMed] [Google Scholar]
- Chu S. H., Hegsted D. M. Adaptive response of lysine and threonine degrading enzymes in adult rats. J Nutr. 1976 Aug;106(8):1089–1096. doi: 10.1093/jn/106.8.1089. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. Ca2+ transport by mammalian mitochondria and its role in hormone action. Am J Physiol. 1985 Dec;249(6 Pt 1):E543–E554. doi: 10.1152/ajpendo.1985.249.6.E543. [DOI] [PubMed] [Google Scholar]
- Ewart H. S., Brosnan J. T. Rapid activation of hepatic glutaminase in rats fed on a single high-protein meal. Biochem J. 1993 Jul 15;293(Pt 2):339–344. doi: 10.1042/bj2930339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewart H. S., Jois M., Brosnan J. T. Rapid stimulation of the hepatic glycine-cleavage system in rats fed on a single high-protein meal. Biochem J. 1992 Apr 15;283(Pt 2):441–447. doi: 10.1042/bj2830441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaussin V., Baquet A., Hue L. Cell shrinkage follows, rather than mediates, the short-term effects of glucagon on carbohydrate metabolism. Biochem J. 1992 Oct 1;287(Pt 1):17–20. doi: 10.1042/bj2870017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P., Quinlan P. T., Armston A. E., Whipps D. E. Mechanisms involved in hormone signal transduction across the mitochondrial membrane. Biochem Soc Trans. 1985 Aug;13(4):659–663. doi: 10.1042/bst0130659. [DOI] [PubMed] [Google Scholar]
- Higashino K., Fujioka M., Yamamura Y. The conversion of L-lysine to saccharopine and alpha-aminoadipate in mouse. Arch Biochem Biophys. 1971 Feb;142(2):606–614. doi: 10.1016/0003-9861(71)90525-x. [DOI] [PubMed] [Google Scholar]
- MYERS D. K., SLATER E. C. The enzymic hydrolysis of adenosine triphosphate by liver mitochondria. I. Activities at different pH values. Biochem J. 1957 Dec;67(4):558–572. doi: 10.1042/bj0670558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovitz P. J., Chuang D. T. The bifunctional aminoadipic semialdehyde synthase in lysine degradation. Separation of reductase and dehydrogenase domains by limited proteolysis and column chromatography. J Biol Chem. 1987 Jul 5;262(19):9353–9358. [PubMed] [Google Scholar]
- Nicoletti M., Guerri C., Grisolia S. Turnover of carbamyl-phosphate synthase, of other mitochondrial enzymes and of rat tissues. Effect of diet and of thyroidectomy. Eur J Biochem. 1977 May 16;75(2):583–592. doi: 10.1111/j.1432-1033.1977.tb11558.x. [DOI] [PubMed] [Google Scholar]
- Noda C., Ichihara A. Purification and properties of L-lysine-alpha-ketoglutarate reductase from rat liver mitochondria. Biochim Biophys Acta. 1978 Aug 7;525(2):307–313. doi: 10.1016/0005-2744(78)90225-5. [DOI] [PubMed] [Google Scholar]
- Peret J., Foustock S., Chanez M., Bois-Joyeux B., Assan R. Plasma glucagon and insulin concentrations and hepatic phosphoenolpyruvate carboxykinase and pyruvate kinase activities during and upon adaptation of rats to a high protein diet. J Nutr. 1981 Jul;111(7):1173–1184. doi: 10.1093/jn/111.7.1173. [DOI] [PubMed] [Google Scholar]
- Porpaczy Z., Sumegi B., Alkonyi I. Interaction between NAD-dependent isocitrate dehydrogenase, alpha-ketoglutarate dehydrogenase complex, and NADH:ubiquinone oxidoreductase. J Biol Chem. 1987 Jul 15;262(20):9509–9514. [PubMed] [Google Scholar]
- Rao V. V., Pan X., Chang Y. F. Developmental changes of L-lysine-ketoglutarate reductase in rat brain and liver. Comp Biochem Physiol B. 1992 Sep;103(1):221–224. doi: 10.1016/0305-0491(92)90435-t. [DOI] [PubMed] [Google Scholar]
- Rao V. V., Tsai M. J., Pan X., Chang Y. F. L-pipecolic acid oxidation in rat: subcellular localization and developmental study. Biochim Biophys Acta. 1993 Jun 24;1164(1):29–35. doi: 10.1016/0167-4838(93)90108-4. [DOI] [PubMed] [Google Scholar]
- Scislowski P. W., Grant G., Harris I., Pickard K., Pusztai A. Urea synthesis in rats fed diet containing kidney beans. Biochem Int. 1992 Oct;28(1):191–197. [PubMed] [Google Scholar]
- Shigesada K., Tatibana M. Role of acetylglutamate in ureotelism. I. Occurrence and biosynthesis of acetylglutamate in mouse and rat tissues. J Biol Chem. 1971 Sep 25;246(18):5588–5595. [PubMed] [Google Scholar]
- Shinno H., Noda C., Tanaka K., Ichihara A. Induction of L-lysine-2-oxoglutarate reductase by glucagon and glucocorticoid in developing and adult rats: in vivo and in vitro studies. Biochim Biophys Acta. 1980 Dec 15;633(3):310–316. doi: 10.1016/0304-4165(80)90190-7. [DOI] [PubMed] [Google Scholar]
- Siess E. A., Brocks D. G., Lattke H. K., Wieland O. H. Effect of glucagon on metabolite compartmentation in isolated rat liver cells during gluconeogenesis from lactate. Biochem J. 1977 Aug 15;166(2):225–235. doi: 10.1042/bj1660225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siess E. A., Wieland O. H. Glucagon-induced stimulation of 2-oxoglutarate metabolism in mitochondria from rat liver. FEBS Lett. 1978 Sep 15;93(2):301–306. doi: 10.1016/0014-5793(78)81126-0. [DOI] [PubMed] [Google Scholar]
- Snodgrass P. J., Lin R. C., Müller W. A., Aoki T. T. Induction of urea cycle enzymes of rat liver by glucagon. J Biol Chem. 1978 Apr 25;253(8):2748–2753. [PubMed] [Google Scholar]
- Takechi T., Okada T., Wakiguchi H., Morita H., Kurashige T., Sugahara K., Kodama H. Identification of N-acetyl-alpha-aminoadipic acid in the urine of a patient with alpha-aminoadipic and alpha-ketoadipic aciduria. J Inherit Metab Dis. 1993;16(1):119–126. doi: 10.1007/BF00711325. [DOI] [PubMed] [Google Scholar]
- Titheradge M. A., Binder S. B., Yamazaki R. K., Haynes R. C., Jr Glucagon treatment stimulates the metabolism of hepatic submitochondrial particles. J Biol Chem. 1978 May 25;253(10):3357–3360. [PubMed] [Google Scholar]
- Titheradge M. A., Picking R. A., Haynes R. C., Jr Physiological concentrations of 2-oxoglutarate regulate the activity of phosphoenolpyruvate carboxykinase in liver. Biochem J. 1992 Aug 1;285(Pt 3):767–771. doi: 10.1042/bj2850767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walajtys-Rhode E., Zapatero J., Moehren G., Hoek J. B. The role of the matrix calcium level in the enhancement of mitochondrial pyruvate carboxylation by glucagon pretreatment. J Biol Chem. 1992 Jan 5;267(1):370–379. [PubMed] [Google Scholar]


