Abstract
Background
Myocardial bridging is a cardiac anomaly where a segment of epicardial coronary arteries runs through the myocardium and can rarely cause MI. Takotsubo syndrome is a stress-induced cardiomyopathy that can mimic MI. Catecholamine surge during stress can contribute to Takotsubo syndrome, but whether this surge can trigger an inconspicuous myocardial bridging to manifest symptomatically remains unclear, and alternately, whether a myocardial bridge might cause worsening of Takotsubo syndrome is also a matter that needs further research.
Case presentation
We report the case of a patient who initially presented with features of acute exacerbation of bronchiectasis and subsequently developed symptoms and ECG features suggestive of acute myocardial infarction. Echocardiography revealed features of takotsubo syndrome, and complete myocardial bridging was revealed via coronary angiography. The patient was managed conservatively with pharmacological treatment, and after a few days, echocardiographic features were reversed. As such, the diagnosis shifted toward Takotsubo syndrome with myocardial stunning due to co-existent myocardial bridging.
Conclusion
We report a rare case of a patient with acute bronchiectasis exacerbation with features suggestive of acute myocardial infarction who had findings of Takotsubo syndrome and complete myocardial bridging. In the beginning, it was difficult to determine whether the symptoms arose due to acute MI resulting from myocardial bridging or were solely due to takotsubo syndrome because of stress from bronchiectasis. Although myocardial bridging is often overlooked as an etiology for acute MI, this case highlights the importance of expanding the differential diagnosis to myocardial bridging in the work-up for the cause of acute MI and how Takotsubo syndrome can mimic acute MI and pose a diagnostic challenge.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-024-04153-x.
Keywords: Myocardial bridging, Takotsubo syndrome, Acute myocardial infarction, Angiography, Echocardiography
Background
Acute myocardial infarction (AMI) occurs due to decreased coronary blood flow, leading to insufficient oxygen supply to the heart and cardiac ischemia. Classically, atherosclerotic plaque rupture and thrombosis can lead to AMI. Other common etiologies of myocardial ischemia include coronary artery embolism, cocaine-induced ischemia, coronary dissection, and coronary vasospasm [1]. Takotsubo syndrome (TS) is a rare cardiovascular disease, with clinical manifestations similar to those of acute myocardial infarction (AMI), but it is characterized by reversible systolic dysfunction of the left ventricle (LV) in the absence of obstructive coronary artery disease (CAD) [2]. Myocardial bridging is a rare coronary anomaly that occurs when cardiac muscle overrides the intramural segment of a major epicardiac coronary artery. Although usually benign, this phenomenon has sometimes been associated with myocardial ischemia, myocardial infarction, ventricular septal rupture, AV conduction block, arrhythmia, and sudden cardiac death. [3, 4] Because of their rarity, the literature relating to the simultaneous occurrence of TS and MB has been limited, and there are conflicting data. [5, 6] This concurrence can pose a diagnostic challenge because TS can mimic AMI, and AMI can rarely occur as a result of myocardial bridging. We present a rare case report of a diagnostic dilemma involving a patient with acute exacerbation of chronic bronchiectasis who developed symptoms suggestive of acute MI with echocardiographic findings of Takotsubo syndrome and angiographic findings of complete myocardial bridging.
Case presentation
A 62-year-old elderly female presented to our emergency department with chief complaints of increasing cough and shortness of breath for 7 days. Her shortness of breath progressed from difficulty walking on level ground with frequent breaks to catch her breath (MMRC grade 2) to breathless even at rest (MMRC grade 4) within a few days. The cough also had an acute onset, was present more during the night, and was productive. She has been diagnosed with post-tuberculosis cystic bronchiectasis for 7 years, underwent thoracotomy and wedge resection of the right upper lobe 2 years back, and was currently being treated with rotahaler salmeterol + fluticasone and rotahaler tiotropium bromide for 2 years. There was no history of recent fever, headache, nausea/vomiting, palpitations, body swelling, loose stool, or loss of consciousness. Her oxygen saturation was 86% in room air and 96% in 4 L of O2 via a nasal prong. Her blood pressure was 90/50 and her pulse rate, respiratory rate, and temperature were within normal limits. A systemic examination revealed bilateral coarse crepitations present diffusely over the lung fields, and no heart murmurs were observed. She was admitted with a diagnosis of acute exacerbation of bronchiectasis and was then managed with IV fluids, nebulized ipratropium bromide, nebulized fluticasone, IV piperacillin-tazobactam, and tablet azithromycin. Oxygen was provided via nasal prong to maintain an oxygen saturation of more than 95%.
