Abstract
The globus pallidus (GPe) of the basal ganglia has been underappreciated due to poor understanding of its cells and circuits. It was assumed that the GPe consisted of a homogenous neuron population primarily serving as a "relay station" for information flowing through the indirect pathway. However, the advent of advanced tools in rodent models has sparked a resurgence in interest in the GPe. Here, we review recent data that have unveiled the cell and circuit complexity of the GPe. These discoveries have revealed that the GPe does not conform to traditional views of the basal ganglia. Particularly, recent evidence confirms that its afferent and efferent connections span both the direct and indirect pathways. Furthermore, the GPe displays broad interconnectivity beyond the basal ganglia, consistent with its emerging multifaceted roles in both motor and non-motor functions. Altogether, recent data prompt new proposals for computational rules of the basal ganglia.
Keywords: cellular diversity, synaptic connectivity, motor control, Parkinson’s disease
Our ability to move is essential to survival. Volitional movements are thought to be controlled by two major forebrain descending pathways—the cortical and basal ganglia circuits. The general organization of the basal ganglia is conserved throughout vertebrate evolution1. In the simplified basal ganglia circuit, the cortex provides excitatory inputs to both the striatum (dStr) and the subthalamic nucleus (STN). They in turn project to the substantia nigra pars reticulata (SNr) and internal globus pallidus (GPi)—the output of the basal ganglia (see Figure 1). As simplified models of the basal ganglia emphasize feedforward circuitry, intrinsic feedback circuits are often ignored. Importantly, although most circuit models assume a homogenous neuronal population, recent studies argue that neuronal heterogeneity is common across the basal ganglia2–10.
Figure 1. Classic model and GPe-centric model of the basal ganglia.
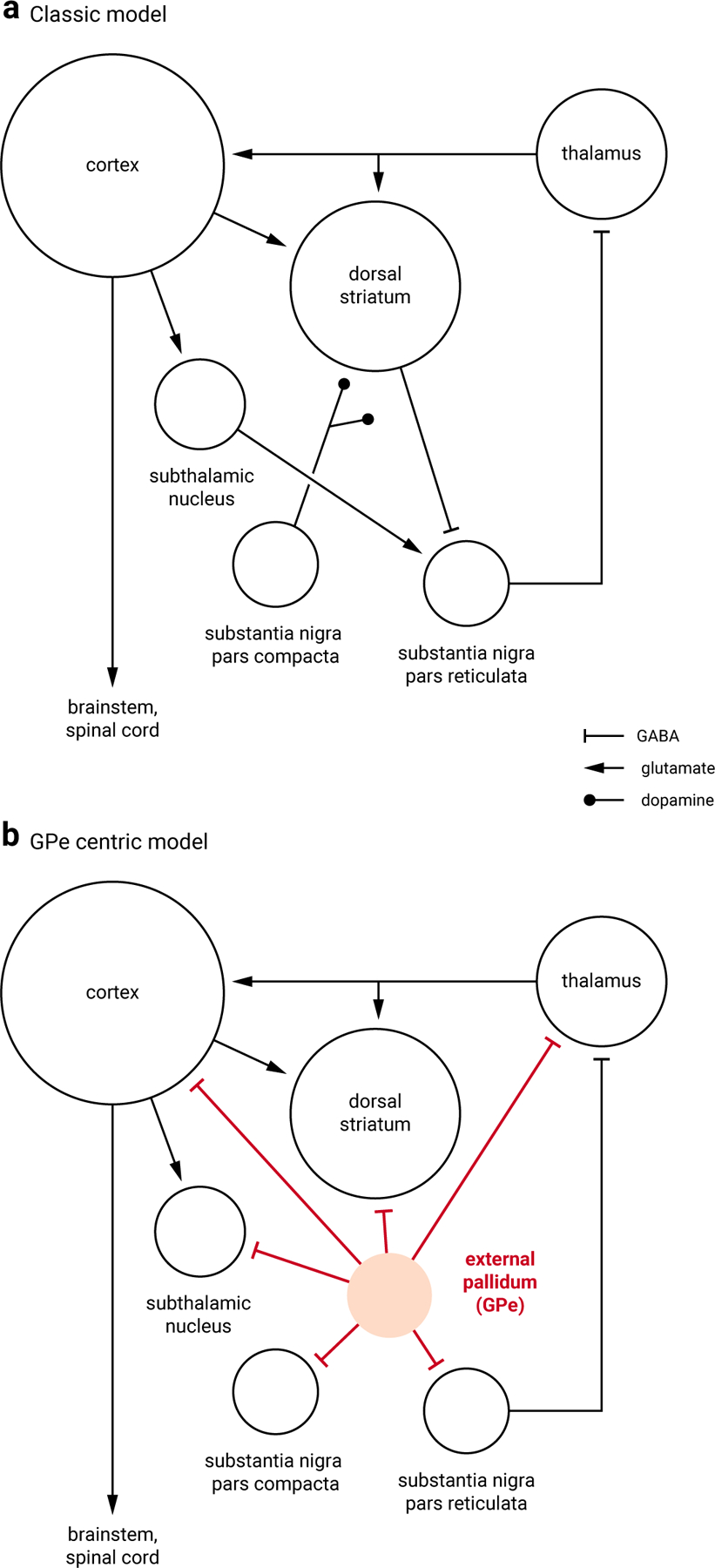
a. A simplified classic circuit diagram showing the key nodes within the basal ganglia; the circuit role of the GPe is often ignored. The dorsal striatum and subthalamic nucleus are the two input stations that receive excitatory cortical inputs; the substantia nigra pars reticulata is the output station that provides inhibitory projection to the thalamus. Dopamine exerts a broad influence over the entire basal ganglia circuit, especially the dorsal striatum. Size of the target areas (circles) is an artistic rendering based on the volume of the brain areas. b. The GPe sends inhibitory projections to all key structures within the circuit. Some of the connections are removed for illustration purposes to highlight the central role of the GPe within the basal ganglia.
The external globus pallidus (GPe), as part of the basal ganglia, was once thought to be a simple relay nucleus. Pioneering studies using crude manipulations yielded conflicting results regarding the function of the GPe. However, by targeting specific cell types with improved precision, recent work in rodents has revealed that the GPe is more complex than once thought. We now know that the GPe has a heterogeneous neuronal makeup, containing populations with distinct characteristics and functions. Critically, there is now direct evidence that GPe neuron activity plays causal roles in movement11–16. These results corroborate neural recordings that show phasic changes in the firing of GPe neurons are associated with body movement17–19.
Historical reviews of the GPe are available elsewhere20–22. Here, we focus on the evolving views of neuron classification in the GPe, with particular emphasis on the recent breakthroughs in the circuit properties and functions of GPe neurons. We review evidence supporting the idea that the unique connectivity of GPe neuron subtypes drives their functions and that these functions go awry in disease conditions.
Classification of Neurons
Principal neuron classes
Heterogeneity in the phenotype of GPe neurons was noted in the early 1970’s. However, as the descriptions of molecularly-defined GPe neuron subtypes were not established until less than a decade ago23,24, it was not possible to correlate the cellular features with the molecular identity of neurons. Using advanced tools in rodent models, it is now established that the near-exclusive expression of the calcium-binding protein parvalbumin (PV) and the transcription factor Npas1 identifies the two separate supersets of GABAergic neuron types in the adult GPe4,13,23–28.
PV-expressing (PV+) neurons and Npas1-expressing (Npas1+) neurons constitute 50% and 30% of the adult GPe, respectively, and are considered as the two principal neuron classes (see Figure 2). They are distinct across multiple modalities, including axonal patterns, synaptic inputs, electrophysiological properties, alterations in disease states, and behavioral roles12–14,25,27–31. The reported abundance of PV+ neurons varies across laboratories in part due to methodological differences (e.g., using cell-specific driver lines, retrograde labeling, fate mapping, and immunohistochemistry)23–27,29,30,32–35. Additionally, some of the observed range in the abundance of PV+ neurons is due to the spatial gradients in their distribution. However, by systematically examining a number of transgenic lines, a recent examination definitively confirmed that PV+ neurons account for approximately 50% of neurons in the GPe28.
Figure 2. Developmental and marker heterogeneity of GPe neurons.
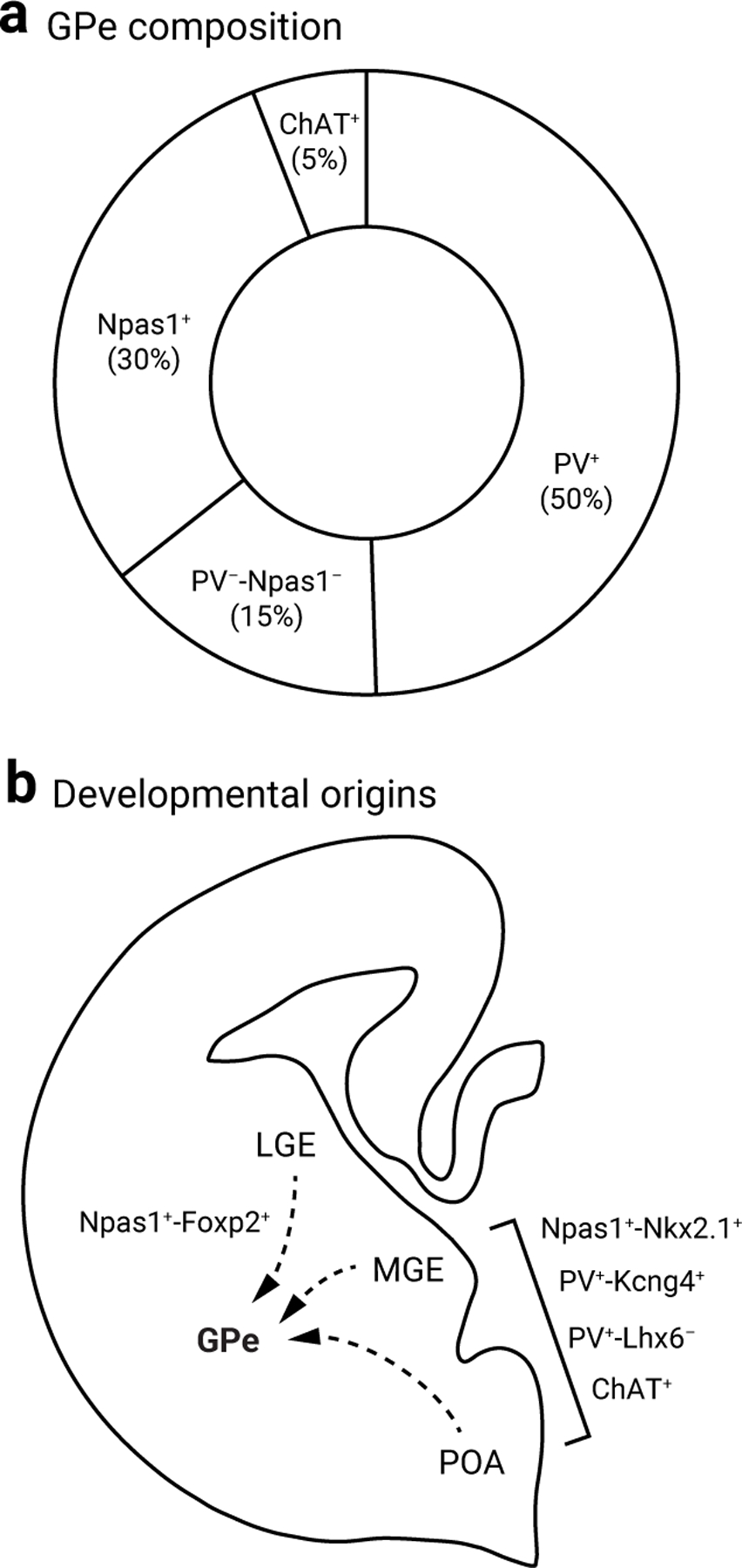
a. Pie chart summarizing the neuronal composition of the rodent GPe. The area of the sectors represents the approximate size of each neuron class. PV+ neurons (which constitute 50% of the GPe) can be subdivided based on their projections to the subthalamic nucleus and substantia nigra pars reticulata vs. parafascicular nucleus. Npas1+ neurons are 30% of the GPe; they can be subdivided into two subclasses that differ in their marker profiles and axonal projections. ChAT+ neurons are ∼5% of the total GPe neuron population, show no overlap with other known classes of GPe neurons, and project broadly to cortical areas. PV−-Npas1− neurons amount to ∼15% of the total GPe and are the least characterized neuron subtype.