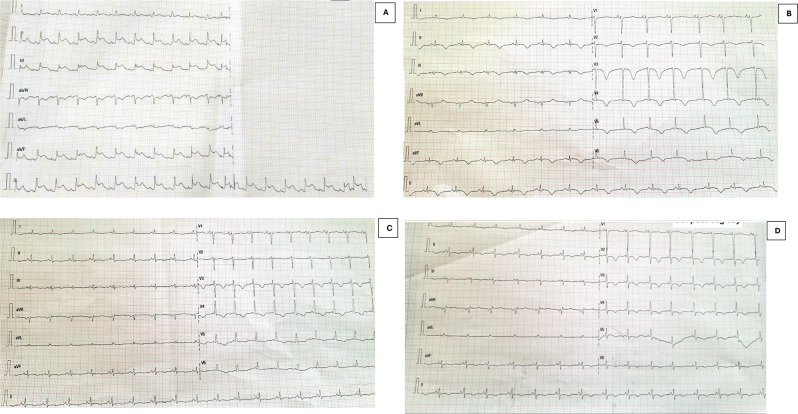
On the second day after admission, the patient complained of chest pain. The chest pain was central, sudden in onset, continuous and crushing in nature, non-radiating, and without any aggravating or relieving factors. An ECG was performed, but the patient was too breathless and agitated to lie down, and as such, only the limb leads were connected, which revealed ST segment elevation in leads II, III, and avF and ST depression in avR {Figure 1 (A)}. The relevant laboratory investigations sent along with their values are given in Table 1. A provisional diagnosis of acute myocardial infarction was made because two criteria were fulfilled; symptoms of ischemia and new ST-segment changes.
Fig. 1.
Serial ECGs of the patient A: ECG of the patient on the day of presentation showing ST levation on the limb leads II, III and avF and ST depression in avR. B: ECG of the patient on day 2 post-coronary angiography with reversal of the ST elevation, inversion of the T waves, microvoltage, and dynamic changes in the anterior leads. C. ECG changes of the patient on day 5 post-coronary angiography with gradual recovery from the anterior lead dynamic changes amplitude of T wave inversion is shallow compared to post-angiography day 2). D. ECG changes of the patient on day 8 post-coronary angiography with gradual recovery from the anterior lead dynamic changes (amplitude of T wave inversion is shallow compared to post-angiography day 5)
Table 1.
Relevant laboratory reports of the patient
| Test | Value | Reference range | |
|---|---|---|---|
| CAG day (before CAG) | Post-CAG day 1 | ||
| Troponin I | 0.1 ng/ml | 3.36 ng/ml | 0-0.3 ng/ml |
| CPK MB | 6 U/L | 25 U/L | 0–24 U/L |
| Procalcitonin | 0.50 ng/ml | < 0.5 – Minor or no significant infection | |
CPK MB: Creatine phosphokinase-MB, CAG: coronary angiography
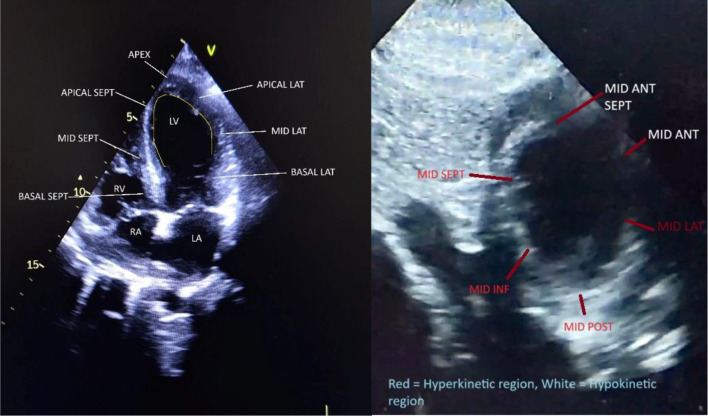
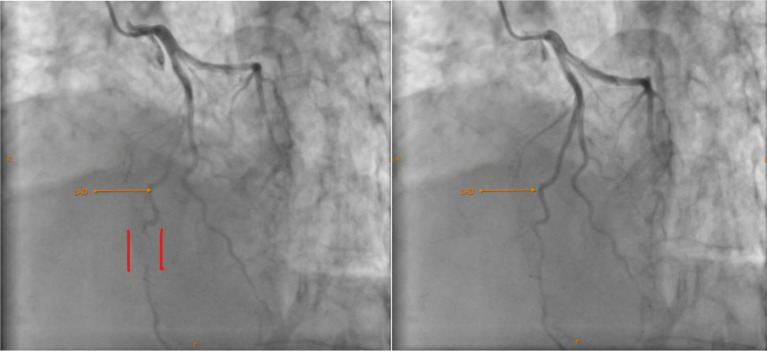
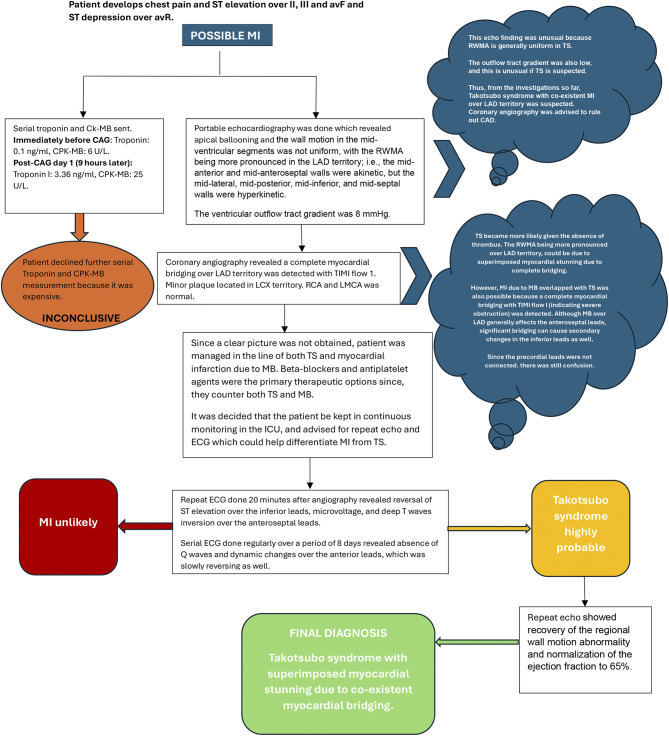
The patient was provided with an aspirin tablet, and an echocardiography was also performed, which revealed ballooning of the left ventricular mid- and apical segments. (Fig. 2, left and Additional file 1) The apical segments were akinetic. However, the wall motion in the mid-ventricular segments was not uniform, with the RWMA being more pronounced in the LAD territory; i.e., the mid-anterior and mid-anteroseptal walls were akinetic, but the mid-lateral, mid-posterior, mid-inferior, and mid-septal walls were hyperkinetic (Fig. 2, right and Additional file 2). The basal segments were hyperkinetic. These findings are clearly illustrated in Fig. 3. The left ventricular ejection fraction was 30–35% and the outflow tract gradient was 8 mm Hg. Given the history of sudden bronchiectasis exacerbation, the echocardiography findings were suspicious for Takotsubo syndrome; however, the unusual occurrence of RWMA being more pronounced in the LAD territory and the normal outflow tract gradient created further confusion. The patient was then transferred to the cardiac catheterization laboratory, coronary angiography was performed via the right radial artery route. Angiography revealed complete bridging of the distal left anterior descending (LAD) artery with TIMI flow grade I. There was a minor plaque in the left circumflex (LCX) artery. The left main coronary artery (LMCA) and the right coronary artery (RCA) were normal. A diagnosis of complete myocardial bridging was reached (Fig. 4 and Additional video file 3). However, it was still not clear whether the patient developed chest pain because of AMI or Takotsubo syndrome. It could be possible that the myocardial stunning from MB caused the unusual RWMA over the LAD territory with the TS. On the other hand, MI overlapping with TS was also possible because a complete myocardial bridging with TIMI flow I (indicating severe obstruction) was detected. Although MB over LAD generally affects the anteroseptal leads, significant bridging can cause secondary changes in the inferior leads as well. The PCI was deferred because of the high rates of in-stent restenosis (ISR) in MBs and the risk of complications such as stent fracture/perforation associated with MBs. The patient was then admitted to the ICU and was planned for optimal medical management with metoprolol to counter TS and/or MB. Phenylephrine was given in the case of hypotension because other inotropes would not be favorable due to TS. The patient was also given clopidogrel and aspirin to counter AMI because of the diagnostic dilemma. It was decided that the patient be monitored on serial ECG and a repeat echocardiography be performed at regular intervals which could reveal any reversal of RWMAs supporting a diagnosis of TS. For the management of bronchiectasis, the patient was given nebulized ipratropium, formoterol and budesonide via a metered-dose inhaler, piperacillin-tazobactam and azithromycin antibiotics, and