b. Diagram of a cross section of the rodent fetal forebrain illustrating the contributions of lateral ganglionic eminence (LGE), medial ganglionic eminence (MGE), and preoptic area (PoA) to GPe neurons subtypes. The MGE and PoA give rise to the majority of GPe neuron subtypes, except Npas1+-Foxp2+ neurons, which are derived from the LGE.
Aside from the two principal neuron classes, neurons lacking expression of either PV or Npas1 amount to 20% of the GPe. This population includes choline acetyltransferase-expressing (ChAT+) neurons, which amount to 5% of the total population27,28,30 and project to the cortex30. The remaining GPe neurons (~15%) have not been rigorously characterized. This neuron population expresses the transcription factor Lhx6 but not Sox6 (Lhx6+-Sox6−)28. Likely due to the relatively low abundance of Lhx6+-Sox6− neurons, no data are available from the previous single-cell transcriptomic dataset4,36, and identifying reliable markers for these neurons has been challenging. Importantly, Lhx6 expression should not be considered as a classifier as it is seen across multiple GPe neuron populations, including in PV+, Npas1+, and PV−-Npas1− neurons. Furthermore, the reported expression of Lhx6 within these subpopulations varies widely across laboratories; some studies show little overlap with other neuron groups, while others show up to a ~95% overlap with other established cell type-specific markers4,11,14,16,25,26,28,29,34 (see Neuron subclasses & Figure 2).
While it is likely that there are additional rare neuron types yet to be discovered, we now have a near-complete description of the major neuron types within the GPe. Importantly, while neurons with phenotypic similarities are observed in primate studies, we cannot yet directly equate neuron subtypes in rodents to higher-order species due to methodological limitations in identifying neuron types in primates37. Nevertheless, future analyses such as transcriptome-wide spatial profiling and single-nucleus RNA-sequencing will be useful for characterizing the rare neuron types in the GPe.
Neuron subclasses
Although PV+ neuron and Npas1+ neuron classes are distinct across multiple modalities, such as electrophysiological and synaptic properties, subtle differences exist within each neuron class. Through harnessing molecular tools to target specific GPe neuron populations, recent studies have identified distinct subclasses of PV+ and Npas1+ neurons with unique properties. Within the Npas1+ class, 60% are Foxp2+ (Npas1+-Foxp2+, also known as ‘arkypallidal’ neurons; see Box 1); these neurons represent the first unique GPe neuron subclass described38. Compelling data show the distinct features of Npas1+-Foxp2+ neurons, such as their developmental origin and their electrophysiological, anatomical, and molecular profiles26. Importantly, as Foxp2 expression is maintained in adults, the Foxp2-Cre and Foxp2-Flp mouse lines have been successfully used across labs to capture this population of neurons14,15,28,39.
Box 1. Evolving classification of GPe neurons.
GPe neurons are often classified into two main groups: ‘arkypallidal’ and ‘prototypic’ neurons. The defining features of these neurons have emerged over time since the early 2000s38,150. Recent evidence confirmed that arkypallidal neurons directly correspond to the Npas1+-Foxp2+ neuron subclass. These neurons have a unique molecular signature, developmental origin, and axonal projection pattern. On the other hand, prototypic neurons (aka non-arkypalldal neurons) encompass a heterogeneous population of neurons that differ widely in their anatomical projections, electrophysiological properties, and functional output, thus limiting the effectiveness of the classification and contributing to less rigorous comparisons within the field. It is important to note that while all PV+ neurons are prototypic, not all prototypic neurons are PV+. Furthermore, not all GPe neurons with ascending projections to the dStr are arkypallidal neurons. We propose the term prototypic be avoided, as it is repeatedly misused, and suggest that GPe neurons be classified using more descriptive designations, such as by molecular signature or principal projection target(s) to avoid confusion.
The remaining neurons within the Npas1+ class are both Nkx2.1+ and Lhx6+. Unlike Npas1+-Foxp2+ neurons that project exclusively to the dStr, Npas1+-Nkx2.1+ neurons project to the midbrain, cortex, and reticular thalamus14,28,30 (see Synaptic Outputs & Figure 3). Accordingly, both Npas1+-Foxp2+ and Npas1+-Nkx2.1+ neuron subtypes should be regarded as bona fide subclasses, as recent single-cell data4,36 further confirmed the unique transcriptomic properties of these two Npas1+ neuron subclasses. Notably, these studies led to the discovery that GPe neurons labeled using the Npr3-Cre driver are molecularly and anatomically consistent with Npas1+-Nkx2.1+ neurons14.
Figure 3. Input to the GPe.
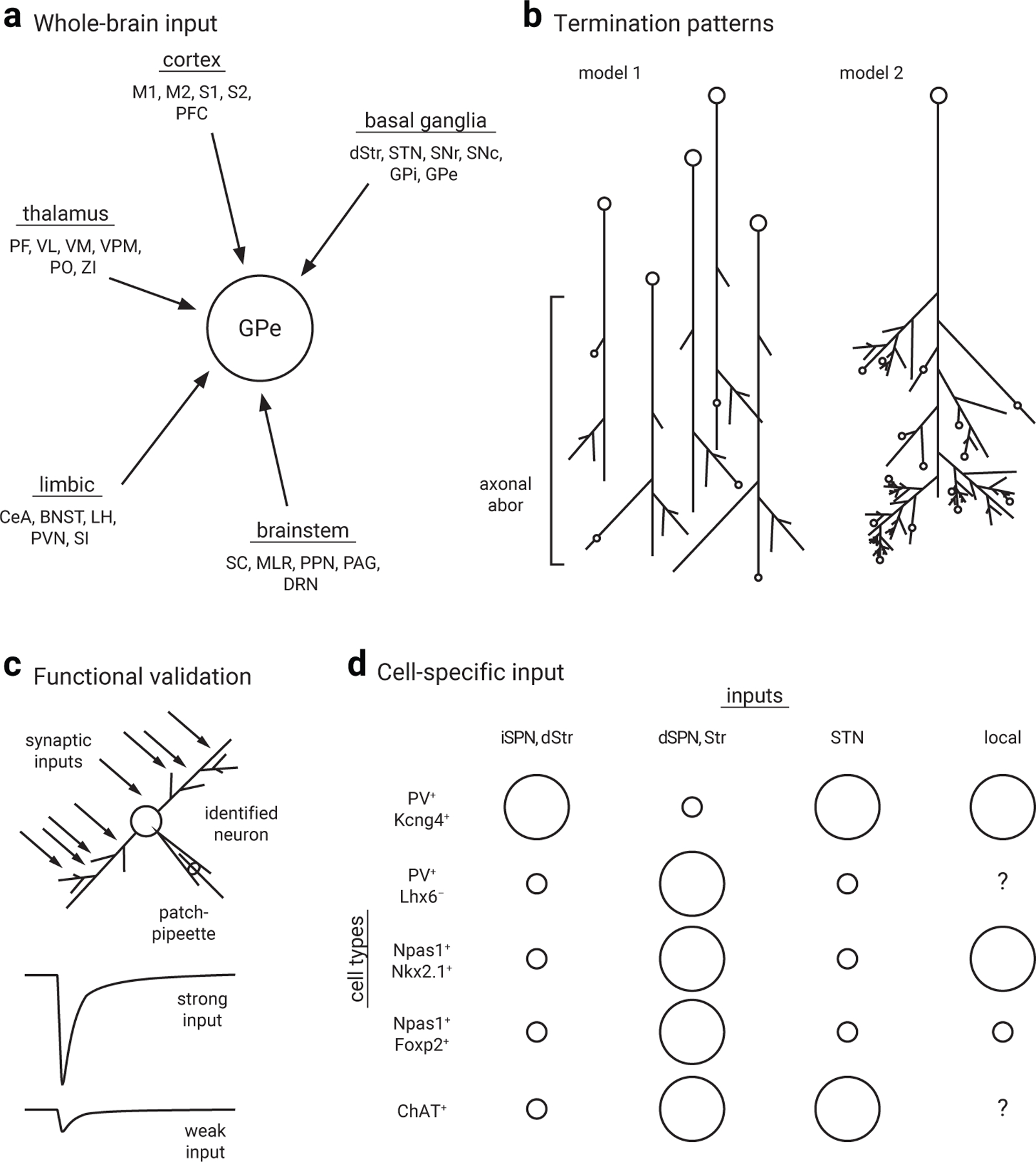
a. Diagram highlighting the diversity of inputs to the GPe across the entire brain. Data are summarized from viral tracing studies16,46–48. Differences are observed across studies due to technical nuances, such as tropism of viral preparations. Connectome results should be interpreted with both the termination patterns (b) and functional properties of inputs in mind. Additional characteristics, such as the physiological properties, chemical nature, and cell-targeting patterns remain to be studied systematically. Abbreviations: BNST, bed nucleus of the stria terminalis; CeA, central nucleus of the amygdala; DRN, dorsal raphe nucleus; dStr, dorsal striatum; GPe, external globus palldius; GPi, internal globus palldius; LH, lateral hypothalamus; M1, primary motor cortex; M2, MLR, mesencephalic locomotor region; PAG, periaqueductal gray; PF, parafascicular nucleus; PFC, prefrontal cortex; PPN, pedunculopontine nucleus; S1, primary sensory cortex; S2, secondary motor cortex; SC, superior colliculus; SI, substantia innominata; SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticulata; STN, subthalamic nucleus; VL, ventrolateral nucleus; VM, ventromedial nucleus; VPM, ventral posteromedial nucleus; ZI, zona incerta.
b. Input to the GPe shows varied termination patterns. Model 1 describes input formed by a large number of neurons giving rise to only a small number of synapses (e.g., striatal projection neurons). Model 2 describes input formed by a small number of neurons that give rise to a large number of synapses (e.g., PV+ neuron local collaterals).
c. While widespread inputs have been mapped via tracing studies, relatively few inputs have been functionally validated. However, a select subset of inputs to the GPe has been characterized using patch-clamp recordings either ex vivo or in vivo. For simplicity, these inputs are categorized into strong vs. weak inputs in both c and d.
d. Simplified visualization of the relative strength of basal ganglia inputs to GPe neuron subtypes. Note that GPe neurons receive substantial direct pathway input, inconsistent with the classic model. Size of the circles is used as a binary categorization to differentiate between strong vs. weak inputs across GPe neuron subtypes; it is an approximation and not intended for comparisons among inputs within a given GPe neuron subtype. Question marks denote inputs that have not been studied; cell-type specificity of inputs from other regions remains to be examined.
In addition to the two distinct Npas1+ neuron subclasses, multiple lines of evidence point to at least two subtypes of PV+ neurons (Figure 2). One of these populations is identified by their axonal projection to STN and SNr. The marker Kcng4 demarcates a subset of these STN-projecting PV+ neurons. However, a marker encompassing all STN-projecting PV+ neurons has yet to be determined14. In addition, parafascicular thalamus (Pf)-projecting PV+ neurons constitute a unique subtype of PV+ neurons within the GPe—they have distinct synaptic partners, intrinsic properties, and behavioral roles16. These PV+ neuron subclasses are further distinguished by Lhx6 expression, which converging evidence suggests is present in the STN-projecting population but is weak or absent in the Pf-projecting population16,29. While it is clear that STN-projecting (PV+-Kcng4+) neurons and Pf-projecting (PV+-Lhx6−) PV+ neurons are unique neuron subclasses, how they assimilate into existing fate-map and transcriptomic data (see also Developmental origins) is unclear. Therefore, it will be paramount for future studies to reconcile how axonal projection profiles align with molecular marker expression within the PV+ neuron class. This in turn should facilitate our goal to map specific neuron types to functions. Altogether, the vast differences in marker expression, axonal projections, and physiological properties between GPe neuron subtypes stand in direct contrast to the traditional model of the GPe serving as a homogenous relay nucleus of the indirect pathway.
Developmental origins
The literature converges on the idea that both the embryonic origins and the congruent expression of transcription factors govern cell specifications40. A set of transcription factors have been previously identified to dictate the formation of the GPe23,24,41–43. More recent studies have extended our understanding of the molecular landscape of the GPe and have collectively provided a firmer foundation on its neuronal composition14,28.
The GPe primarily arises from the medial and lateral ganglionic eminence (MGE and LGE)23,24,26,44 (see Figure 2). Immunolabeling for Nkx2.1 can be used to identify MGE-derived neurons. However, as only 90% of neurons that arise from the Nkx2.1 lineage maintain Nkx2.1 protein expression in adulthood, this approach is not faithful28. While PV+ neurons are believed to largely arise from the MGE, their expression of MGE-markers varies—over 80% express Nkx2.1 whereas only ~30–40% maintain Lhx6 in adulthood16,27,28. This is consistent with data suggesting the existence of two pools of PV+ neurons that are produced with different temporal patterns and occupy slightly different, but otherwise largely overlapping spatial domains within the GPe44.
Npas1+ neurons are derived from both the MGE and LGE; MGE-derived Npas1+ neurons are Nkx2.1+ and Lhx6+, while LGE-derived Npas1+ neurons are Foxp2+23–26,28. Although developmental origins likely influence transcriptional programs that control the specifications of GPe neurons, Npas1+-Nkx2.1+ neurons and Npas1+-Foxp2+ neurons have similar electrophysiological properties despite their differing origins28. In contrast, PV+ neurons and Npas1+-Nkx2.1+ neurons have striking differences in their electrophysiological and synaptic properties despite sharing a common developmental derivation (see Synaptic Inputs).
In addition to the MGE and LGE, a small fraction of GPe neurons are derived from the preoptic area (PoA) and descend from the Dbx1-lineage (Dbx1+)23,28,42,43,45. The Dbx1+ population contains neurons that express, to varying degrees, all established GPe neuron markers28. This is consistent with the literature that PoA progenitors give rise to a small but diverse contingent set of neurons42,43. In particular, most Dbx1+ neurons are Sox6-expressing; they primarily express PV (~70%) and to a lesser extent Npas1 (~10%). Accordingly, Dbx1+ neurons do not correspond to the Lhx6+-Sox6− population, which is PV−-Npas1− (see Principal neuron classes). Despite their lineage difference, PV+-Dbx1+ neurons display a phenotype similar to the general PV+ population23,24. The wide diversity of molecular marker expression seen in PoA-derived neurons further indicates that the developmental origin of GPe neurons should not be considered a predictor of their eventual properties. Altogether, recent studies argue that while developmental origin is an important consideration for neuronal identification, neuronal birthplace itself does not dictate the physiological and synaptic characteristics of GPe neurons.
Synaptic Inputs
The classic model asserts that the GPe is an intrinsic nucleus within the basal ganglia circuit, receiving inputs exclusively from the dStr and the STN. However, accumulating evidence demonstrates that the GPe receives diverse inputs from additional sources (Figure 3). In particular, direct cortical inputs to the GPe reject the hierarchical organization proposed in the classic model. Additionally, the recent generation of whole-brain input maps16,28,46–48 reaffirms that the GPe integrates a wide variety of inputs across a number of systems and should therefore no longer be considered an intrinsic nucleus. As the size of inputs in these studies is estimated from cell counts, results cannot be easily interpreted without considering the termination patterns or the physiological properties of the inputs. Regardless, the intersection of a complex set of inputs at the GPe allows the transformation of salient cues to motoric responses. Additionally, inputs from non-basal ganglia regions support the notion that the GPe participates in non-motor functions.
Though different markers and classification schemes are used in the literature, the data from ‘prototypic’ and ‘arkypallidal’ neurons coincide with the PV+ and Npas1+ neuron classifications, respectively (see Box 1). For simplicity, research findings are discussed in relation to these principal neurons in the following sections. Consistent with the idea that PV+ neurons and Npas1+ neurons receive distinct sets of inputs, the two neuron classes have different electrophysiological properties14,27,28, suggesting a different mode of input-output transformation between them. The differences in the electrophysiological properties of neuron subclasses are more subtle, and it is still unknown how the complement of inputs varies substantially between neuron subclasses.
GABAergic inputs
The dStr is, by far, the largest input to the GPe—the number of input neurons from the dStr constitutes ~80% of the total projection to the GPe and is at least an order of magnitude larger than that from other brain regions47,49. This observation is consistent with the earlier finding that GABAergic synapses amount to over 80% of all synapses in the GPe20.
Contrary to the simplified basal ganglia model, it has been known since the 1980s that axons from both direct-pathway spiny projection neurons (dSPNs) and indirect-pathway SPNs (iSPNs) converge at the GPe. While iSPN axons terminate exclusively in the GPe, modern techniques confirm that dSPN axons collateralize in both the GPe and the SNr47,49–54; these studies solidify the fact that the GPe receives notable direct pathway input and demonstrate the physiological and targeting properties of dSPN collaterals. Nevertheless, the indirect pathway remains the predominant input to the GPe. While previous studies showed that dSPNs provide roughly half the number of boutons compared to iSPNs in the GPe, more recent estimations of the number of boutons formed by iSPNs is three to eight times higher than that formed by dSPNs47,49. Both viral-tracing and patch-clamp studies demonstrate that iSPNs strongly target canonical STN-projecting PV+ (PV+-Kcng4+) neurons, whereas dSPNs largely target Npas1+ neurons, Pf-projecting PV+ (PV+-Lhx6−) neurons, and ChAT+ neurons15,16,30,39,47,55,56.
GPe neurons provide lateral GABAergic inhibition to neighboring neurons through their local axon collaterals14,15,31,39,56–61. These collaterals give rise to a large number of local synapses, in contrast to the relatively few synapses typically formed by input from individual SPNs59. They have not been studied in great detail until recently due to the inherent difficulty in identifying and selectively activating individual classes of GPe neurons and their collateral axons. These technical challenges have been overcome by combining transgenic and optogenetic approaches. Although subtle nuances exist across studies, three key findings have recently emerged. First, PV+ neurons provide the largest local input. Second, Npas1+-Foxp2+ neurons do not produce appreciable levels of local connections. Third, the local collaterals can strongly inhibit neighboring neurons and thus are integral to both local circuit dynamics and downstream network effects14,15,31,39,56,57. While further studies are needed to investigate the functional relevance of the unique organization of the local collaterals, the existence of local inhibitory networks creates an anatomical substrate for feedback and functional opponency across neuron subtypes.
Glutamatergic inputs
Whole-brain input maps verify that the STN is one of the primary glutamatergic inputs innervating the GPe16,46,47. However, the cellular targeting properties of this input were not known until recently. Three independent studies have convincingly demonstrated that STN inputs target PV+ neurons more strongly relative to Npas1+ neurons13,15,39. While the neuronal makeup of the STN was generally thought to be homogeneous, recent studies show that STN neurons are more heterogeneous than previously expected6,9,58,62–64. Therefore, it will be important to determine if PV+-targeting neurons within the STN are distinct from those targeting Npas1+ neurons.
Recent studies highlight the existence of cortical inputs to the GPe. Importantly, viral-tracing data argue that the spatially-distributed cortical inputs altogether form the largest source of excitatory inputs to the GPe16,28,65–68, in contrast to the traditional model, which assumes dominant glutamatergic input from the STN. These cortical inputs arise primarily from pyramidal tract neurons9,28,67. However, the precise downstream targets require further characterization, as data from recent studies are somewhat conflicting. While a recent study suggests that secondary motor cortex input selectively targets Npas1+-Foxp2+ neurons65, data from others show that cortical innervations do not target specific neuron subtypes9,16,28.
In addition to the STN and cortex, the Pf and pedunculopontine nucleus send glutamatergic projections to the GPe13,16,47,69–71, two sources that are omitted from the classic model. These inputs do not show a biased connectivity pattern across PV+ neuron and Npas1+ neuron classes13. Taken together, the most recent data indicate that the GPe integrates a wide array of synaptic inputs, including from sources beyond traditional basal ganglia nuclei.
Neuromodulatory inputs
The GPe receives substantial dopaminergic input—recent viral tracing data show that this input arises primarily from the substantia nigra pars compacta, though we have yet to determine the precise neuronal subpopulation responsible3,16,46,47. However, this is likely an underestimation of the total dopamine input; earlier findings suggest that a much broader area, including the substantia nigra pars compacta, ventral tegmental area, and retrorubral area, give rise to the dopaminergic innervations in the GPe62,72–79.
The GPe is at the intersection of motor-anxiety circuits, as tracing studies reveal inputs from corticotropin releasing factor-expressing neurons in the paraventricular thalamus and the central amygdala16,46–48,80. While the signaling partners of this input remain to be explored, strong evidence argues that PV+ neurons and their projections are a major target as they robustly express Crfr146,48,80,81. Notably, the central amygdala forms one of the largest extrinsic inputs targeting the GPe47, and this projection has been recently implicated in fear learning80.