MgSO4. The patient was monitored continuously, and a repeat ECG done 20 min after angiography showed a reversal of the ST segment elevation. Successive ECG showed reversal of the inferior lead ST elevation, inversion of the T waves, QT prolongation, micro voltage, absence of Q waves, and dynamic changes in the anterior leads. The amplitude of the T wave inversion gradually decreased on the anterior leads on serial ECG, and this was indicative of myocardial recovery from TS. It suggests that the acute phase of the TS was resolving, and the myocardial stunning was improving {Figure 1 (B, C,D)}. Daily ECG monitoring was performed until day 8. The patient was also advised for serial troponin and CPK-MB measured, however, they refused because of financial constraints. Repeat echocardiography was also performed on day 8, which showed recovery of the regional wall motion abnormality (Fig. 5) and normalization of the ejection fraction to 65%. A diagnosis of Takotsubo syndrome was then made with superimposed myocardial stunning from the MB. The patient gradually improved with medical management, and on day 9, the patient was discharged with the following medications to continue: metoprolol, spironolactone, sacubitril valsartan, and was planned for routine follow-up. During discharge, aspirin and clopidogrel were also prescribed for 15 days. The patient was also prescribed routine medications for bronchiectasis after adjustment of the dose. An elaborative figure demonstrating the sequential diagnostic workup of the patient is given in Fig. 6.
Fig. 2.
Four chamber echocardiography (left) of the patient showing ballooning of the left ventricular mid and apical segments – (yellow line overlay over LV) and Parasternal short axis view (right) showing the akinetic (white) and Hyperkinetic (red) regions. Further demonstration in Additional File 2 and 3. LV: Left ventricle, RV: Right ventricle, LA: Left atrium, RA: Right atrium, ANT: Anterior, POST: Posterior, LAT: Lateral, INF: Inferior, SEPT: Septal
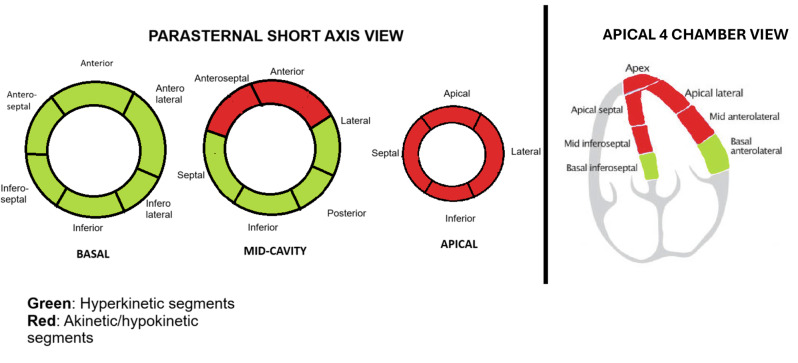
Fig. 3.
Illustration showing the hyperkinetic and akinetic/hypokinetic segments in the parasternal short axis view (left) and the four chamber view (right). Green overlay: Hyperkinetic segments, Red overlay: Akinetic/hypokinetic segments
Fig. 4.
Coronary angiography of the patient showing Myocardial bridging. Left: bridged segment during systole (between the two red lines) Right: normal flow during diastole
Fig. 5.