Altogether, whole-brain connectome analyses suggest a diverse neuromodulatory control at the GPe level3,16,46,47,82 and reinforce the notion that the GPe is a critical node that interfaces signals from a number of systems (see Figure 3). In turn, neuromodulatory control of GPe neurons can influence both motor and non-motor behavior within the same circuit.
Synaptic Outputs and Functions
Although major efferent projections from the GPe have been mapped previously20–22, the circuit elements involved were not previously known. By gaining genetic access to unique neuron types within the mouse GPe, researchers have provided detailed circuit dissections for GPe neuron outputs and their functional implications (see Figure 4). These studies collectively reveal that changes in the activity of GPe neurons in vivo are not merely passive responses to movement—in other words, GPe neurons have the capacity to tune or maintain ongoing actions. Furthermore, increasing evidence indicates GPe neuron involvement in non-motor roles, including learning16, limbic function2,46,48,80,81,83, sleep84, and the processing of sensory and reward cues37,56. It is probable that interconnections with classic basal ganglia nuclei (such as dStr and STN) are involved in multiple functions, consistent with the “parallel processing” model (see Figure 5). Additionally, more recently discovered connections (i.e., cortex and thalamus) play important roles in both motor and non-motor functions. Altogether, anatomical and functional data collectively emphasize that the GPe should not be considered merely an intrinsic nucleus within the larger basal ganglia.
Figure 4. Distinct efferent projections of GPe neuron subtypes.
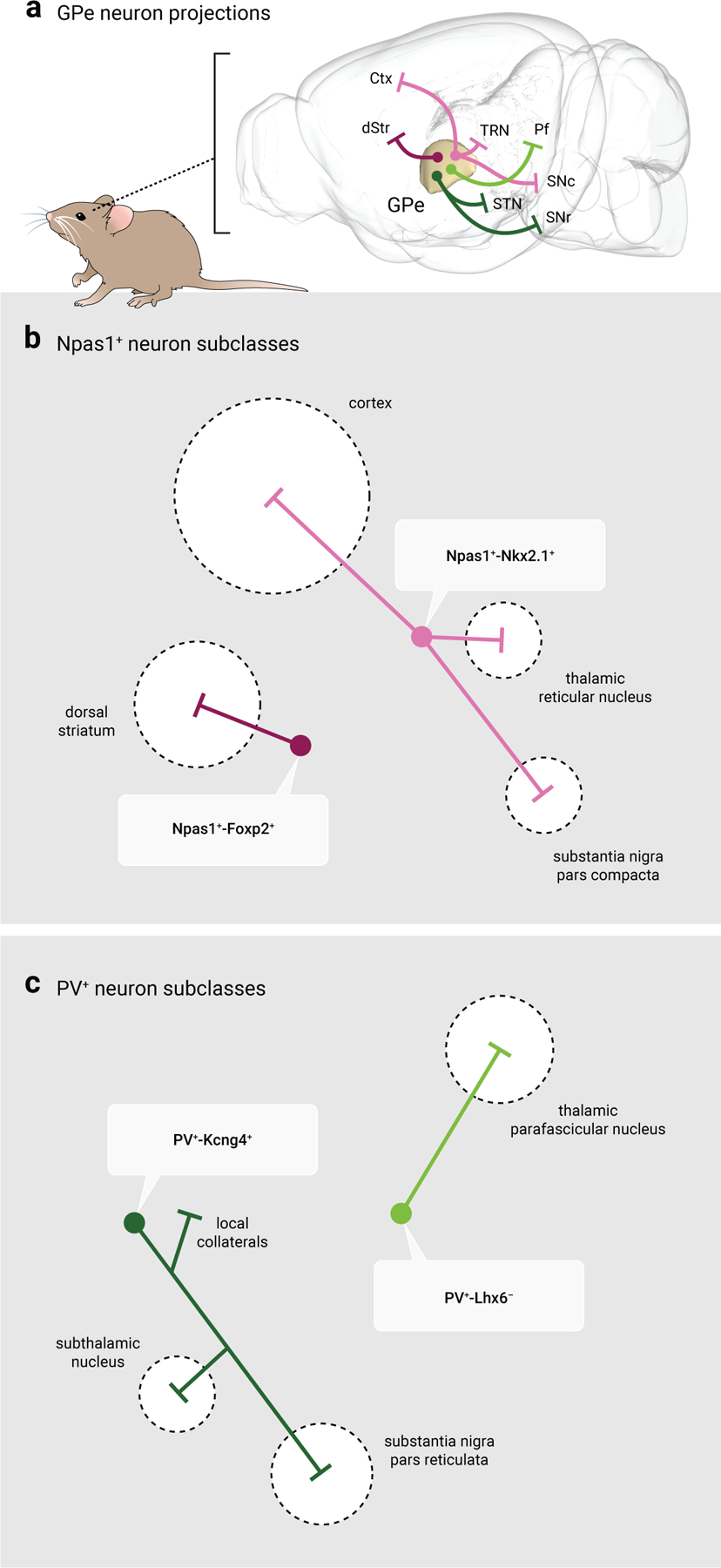
a. Map displaying the major projection targets of PV+ neurons and Npas1+ neurons. Note that GPe neuron projections are widespread and extend beyond the traditional basal ganglia nuclei, in contrast to the classic indirect pathway model. Magenta: Npas1+-Foxp2+ neurons; Pink: Npas1+-Nkx2.1+ neurons; Dark green: PV+-Kcng4+ neurons; Light green: PV+-Lhx6− neurons. Abbreviations: Ctx, cortex; dStr, dorsal striatum; Pf, thalamic parafascicular nucleus; SNr, substantia nigra pars reticulata; SNc, substantia nigra pars compacta; STN, subthalamic nucleus; TRN, thalamic reticular nucleus.
b. Schematic of the projection targets of Npas1+ neuron subtypes. Npas1+-Foxp2+ neurons represent 60% of the total Npas1+ neuron class and project exclusively to the dorsal striatum, targeting spiny projection neurons. Npas1+-Nkx2.1+ neurons represent 40% of the total Npas1+ neuron class and project to the cortex, thalamic reticular nucleus, and substantia nigra pars compacta.
c. Schematic of the projection targets of identified PV+ neuron subtypes. PV+-Kcng4+ neurons overlap with canonical PV+ neurons; they project to the subthalamic nucleus, substantia nigra pars reticulata and provide collateral inhibition to local GPe neurons. PV+-Lhx6− neurons project to the thalamic parafascicular nucleus. Size of the target areas (dotted circles) in b, c are artistic renderings based on the volume of the target areas and do not reflect the axonal density, synaptic strength, or contacts formed by GPe neuron subclasses.
Figure 5. Cellular and circuit substrates for functional regulation by GPe neurons.
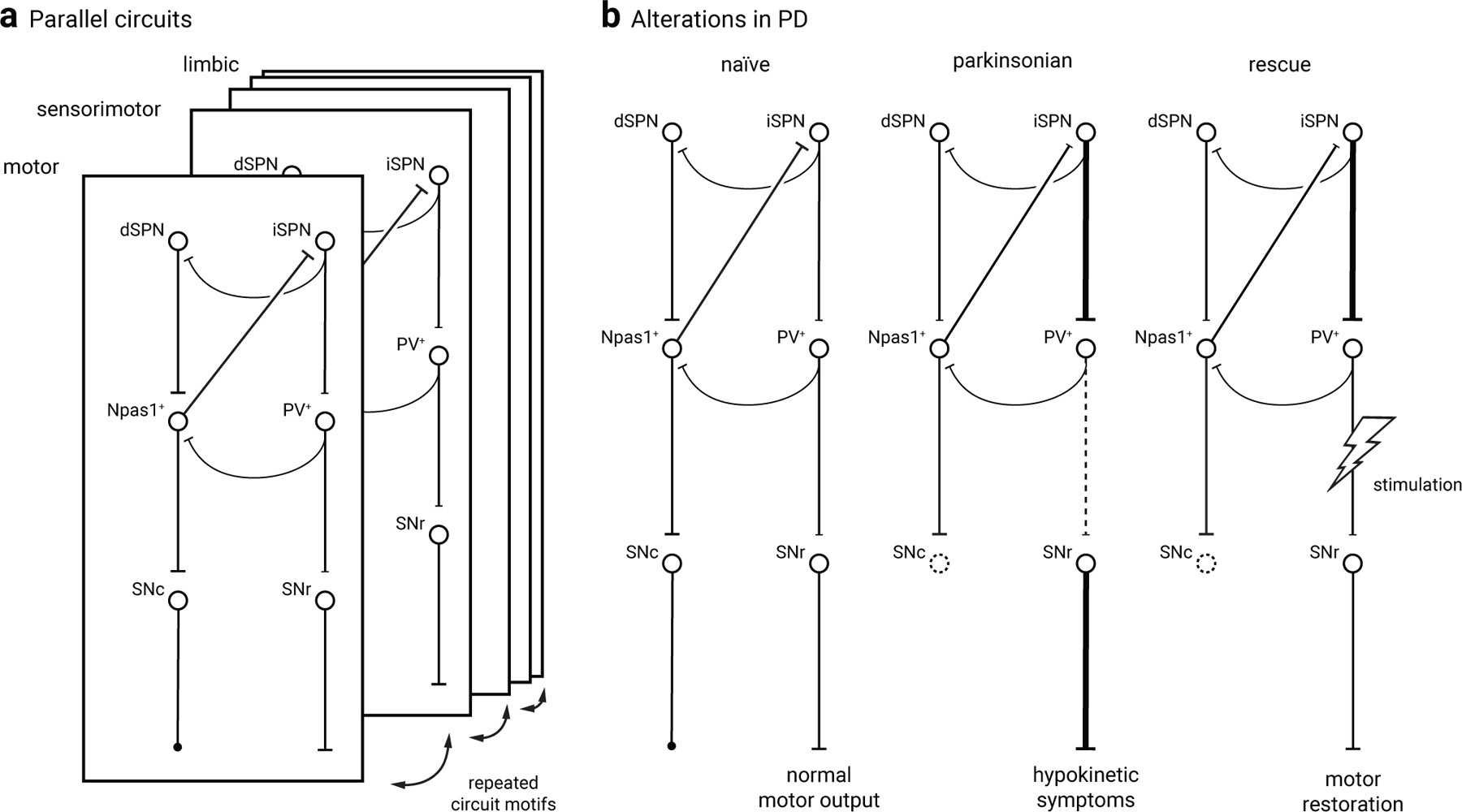
a. A simplified diagram of the GPe and its associated circuit. PV+ neurons and Npas1+ neurons are illustrated as the principal cell classes within the GPe and are differentially organized with the input and output of the basal ganglia. Consistent with their distinct electrophysiological properties, PV+ neurons and Npas1+ neurons have unique behavioral roles: PV+ neurons are motor-promoting, while Npas1+ neurons are motor-inhibiting. The PV+ neuron and Npas1+ neuron classes jointly tune motor behavior through a push-pull mechanism; local inhibitory connections between these neuron classes may control this process. Importantly, the same circuit motif is conserved across motor, sensorimotor, and limbic domains of the basal ganglia to support parallel processing of information.
b. A proposed model of circuit alterations in 6-OHDA lesioned mice and therapeutic rescue via PV+ neuron stimulation. PV+ neuron output to the SNr is functionally weakened (dotted line) in the absence of dopamine. Stimulation of the PV+ neuron output has been shown to restore motoric function in mouse models of Parkinson’s disease, putatively through restoring a normal level SNr output (e.g.149).
PV+ neurons
PV+ neurons form the principal inhibitory innervation to the STN25,27,29,32,33,35. Consistent with this, optogenetic and chemogenetic stimulation of pan-PV+ neurons or selectively STN-projecting GPe neuron subsets promote movement11,13,14,16. In addition, this effect can be recapitulated by direct inhibition of STN neurons13. These findings agree with the well-established relationship between STN activity and movement suppression85–93. Along with the projection to the STN, canonical PV+ (PV+-Kcng4+) neurons also collateralize within the SNr and GPi14,16,94. In addition to locomotion, modulation of PV+ neuron collaterals in the SNr induces avoidance behavior48. These findings are consistent with the idea that both motor and non-motor signals are processed in parallel within the basal ganglia.
Although earlier studies examined the electrophysiological and anatomical properties of the STN-GPe network, the cell-type specificity of this network was unknown until recently. The latest findings collectively emphasize a closed reciprocal loop formed between the STN and PV+ neurons (see Synaptic Inputs), adding critical insight into the cellular constituents involved. The recurrent loop formed between the STN and PV+ neurons has strong implications for the pathological network activity observed in Parkinson’s disease (PD) (see Disease Alterations). However, it is important to note that optogenetic inhibition of PV+ neurons does not yield any motor responses11, suggesting the possibility of compensatory network mechanisms, such as the involvement of the local collaterals. Alternatively, the basal ganglia may control motor output by computation through a cascade of logic gates that operate on multiple rules. This idea is consistent with the co-activation of dSPNs and iSPNs during volitional movement95–97.
Additionally, a small subset of PV+ neurons target the Pf (see Neuron subclasses). These non-canonical PV+ (PV+-Lhx6−) neurons are not involved in motor control, but are associated with reversal learning16. Given that STN-projecting (PV+-Kcng4+) and Pf-projecting (PV+-Lhx6−) PV+ neurons receive distinct sets of synaptic inputs, their respective roles in motor and non-motor functions reinforce the concept that GPe neuron subtypes enact their functions through their unique circuit architecture.
Npas1+-Foxp2+ neurons
Based on relative spike timing, earlier literature inferred that Npas1+-Foxp2+ neurons mediate motor inhibition by broadcasting an inhibitory signal across the dStr98—their exclusive projection target. However, this causality had not been demonstrated. Importantly, recent optogenetic and chemogenetic approaches demonstrate that stimulation of either pan-Npas1+ neurons or the Npas1+-Foxp2+ subset leads to motor inhibition during both spontaneous ambulation and a trained-task12–15, further establishing that GPe neurons directly modulate motor behavior. Additionally, optogenetic inhibition of Npas1+ neurons leads to motor promotion13, arguing that the observed phenotype with excitatory actuators is not a spurious gain-of-function. As discussed earlier, Npas1+-Foxp2+ neurons do not form an extensive local collateral network within the GPe; it is therefore likely that they exert their circuit effects within their terminal field (i.e., within the dStr). While Npas1+-Foxp2+ axons impinge on dendrites of SPNs12,38, the biological significance of this anatomical arrangement has not been examined.
Npas1+-Nkx2.1+ neurons
In contrast with Npas1+-Foxp2+ neurons, the projection and function of Npas1+-Nkx2.1+ neurons are less well-characterized. These neurons project to the midbrain, cortex, and reticular thalamus14,28,30. However, it is unclear if a single Npas1+-Nkx2.1+ neuron projects to multiple brain regions or if separate neurons are responsible for each of these projections.
The projection to the midbrain agrees with previous electrophysiological, neurochemical, and viral tracing data99–103. While there is evidence suggesting PV+ neurons target the midbrain99, additional studies strongly argue that this projection is more consistent with the axonal projection patterns of Cre-expressing neurons from the Lhx6 driver29,104; the discrepancies may be related to the nuances of rabies virus tracing105. While this projection is likely constituted by Npas1+-Nkx2.1+ neurons, it remains to be determined if other neurons target midbrain dopamine neurons. More importantly, the behavioral relevance of this projection awaits further clarification. In particular, the feedback loop formed between GPe and midbrain dopamine neurons (see also Neuromodulatory inputs) may create complex circuit effects.
The GPe projections to the cortex arise from Npas1+-Nkx2.1+ neurons and ChAT+ neurons28,30. A mixture of neurons in the cortex has been shown to receive this GPe input30,106,107. While the functions of these projections await further characterization, recent data suggest that they are involved in regulating sleep84,108,109. Additionally, indirect evidence suggests that Npas1+-Nkx2.1+ neurons may contribute to other non-motor functions. Specifically, Nkx2.1+ neurons that are PV− and Foxp2− innervate lateral habenula-targeting neurons in the internal globus pallidus; however, it is possible that these neurons are Lhx6+-Sox6− rather than Npas1+. Nonetheless, these data further implicate the contribution of GPe neurons to the limbic functions of the basal ganglia2,83.
Disease Alterations
The selection of desired actions and concomitant suppression of competing actions is key to volitional movement. Disruption of the ability to facilitate desired movements and inhibit unwanted movements results in disorders such as PD, Huntington’s disease (HD), and dystonia. As GPe neurons are critically involved in motor control, we survey the emerging observations of the GPe in these disease conditions.
Parkinson’s disease
The loss of nigral dopamine neurons in PD leads to widespread basal ganglia circuit alterations. GPe neuron activity is altered following the loss of dopamine, shifting from autonomous decorrelated pacemaking to highly synchronous burst firing. This aberrant GPe neuron activity correlates with the emergence of motor symptoms in both animal models and human patients110–113, emphasizing the importance of the GPe in PD. Additionally, one of the hallmarks of PD is the widespread development of synchronized basal ganglia activity known as β-oscillations (13–30 Hz), which are implicated in motor suppression114–118. Recent evidence indicates that the GPe plays a critical role in the development of pathological β-oscillations. Specifically, optogenetic inhibition of GPe neurons robustly suppresses β-oscillations in chronic 6-OHDA lesioned rodents, a well-established model of PD. Additionally, optogenetic patterning of GPe neuronal firing at β frequency leads to key circuit changes reminiscent of naturally occurring β-oscillations119.
Recent studies using animal models of PD show that dopamine neuron loss has cell type-specific effects in the GPe. Following chronic 6-OHDA lesion, Npas1+ neurons display a reduction in spontaneous activity (where fast transmission was intact) ex vivo27. More recent studies suggest that the hypoactivity of Npas1+ neurons is at least in part due to changes in GABAergic signaling rather than alterations in their intrinsic properties13. In contrast, following acute or chronic 6-OHDA administration, PV+ neurons display only subtle or no detectable changes in their firing compared to control mice ex vivo16,27,120.
Synaptic inputs to GPe neurons are altered in models of PD. Following chronic 6-OHDA lesion, STN input targeting PV+ neurons is weakened in ex vivo slices. This alteration is likely due to a net loss in synaptic contacts and the associated postsynaptic receptors. In contrast, STN-Npas1+ input is unaltered. These results agree with earlier findings that AMPA and NMDA receptors are downregulated in rodent models of PD13. Furthermore, both systemic administration and GPe-specific application of NMDA antagonists are effective in ameliorating motor symptoms in animal models of PD121–123, arguing that the initial alteration may be useful for compensating the hyperactivity of STN input in vivo; however, the loss of NMDA receptor activity is likely maladaptive. The altered glutamate imbalance is further exacerbated by the disrupted glutamate homeostasis that is normally maintained by local astrocytes124.
In addition to STN input, striatal input to the GPe is also altered in the chronic 6-OHDA model. An ex vivo examination of dStr-GPe input revealed a net increase in the strength of the total striatal input to the GPe following chronic 6-OHDA lesion124. Although this finding is consistent with the prediction from the classic model, the observed alteration was mediated by a local disruption of glutamate homeostasis within the GPe. However, a more recent examination reveals that the indirect-pathway inputs to PV+ neurons are unchanged. This contrasts with the direct-pathway input to Npas1+ neurons, which is strengthened following chronic 6-OHDA lesion47. As the ascending Npas1+ projections to the striatum are strengthened under the same condition12, it is likely that this recurrent loop contributes to the hypokinetic symptoms of PD. It is unclear at this point why there is a discrepancy between studies; it is worth noting that the method of stimulation (electrical vs. optogenetic) can be a key disparity.
The loss of dopamine neurons alters efferent projections and in vivo function of GPe neurons. For example, the GPe-STN projection is strengthened following chronic 6-OHDA lesion125. This process is heterosynaptically induced, such that excessive cortical input to STN neurons leads to an increase in the strength of the GPe-STN projection126, indicating that GPe output can be regulated by wider basal ganglia circuit alterations in pathological states. Additionally, the PV+-SNr projection is weakened in 6-OHDA mice via a reduction in presynaptic transmitter release16, suggesting this alteration may contribute to hypokinetic symptoms in PD. Importantly, optogenetic activation of pan-PV+ neurons or STN-projecting PV+ (PV+-Kcng4+) neurons rescues motor deficits in both acute11,16 and chronic13 6-OHDA animals. These results strongly argue for the adaptive nature of the GPe outputs. Furthermore, following partial dopamine loss, chemogenetic inhibition of Pf-projecting PV+ (PV+-Lhx6−) neurons rescues impairment in a reversal learning task16, suggesting that these neurons may have therapeutic potential for the cognitive symptoms of PD.
Remarkably, targeting PV+ neurons has profound effects on rescuing locomotor activity in preclinical models of PD. Although optogenetic stimulation of PV+ neurons produces reliable locomotor rescue in 6-OHDA models of PD across multiple laboratories, the duration of the motor improvement following GPe neuron stimulation varies across studies (see Figure 5). For example, in a chronic 6-OHDA model, the motor improvement following PV+ neuron stimulation only lasts for the duration of the optical stimulation13. However, studies using acute 6-OHDA models report motor improvements that persist beyond the duration of the stimulation11,16,127, suggesting that the recovery period following 6-OHDA lesion may be of critical importance in determining whether motor-promoting effects of PV+ neuron stimulation are acute or persistent. Furthermore, these studies differ in the duration and intensity of their stimulation of PV+ neurons, thus making it difficult to compare results across laboratories. Therefore, it is important for future studies to carefully consider the translational applicability of different 6-OHDA lesion models and stimulation paradigms when interpreting functional results.
Huntington’s disease
HD is an inherited neurodegenerative movement disorder that leads to basal ganglia dysfunction. Currently, the precise roles of GPe neurons in HD are still unclear. However, hyperactivity of the GPe is presumed to underlie s-ome of the behavioral symptoms of HD128,129, and deep brain stimulation of the GPe provides symptomatic relief in human patients130.