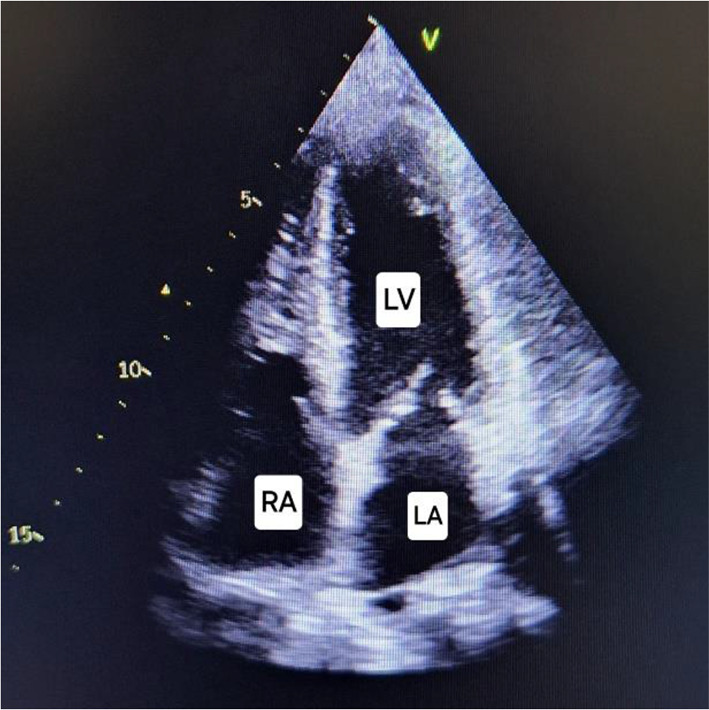
Four chamber echocardiography during systole showing normalization of features after medical management Day 8 (right). LV: Left ventricle, RV: Right ventricle, LA: Left atrium, RA: Right atrium
Fig. 6.
Diagnostic workup of the patient
Discussion
Takotsubo syndrome and myocardial bridging are extremely rare anomalies. TS is more common in females than in males, as documented by The International Takotsubo Registry across 26 centers in Europe and the United States which demonstrated a female percentage reaching 89.8%, with a mean age of 66.8 years [7]. TS accounts for 1–3% of acute coronary syndrome and 5–6% of ST-segment elevation myocardial infarction in women [8]. The incidence and prevalence of MB per population vary widely based on the diagnostic modality used. As such, coronary angiography revealed an estimated frequency ranging from 1.5 to 16%, but it has been reported to be as high as 80% in some autopsy series [4]. The association between TS and MB has been studied, but the results have shown conflicting findings. Compared with controls, TS patients had a greater prevalence of MB (40% vs. 8%; p < 0.001) during CAG in the study by Migliore F et al [5]. However, another larger study revealed a comparable prevalence of MBs in TS patients and controls [6].
Despite several hypotheses, the exact patho-mechanism of TS remains unknown. Nevertheless, the most widely accepted theories are “catecholamine-induced cardiotoxicity”, with serious emotional stress as a precipitant and “dysfunction in the coronary microvasculature”, leading to impaired myocardial perfusion [9]. In 85% of cases, TS is triggered by an emotionally or physically stressful event that precedes the onset of symptoms. Common physical stressors reported are acute asthma, chemotherapy-induced, surgical stress, and cerebrovascular events such as stroke, whereas common emotional stressors included the death of a relative, problems in relationships, financial stressors and anger [10]. Our patient had a history of acute exacerbation of bronchiectasis, which could have been the potential stressor. MBs, on the other hand, are usually small and considered benign, but, they are often considered a strong predisposing factor for atherosclerotic plaque in the segment proximal to the bridge because of consequential hemodynamic and structural changes such as myocardial malperfusion, disturbance of blood flow, and lipid deposition abnormalities [3]. Since myocardial perfusion occurs mainly during diastole, systolic compression by the myocardial bridge cannot be the only cause of ischemia. When analyzing the effect of myocardial bridging on atherosclerosis localization, Masuda et al [11]. reported that the proximal or distal segments had a greater extent of atherosclerosis than did the bridged segment. Intravascular ultrasound (IVUS) studies have also shown that squeezing of the bridge during systole produces retrograde flow against the anterograde flow in the proximal segment, leading to high pressure, and a characteristic pressure termed the “sucking phenomenon” is observed in the bridged segment during end diastole [12]. Ge et al [12]. also reported that the pressure proximal to the myocardial bridge was greater than the aortic pressure, and suggested that the disturbance of blood flow and high wall stress proximal to the myocardial bridge were the main contributors to the development of atherosclerosis in the proximal segment.