Contrary to the widespread loss of SPNs in the striatum, there is typically no GPe neuron loss in human patients until late in the disease progression131,132. Consistent with this observation, GPe neuron loss is only observed at 18 months in the Q175 mouse model of HD. Although the abundance of PV+ neurons is maintained for up to 18 months, the abundance of Npas1+-Foxp2+ neurons is significantly reduced at 18 months, but not at 6 or 12 months133. It will be important to determine if the susceptibility of Npas1+-Foxp2+ neurons is associated with pathological hallmarks of HD, such as neuronal intranuclear inclusions and mutant huntingtin load.
Increases in α1, β2/3, and γ2 GABAA receptor subunits on GPe neurons are observed in human patients134. Additionally, increased postsynaptic responses in GPe neurons to striatal inputs are seen across the Q175, YAC128, and R6/2 models of HD135,136. The data from mouse models argue that increased striatal responses are independent of cell loss in the dStr. Instead, this alteration is likely a homeostatic reaction to decreased iSPN input, as the coupling between cortical input and iSPNs is weakened137–139. Although the intrinsic properties of PV− neurons are unaltered, their activity in vivo is suppressed by the increased collateral input from PV+ neurons140.
Dystonia
Dystonia is characterized by uncontrollable muscle contractions, resulting in involuntary and abnormal movements and postures141,142. Data from animal models and human patients suggest that abnormal GPe neuron activity contributes to dystonia symptoms143–147. Importantly, in the DYT1 mouse model of dystonia, there is reduced firing of PV+ neurons due to impaired function of HCN channels. Pharmacological enhancement of HCN channel activity restores physiological PV+ neuron pacemaking and improves motor performance148. These data suggest GPe neurons play important roles in dystonia symptomatology and highlight PV+ neurons as potential therapeutic targets in some forms of dystonia. However, the roles of other neuron subtypes in dystonia models remain to be examined.
Concluding Remarks
Methodological revolutions have greatly advanced our understanding of the GPe. Its complex neuron types, diverse synaptic inputs, and widespread axonal projection patterns argue that the architecture of GPe does not conform to the traditional direct-indirect model of the basal ganglia. In particular, new data that show interconnections between the GPe and “upstream nuclei” (such as the cortex and dStr) go against the hierarchical organization of the classic model. Furthermore, the causal roles that GPe neurons play in movement strongly argue that new theories of how the basal ganglia compute motor commands are needed. For example, can neural signals from the GPe veto decisions that are already committed at the cortical, striatal, or STN level? Rules governing these outcomes are likely brain state-dependent; therefore, it will be important to establish the actions of various neuromodulators within the GPe with behavioral context.
We are still in the beginning stages of understanding the full behavioral capacity of the GPe, especially the non-motor aspects. Brain-wide inputs to the GPe have now been mapped; given the extent of connectivity between non-classic motor regions, it is conceivable that the GPe is involved in further undiscovered non-motor functions. Future characterization of physiological properties and activation patterns of these inputs in relation to natural behaviors will help further pinpoint the precise roles of the GPe. This should be a reasonable goal as we make major headways in computer vision techniques, artificial intelligence, and biosensor development. Finally, while a consensus on GPe neuron subtypes and their functions is emerging, we need to develop more powerful models that consider how they integrate with specific action selection networks both within and beyond the basal ganglia.
| Neuron class (% of GPe neurons) | Neuron subclass (% of principal class) | Marker expression; (additional feature) | Validated driver and reporter line(s) | Neurotransmitter(s) |
|---|---|---|---|---|
| PV+ (50%) | aPV+-Kcng4+4,14 | bEtv1, Lhx6, Nkx2.1, Npy2r, Scna4b4,11,14,16,23–28,32–34 | PV-Cre, PV-Flp, PV-tdTomato, cKcng4-Cre14,16,27–29,33,35 | GABA |
| PV+-Lhx6−16,29 | Nkx2.1, Scna4b4,16 | PV-Cre, PV-Flp16 | GABA | |
| Npas1+ (30%) | Npas1+-Foxp2+26 (60%) | Meis2, bPenk, Sox64,25,26,28,38 | Npas1-Cre, cFoxp2-Cre, cFoxp2-Flp14,15,27,28,39 | GABA |
| Npas1+-Nkx2.1+26,28 (40%) | Lhx6, Npr3, Npy2r, Sox64,14,23–28 | Npas1-Cre, cNpr3-Cre, Lhx6-GFP14,28 | GABA | |
| ChAT+ (5%) | N/A | Nkx2.123,25 | ChAT-Cre27,30 | acetylcholine, GABA30 |
| PV−-Npas1− (15%) | N/A | Lhx6; (Sox6−)28 | unknown | unknown |
Footnotes:
Kcng4 demarcates a subset of canonical STN-projecting PV+ neurons. However, a marker encompassing all STN-projecting PV+ neurons has yet to be determined.
alternative names: Etv1, ER81; Penk.
subclass-specific driver.
Acknowledgments
We thank past and current members of the Chan Lab for their creativity and dedication to our understanding of the pallidum. This work was supported by NIH NS069777 (CSC), MH112768 (CSC), NS097901 (CSC), MH109466 (CSC), NS088528 (CSC), AG020506 (AP), and a Parkinson’s Foundation postdoctoral fellowship (CDC). We apologize for not being able to cite earlier studies because of limits on the citation number.
References
- 1.Grillner S & El Manira A Current Principles of Motor Control, with Special Reference to Vertebrate Locomotion. Physiol. Rev 100, 271–320 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Wallace ML et al. Genetically Distinct Parallel Pathways in the Entopeduncular Nucleus for Limbic and Sensorimotor Output of the Basal Ganglia. Neuron 94, 138–152.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poulin J-F et al. Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nat. Neurosci 21, 1260–1271 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saunders A et al. Molecular Diversity and Specializations among the Cells of the Adult Mouse Brain. Cell 174, 1015–1030.e16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welch JD et al. Single-Cell Multi-omic Integration Compares and Contrasts Features of Brain Cell Identity. Cell 177, 1873–1887.e17 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallén-Mackenzie Å et al. Spatio-molecular domains identified in the mouse subthalamic nucleus and neighboring glutamatergic and GABAergic brain structures. Commun. Biol 3, 338 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McElvain LE et al. Specific populations of basal ganglia output neurons target distinct brain stem areas while collateralizing throughout the diencephalon. Neuron (2021) doi: 10.1016/j.neuron.2021.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyamoto Y & Fukuda T The habenula-targeting neurons in the mouse entopeduncular nucleus contain not only somatostatin-positive neurons but also nitric oxide synthase-positive neurons. Brain Struct. Funct (2021) doi: 10.1007/s00429-021-02264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeon H et al. Topographic connectivity and cellular profiling reveal detailed input pathways and functionally distinct cell types in the subthalamic nucleus. Cell Rep 38, 110439 (2022). [DOI] [PubMed] [Google Scholar]
- 10.Liu D et al. A common hub for sleep and motor control in the substantia nigra. Science 367, 440–445 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Mastro KJ et al. Cell-specific pallidal intervention induces long-lasting motor recovery in dopamine-depleted mice. Nat. Neurosci 20, 815–823 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glajch KE et al. Npas1+ Pallidal Neurons Target Striatal Projection Neurons. J. Neurosci 36, 5472–5488 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pamukcu A et al. Parvalbumin+ and Npas1+ Pallidal Neurons Have Distinct Circuit Topology and Function. J. Neurosci 40, 7855–7876 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui Q et al. Dissociable Roles of Pallidal Neuron Subtypes in Regulating Motor Patterns. J. Neurosci. Off. J. Soc. Neurosci 41, 4036–4059 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aristieta A et al. A Disynaptic Circuit in the Globus Pallidus Controls Locomotion Inhibition. Curr. Biol. CB 31, 707–721.e7 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Lilascharoen V et al. Divergent pallidal pathways underlying distinct Parkinsonian behavioral deficits. Nat. Neurosci 24, 504–515 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arkadir D, Morris G, Vaadia E & Bergman H Independent coding of movement direction and reward prediction by single pallidal neurons. J. Neurosci 24, 10047–10056 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida A & Tanaka M Two Types of Neurons in the Primate Globus Pallidus External Segment Play Distinct Roles in Antisaccade Generation. Cereb. Cortex N. Y. N 1991 26, 1187–1199 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Gu BM, Schmidt R & Berke JD Globus pallidus dynamics reveal covert strategies for behavioral inhibition. Elife 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kita H Globus pallidus external segment. Prog. Brain Res 160, 111–133 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Jaeger D & Kita H Functional connectivity and integrative properties of globus pallidus neurons. Neuroscience 198, 44–53 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hegeman DJ, Hong ES, Hernández VM & Chan CS The external globus pallidus: progress and perspectives. Eur. J. Neurosci 43, 1239–1265 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nóbrega-Pereira S et al. Origin and molecular specification of globus pallidus neurons. J. Neurosci 30, 2824–2834 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flandin P, Kimura S & Rubenstein JLR The progenitor zone of the ventral medial ganglionic eminence requires Nkx2–1 to generate most of the globus pallidus but few neocortical interneurons. J. Neurosci 30, 2812–2823 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdi A et al. Prototypic and arkypallidal neurons in the dopamine-intact external globus pallidus. J. Neurosci 35, 6667–6688 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dodson PD et al. Distinct developmental origins manifest in the specialized encoding of movement by adult neurons of the external globus pallidus. Neuron 86, 501–513 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernández VM et al. Parvalbumin+ Neurons and Npas1+ Neurons Are Distinct Neuron Classes in the Mouse External Globus Pallidus. J. Neurosci 35, 11830–11847 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abecassis ZA et al. Npas1+-Nkx2.1+ Neurons Are an Integral Part of the Cortico-pallido-cortical Loop. J. Neurosci 40, 743–768 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mastro KJ, Bouchard RS, Holt HAK & Gittis AH Transgenic mouse lines subdivide external segment of the globus pallidus (GPe) neurons and reveal distinct GPe output pathways. J. Neurosci 34, 2087–2099 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saunders A et al. A direct GABAergic output from the basal ganglia to frontal cortex. Nature 521, 85–89 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgs MH, Jones JA, Chan CS & Wilson CJ Periodic unitary synaptic currents in the mouse globus pallidus during spontaneous firing in slices. J. Neurophysiol 125, 1482–1500 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizutani K, Takahashi S, Okamoto S, Karube F & Fujiyama F Substance P effects exclusively on prototypic neurons in mouse globus pallidus. Brain Struct. Funct 222, 4089–4110 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Oh Y-M et al. Using a novel PV-Cre rat model to characterize pallidonigral cells and their terminations. Brain Struct. Funct 222, 2359–2378 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Abrahao KP & Lovinger DM Classification of GABAergic neuron subtypes from the globus pallidus using wild-type and transgenic mice. J. Physiol 596, 4219–4235 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saunders A, Huang KW & Sabatini BL Globus Pallidus Externus Neurons Expressing parvalbumin Interconnect the Subthalamic Nucleus and Striatal Interneurons. PloS One 11, e0149798 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeisel A et al. Molecular Architecture of the Mouse Nervous System. Cell 174, 999–1014.e22 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katabi S, Adler A, Deffains M & Bergman H Dichotomous activity and function of neurons with low- and high-frequency discharge in the external globus pallidus of non-human primates. Cell Rep 42, 111898 (2023). [DOI] [PubMed] [Google Scholar]
- 38.Mallet N et al. Dichotomous organization of the external globus pallidus. Neuron 74, 1075–1086 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ketzef M & Silberberg G Differential Synaptic Input to External Globus Pallidus Neuronal Subpopulations In Vivo. Neuron 109, 516–529.e4 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Fishell G & Kepecs A Interneuron Types as Attractors and Controllers. Annu. Rev. Neurosci 43, 1–30 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Long JE, Cobos I, Potter GB & Rubenstein JLR Dlx1&2 and Mash1 transcription factors control MGE and CGE patterning and differentiation through parallel and overlapping pathways. Cereb. Cortex N. Y. N 1991 19 Suppl 1, i96–106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gelman DM et al. The embryonic preoptic area is a novel source of cortical GABAergic interneurons. J. Neurosci 29, 9380–9389 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gelman D et al. A wide diversity of cortical GABAergic interneurons derives from the embryonic preoptic area. J. Neurosci 31, 16570–16580 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y-JJ et al. Single-cell RNA sequencing identifies distinct mouse medial ganglionic eminence cell types. Sci. Rep 7, 45656 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poulin J-F et al. PRISM: A Progenitor-Restricted Intersectional Fate Mapping Approach Redefines Forebrain Lineages. Dev. Cell 53, 740–753.e3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hunt AJ et al. Paraventricular hypothalamic and amygdalar CRF neurons synapse in the external globus pallidus. Brain Struct. Funct 223, 2685–2698 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cui Q et al. Striatal Direct Pathway Targets Npas1+ Pallidal Neurons. J. Neurosci (2021) doi: 10.1523/JNEUROSCI.2306-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang S et al. Tripartite extended amygdala-basal ganglia CRH circuit drives locomotor activation and avoidance behavior. Sci. Adv 8, eabo1023 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujiyama F et al. Exclusive and common targets of neostriatofugal projections of rat striosome neurons: a single neuron-tracing study using a viral vector. Eur. J. Neurosci 33, 668–677 (2011). [DOI] [PubMed] [Google Scholar]
- 50.Wu Y, Richard S & Parent A The organization of the striatal output system: a single-cell juxtacellular labeling study in the rat. Neurosci. Res 38, 49–62 (2000). [DOI] [PubMed] [Google Scholar]
- 51.Lévesque M & Parent A The striatofugal fiber system in primates: a reevaluation of its organization based on single-axon tracing studies. Proc. Natl. Acad. Sci. U. S. A 102, 11888–11893 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cazorla M et al. Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry. Neuron 81, 153–164 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okamoto S et al. Overlapping Projections of Neighboring Direct and Indirect Pathway Neostriatal Neurons to Globus Pallidus External Segment. iScience 101409 (2020) doi: 10.1016/j.isci.2020.101409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Foster NN et al. The mouse cortico-basal ganglia-thalamic network. Nature 598, 188–194 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuan X-S et al. Striatal adenosine A2A receptor neurons control active-period sleep via parvalbumin neurons in external globus pallidus. eLife 6, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johansson Y & Ketzef M Sensory processing in external globus pallidus neurons. Cell Rep 42, 111952 (2023). [DOI] [PubMed] [Google Scholar]
- 57.Jones JA, Higgs MH, Olivares E, Peña J & Wilson CJ Spontaneous Activity of the Local GABAergic Synaptic Network Causes Irregular Neuronal Firing in the External Globus Pallidus. J. Neurosci. Off. J. Soc. Neurosci 43, 1281–1297 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sato F, Parent M, Levesque M & Parent A Axonal branching pattern of neurons of the subthalamic nucleus in primates. J. Comp. Neurol 424, 142–152 (2000). [DOI] [PubMed] [Google Scholar]
- 59.Sadek AR, Magill PJ & Bolam JP A single-cell analysis of intrinsic connectivity in the rat globus pallidus. J. Neurosci 27, 6352–6362 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bugaysen J, Bar-Gad I & Korngreen A Continuous modulation of action potential firing by a unitary GABAergic connection in the globus pallidus in vitro. J. Neurosci 33, 12805–12809 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fujiyama F et al. A single-neuron tracing study of arkypallidal and prototypic neurons in healthy rats. Brain Struct. Funct 221, 4733–4740 (2016). [DOI] [PubMed] [Google Scholar]
- 62.Parent A et al. Organization of the basal ganglia: the importance of axonal collateralization. Trends Neurosci 23, S20–27 (2000). [DOI] [PubMed] [Google Scholar]
- 63.Koshimizu Y, Fujiyama F, Nakamura KC, Furuta T & Kaneko T Quantitative analysis of axon bouton distribution of subthalamic nucleus neurons in the rat by single neuron visualization with a viral vector. J. Comp. Neurol 521, 2125–2146 (2013). [DOI] [PubMed] [Google Scholar]
- 64.Xiao C et al. Nicotinic receptor subtype-selective circuit patterns in the subthalamic nucleus. J Neurosci 35, 3734–3746 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karube F, Takahashi S, Kobayashi K & Fujiyama F Motor cortex can directly drive the globus pallidus neurons in a projection neuron type-dependent manner in the rat. eLife 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garcia AF, Crummy EA, Webb IG, Nooney MN & Ferguson SM Distinct populations of cortical pyramidal neurons mediate drug reward and aversion. Nat. Commun 12, 182 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.BRAIN Initiative Cell Census Network (BICCN). A multimodal cell census and atlas of the mammalian primary motor cortex. Nature 598, 86–102 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muñoz-Castañeda R et al. Cellular anatomy of the mouse primary motor cortex. Nature 598, 159–166 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parent M & Parent A Single-axon tracing and three-dimensional reconstruction of centre median-parafascicular thalamic neurons in primates. J. Comp. Neurol 481, 127–144 (2005). [DOI] [PubMed] [Google Scholar]
- 70.Yasukawa T, Kita T, Xue Y & Kita H Rat intralaminar thalamic nuclei projections to the globus pallidus: a biotinylated dextran amine anterograde tracing study. J. Comp. Neurol 471, 153–167 (2004). [DOI] [PubMed] [Google Scholar]
- 71.Kita H, Nambu A, Kaneda K, Tachibana Y & Takada M Role of ionotropic glutamatergic and GABAergic inputs on the firing activity of neurons in the external pallidum in awake monkeys. J. Neurophysiol 92, 3069–3084 (2004). [DOI] [PubMed] [Google Scholar]
- 72.Jan C et al. Dopaminergic innervation of the pallidum in the normal state, in MPTP-treated monkeys and in parkinsonian patients. Eur. J. Neurosci 12, 4525–4535 (2000). [PubMed] [Google Scholar]
- 73.Prensa L, Cossette M & Parent A Dopaminergic innervation of human basal ganglia. J. Chem. Neuroanat 20, 207–213 (2000). [DOI] [PubMed] [Google Scholar]
- 74.Prensa L & Parent A The nigrostriatal pathway in the rat: A single-axon study of the relationship between dorsal and ventral tier nigral neurons and the striosome/matrix striatal compartments. J. Neurosci 21, 7247–7260 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Debeir T et al. Effect of intrastriatal 6-OHDA lesion on dopaminergic innervation of the rat cortex and globus pallidus. Exp. Neurol 193, 444–454 (2005). [DOI] [PubMed] [Google Scholar]
- 76.Anaya-Martinez V, Martinez-Marcos A, Martinez-Fong D, Aceves J & Erlij D Substantia nigra compacta neurons that innervate the reticular thalamic nucleus in the rat also project to striatum or globus pallidus: implications for abnormal motor behavior. Neuroscience 143, 477–486 (2006). [DOI] [PubMed] [Google Scholar]
- 77.Smith Y & Villalba R Striatal and extrastriatal dopamine in the basal ganglia: an overview of its anatomical organization in normal and Parkinsonian brains. Mov. Disord. Off. J. Mov. Disord. Soc 23 Suppl 3, S534–547 (2008). [DOI] [PubMed] [Google Scholar]
- 78.Dopeso-Reyes IG et al. Calbindin content and differential vulnerability of midbrain efferent dopaminergic neurons in macaques. Front. Neuroanat 8, 146 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aransay A, Rodríguez-López C, García-Amado M, Clascá F & Prensa L Long-range projection neurons of the mouse ventral tegmental area: a single-cell axon tracing analysis. Front. Neuroanat 9, 59 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Giovanniello J et al. A Central Amygdala-Globus Pallidus Circuit Conveys Unconditioned Stimulus-Related Information and Controls Fear Learning. J. Neurosci 40, 9043–9054 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sztainberg Y, Kuperman Y, Justice N & Chen A An anxiolytic role for CRF receptor type 1 in the globus pallidus. J. Neurosci 31, 17416–17424 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pollak Dorocic I et al. A whole-brain atlas of inputs to serotonergic neurons of the dorsal and median raphe nuclei. Neuron 83, 663–78 (2014). [DOI] [PubMed] [Google Scholar]
- 83.Stephenson-Jones M et al. A basal ganglia circuit for evaluating action outcomes. Nature 539, 289–293 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qiu MH, Chen MC, Wu J, Nelson D & Lu J Deep brain stimulation in the globus pallidus externa promotes sleep. Neuroscience 322, 115–120 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wessel JR & Aron AR On the Globality of Motor Suppression: Unexpected Events and Their Influence on Behavior and Cognition. Neuron 93, 259–280 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fife KH et al. Causal role for the subthalamic nucleus in interrupting behavior. eLife 6, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schmidt R, Leventhal DK, Mallet N, Chen F & Berke JD Canceling actions involves a race between basal ganglia pathways. Nat. Neurosci 16, 1118–1124 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hamani C, Saint-Cyr JA, Fraser J, Kaplitt M & Lozano AM The subthalamic nucleus in the context of movement disorders. Brain J. Neurol 127, 4–20 (2004). [DOI] [PubMed] [Google Scholar]
- 89.Aron AR et al. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J. Neurosci 27, 11860–11864 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aron AR & Poldrack RA Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J. Neurosci 26, 2424–2433 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eagle DM et al. Stop-signal reaction-time task performance: role of prefrontal cortex and subthalamic nucleus. Cereb. Cortex N. Y. N 1991 18, 178–188 (2008). [DOI] [PubMed] [Google Scholar]
- 92.Schweizer N et al. Limiting glutamate transmission in a Vglut2-expressing subpopulation of the subthalamic nucleus is sufficient to cause hyperlocomotion. Proc. Natl. Acad. Sci. U. S. A 111, 7837–7842 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Adam EM, Johns T & Sur M Dynamic control of visually guided locomotion through corticosubthalamic projections. Cell Rep 40, 111139 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sato F, Lavallée P, Lévesque M & Parent A Single-axon tracing study of neurons of the external segment of the globus pallidus in primate. J. Comp. Neurol 417, 17–31 (2000). [PubMed] [Google Scholar]
- 95.Cui G et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature 494, 238–242 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Markowitz JE et al. The Striatum Organizes 3D Behavior via Moment-to-Moment Action Selection. Cell 174, 44–58.e17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maltese M, March JR, Bashaw AG & Tritsch NX Dopamine differentially modulates the size of projection neuron ensembles in the intact and dopamine-depleted striatum. eLife 10, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mallet N et al. Arkypallidal Cells Send a Stop Signal to Striatum. Neuron 89, 308–316 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Beier KT et al. Rabies screen reveals GPe control of cocaine-triggered plasticity. Nature 549, 345–350 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A & Uchida N Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74, 858–873 (2012). [DOI] [PubMed] [Google Scholar]
- 101.Lee CR, Abercrombie ED & Tepper JM Pallidal control of substantia nigra dopaminergic neuron firing pattern and its relation to extracellular neostriatal dopamine levels. Neuroscience 129, 481–489 (2004). [DOI] [PubMed] [Google Scholar]
- 102.Cobb WS & Abercrombie ED Relative involvement of globus pallidus and subthalamic nucleus in the regulation of somatodendritic dopamine release in substantia nigra is dopamine-dependent. Neuroscience 119, 777–786 (2003). [DOI] [PubMed] [Google Scholar]
- 103.Brazhnik E, Shah F & Tepper JM GABAergic afferents activate both GABAA and GABAB receptors in mouse substantia nigra dopaminergic neurons in vivo. J. Neurosci 28, 10386–10398 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Evans RC et al. Functional Dissection of Basal Ganglia Inhibitory Inputs onto Substantia Nigra Dopaminergic Neurons. Cell Rep 32, 108156 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Luo L, Callaway EM & Svoboda K Genetic Dissection of Neural Circuits: A Decade of Progress. Neuron 98, 256–281 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ahrlund-Richter S et al. A whole-brain atlas of monosynaptic input targeting four different cell types in the medial prefrontal cortex of the mouse. Nat. Neurosci 22, 657–668 (2019). [DOI] [PubMed] [Google Scholar]
- 107.Clayton KK et al. Auditory Corticothalamic Neurons Are Recruited by Motor Preparatory Inputs. Curr Biol (2020) doi: 10.1016/j.cub.2020.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vetrivelan R, Qiu M-H, Chang C & Lu J Role of Basal Ganglia in sleep-wake regulation: neural circuitry and clinical significance. Front. Neuroanat 4, 145 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lazarus M, Chen J-F, Urade Y & Huang Z-L Role of the basal ganglia in the control of sleep and wakefulness. Curr. Opin. Neurobiol 23, 780–785 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dwi Wahyu I, Chiken S, Hasegawa T, Sano H & Nambu A Abnormal Cortico-Basal Ganglia Neurotransmission in a Mouse Model of l-DOPA-Induced Dyskinesia. J. Neurosci 41, 2668–2683 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Raz A, Vaadia E & Bergman H Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of parkinsonism. J. Neurosci 20, 8559–8571 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Magill PJ, Bolam JP & Bevan MD Dopamine regulates the impact of the cerebral cortex on the subthalamic nucleus-globus pallidus network. Neuroscience 106, 313–330 (2001). [DOI] [PubMed] [Google Scholar]
- 113.Chan CS et al. HCN channelopathy in external globus pallidus neurons in models of Parkinson’s disease. Nat. Neurosci 14, 85–92 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leventhal DK et al. Basal ganglia beta oscillations accompany cue utilization. Neuron 73, 523–536 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kuhn AA, Kupsch A, Schneider GH & Brown P Reduction in subthalamic 8–35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease. Eur. J. Neurosci 23, 1956–60 (2006). [DOI] [PubMed] [Google Scholar]
- 116.Gatev P, Darbin O & Wichmann T Oscillations in the basal ganglia under normal conditions and in movement disorders. Mov. Disord. Off. J. Mov. Disord. Soc 21, 1566–1577 (2006). [DOI] [PubMed] [Google Scholar]
- 117.Little S & Brown P The functional role of beta oscillations in Parkinson’s disease. Parkinsonism Relat. Disord 20 Suppl 1, S44–48 (2014). [DOI] [PubMed] [Google Scholar]
- 118.Stein E & Bar-Gad I β oscillations in the cortico-basal ganglia loop during parkinsonism. Exp. Neurol 245, 52–59 (2013). [DOI] [PubMed] [Google Scholar]
- 119.Crompe B. de la et al. The globus pallidus orchestrates abnormal network dynamics in a model of Parkinsonism. Nat. Commun 11, 1570 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kovaleski RF et al. Dysregulation of external globus pallidus-subthalamic nucleus network dynamics in parkinsonian mice during cortical slow-wave activity and activation. J. Physiol 598, 1897–1927 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Starr MS, Starr BS & Kaur S Stimulation of basal and L-DOPA-induced motor activity by glutamate antagonists in animal models of Parkinson’s disease. Neurosci. Biobehav. Rev 21, 437–446 (1997). [DOI] [PubMed] [Google Scholar]
- 122.Kelsey JE et al. NMDA receptor antagonists ameliorate the stepping deficits produced by unilateral medial forebrain bundle injections of 6-OHDA in rats. Psychopharmacology (Berl.) 175, 179–188 (2004). [DOI] [PubMed] [Google Scholar]
- 123.Liu J et al. Facilitation of GluN2C-containing NMDA receptors in the external globus pallidus increases firing of fast spiking neurons and improves motor function in a hemiparkinsonian mouse model. Neurobiol. Dis 150, 105254 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cui Q et al. Blunted mGluR Activation Disinhibits Striatopallidal Transmission in Parkinsonian Mice. Cell Rep 17, 2431–2444 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fan KY, Baufreton J, Surmeier DJ, Chan CS & Bevan MD Proliferation of external globus pallidus-subthalamic nucleus synapses following degeneration of midbrain dopamine neurons. J. Neurosci 32, 13718–13728 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chu HY, Atherton JF, Wokosin D, Surmeier DJ & Bevan MD Heterosynaptic regulation of external globus pallidus inputs to the subthalamic nucleus by the motor cortex. Neuron 85, 364–76 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Spix TA et al. Population-specific neuromodulation prolongs therapeutic benefits of deep brain stimulation. Science 374, 201–206 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Starr PA, Kang GA, Heath S, Shimamoto S & Turner RS Pallidal neuronal discharge in Huntington’s disease: support for selective loss of striatal cells originating the indirect pathway. Exp. Neurol 211, 227–233 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Temel Y et al. Motor and cognitive improvement by deep brain stimulation in a transgenic rat model of Huntington’s disease. Neurosci. Lett 406, 138–141 (2006). [DOI] [PubMed] [Google Scholar]
- 130.Beste C et al. Behavioral and neurophysiological evidence for the enhancement of cognitive control under dorsal pallidal deep brain stimulation in Huntington’s disease. Brain Struct. Funct 220, 2441–2448 (2015). [DOI] [PubMed] [Google Scholar]
- 131.Reiner A, Dragatsis I & Dietrich P Genetics and neuropathology of Huntington’s disease. Int. Rev. Neurobiol 98, 325–372 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Waldvogel HJ, Kim EH, Tippett LJ, Vonsattel J-PG & Faull RLM The Neuropathology of Huntington’s Disease. Curr. Top. Behav. Neurosci 22, 33–80 (2015). [DOI] [PubMed] [Google Scholar]
- 133.Deng Y, Wang H, Joni M, Sekhri R & Reiner A Progression of basal ganglia pathology in heterozygous Q175 knock-in Huntington’s disease mice. J. Comp. Neurol 529, 1327–1371 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Waldvogel HJ & Faull RLM The diversity of GABA(A) receptor subunit distribution in the normal and Huntington’s disease human brain. Adv. Pharmacol. San Diego Calif 73, 223–264 (2015). [DOI] [PubMed] [Google Scholar]
- 135.Perez-Rosello T et al. Enhanced striatopallidal gamma-aminobutyric acid (GABA)A receptor transmission in mouse models of huntington’s disease. Mov. Disord. Off. J. Mov. Disord. Soc 34, 684–696 (2019). [DOI] [PubMed] [Google Scholar]
- 136.Barry J, Akopian G, Cepeda C & Levine MS Striatal Direct and Indirect Pathway Output Structures Are Differentially Altered in Mouse Models of Huntington’s Disease. J. Neurosci 38, 4678–4694 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Plotkin JL et al. Impaired TrkB receptor signaling underlies corticostriatal dysfunction in Huntington’s disease. Neuron 83, 178–188 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sebastianutto I, Cenci MA & Fieblinger T Alterations of striatal indirect pathway neurons precede motor deficits in two mouse models of Huntington’s disease. Neurobiol. Dis 105, 117–131 (2017). [DOI] [PubMed] [Google Scholar]
- 139.Carrillo-Reid L et al. Mutant huntingtin enhances activation of dendritic Kv4 K+ channels in striatal spiny projection neurons. eLife 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Callahan JW, Wokosin DL & Bevan MD Dysregulation of the Basal Ganglia Indirect Pathway in Early Symptomatic Q175 Huntington’s Disease Mice. J. Neurosci. Off. J. Soc. Neurosci 42, 2080–2102 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Breakefield XO et al. The pathophysiological basis of dystonias. Nat. Rev. Neurosci 9, 222–234 (2008). [DOI] [PubMed] [Google Scholar]
- 142.Tanabe LM, Kim CE, Alagem N & Dauer WT Primary dystonia: molecules and mechanisms. Nat. Rev. Neurol 5, 598–609 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Starr PA et al. Spontaneous pallidal neuronal activity in human dystonia: comparison with Parkinson’s disease and normal macaque. J. Neurophysiol 93, 3165–3176 (2005). [DOI] [PubMed] [Google Scholar]
- 144.Chiken S, Shashidharan P & Nambu A Cortically evoked long-lasting inhibition of pallidal neurons in a transgenic mouse model of dystonia. J. Neurosci 28, 13967–13977 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Baron MS, Chaniary KD, Rice AC & Shapiro SM Multi-neuronal recordings in the Basal Ganglia in normal and dystonic rats. Front. Syst. Neurosci 5, 67 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nambu A et al. Reduced pallidal output causes dystonia. Front. Syst. Neurosci 5, 89 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nishibayashi H et al. Cortically evoked responses of human pallidal neurons recorded during stereotactic neurosurgery. Mov. Disord. Off. J. Mov. Disord. Soc 26, 469–476 (2011). [DOI] [PubMed] [Google Scholar]
- 148.Sciamanna G et al. Optogenetic Activation of Striatopallidal Neurons Reveals Altered HCN Gating in DYT1 Dystonia. Cell Rep 31, 107644 (2020). [DOI] [PubMed] [Google Scholar]
- 149.Assaf F & Schiller Y A chemogenetic approach for treating experimental Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc 34, 469–479 (2019). [DOI] [PubMed] [Google Scholar]
- 150.Mallet N et al. Parkinsonian beta oscillations in the external globus pallidus and their relationship with subthalamic nucleus activity. J. Neurosci 28, 14245–14258 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]