Morphologically, MBs are mostly localized on the middle segment of the left anterior descending artery (LAD) [13], but they have been reported in diagonal and marginal branches in 18% and 40% of cases, respectively [3]. Carrascosa P et al [14]. reported two types of bridging: (1) complete bridging (55%), where the artery segment is entirely covered by myocardium, and (2) incomplete bridging (45%), where the artery is covered only by a thin layer of connective and fatty tissue. The morphological subtypes of TS include the apical ballooning type (81.7%), mid-ventricular wall motion pattern (14.6%), basal wall motion pattern (2.2%), and focal wall motion pattern (1.5%)[15]. Our patient had a complete MB on the distal segment of the left anterior descending artery, and her echocardiography showed an apical ballooning type of TS.
MB is typically asymptomatic; however, it has also been associated with MI, ACS, stress cardiomyopathy, ventricular arrhythmias, and sudden cardiac death [4]. The diagnosis can be made when a significant portion or the entire segment of the coronary artery is embedded within the myocardium. This may be evident intraoperatively, during catheterization, using other imaging modalities, or during autopsy. Diagnostic techniques for an MB include CAG, IVUS, coronary computed tomography angiography, optical coherence tomography (OCT), and intracoronary Doppler studies [16]. CAG is the gold standard for diagnosing MB with the typical “milking effect”/systolic narrowing visible during systolic compression of the tunneled segment [3]. The “milking effect” requires a ≥ 70% reduction in the minimal luminal diameter during systole and a persistent ≥ 35% reduction in the minimal luminal diameter during mid- to late-diastole [3]. OCT is superior to CAG for studying MB due to its detailed morphological analysis of coronary arteries and associated plaques. However, its limited penetration and rapid fiber pullback make it less ideal for detecting MB [17]. The diagnosis of complete MB in our patient was made via demonstration of the classical milking effect on the distal portion of the LAD on CAG with TIMI flow I.
A meticulous approach is required to diagnose TS, as it presents similarly to CAD, with chest pain and dyspnea as the primary complaints, but a recent history of intense physical or emotional stress might raise more suspicion, especially when the symptoms and ECG findings are out of proportion to the degree of cardiac biomarker elevation. The diagnosis is usually established using the troponin, ECG, and echocardiographic abnormalities [9]. ECG findings for TS are nonspecific, but the precordial leads likely demonstrate ST-segment elevation [2]. In TS, the ST elevation is diffusely prevalent, occurring mostly in the anterior and anteroseptal leads and there is usually ST depression in avR [18]. A study was conducted to demonstrate the efficacy of ECG in differentiating TS from MI which found that the presence of ST depression in avR along with ST elevation in any two of the inferior leads was 14% sensitive, 96% specific with a positive predictive value of 89% and a negative predictive value of 52% for TS. This study further established an algorithm that showed that ST depression in avR and ST elevation in inferior leads could diagnose TS with > 95% specificity [18]. However, our initial ECG only visualized the limb leads and the precordial leads were not connected so, any interpretation made from ECG was inconclusive. Additionally, myocardial bridging over LAD cannot be ruled out solely based on ST elevation in the inferior leads if the precordial lead findings are absent. Although MBs primarily affect the segment supplied by the bridged artery, they can have broader implications due to altered hemodynamics and impaired perfusion [19]. QT prolongation is also a common finding in TS [18]. Our patient also demonstrated QT prolongation on ECG done on the 5th day post-CAG. Cardiac biomarkers such as troponin I and creatine kinase increase in TS, but this increase is usually disproportionate to ECG changes and wall motion abnormalities [2]. TTE is often the first imaging investigation used in patients suspected of having TS. The apical ballooning pattern of the LV is the most specific change that occurs in the majority of patients with TS (81.7%), because of apical akinesia/dyskinesia and basal hyperkinesia [15]. In TS, RWMAs are not restricted to a single epicardial coronary artery territory, unlike in AMI. While both AMI and TS demonstrate LV systolic dysfunction in the acute phase, TS shows reversibility of this dysfunction followed by a complete recovery of LV function compared to AMI [2]. These findings are useful for differentiating TS from AMI. CAG is usually performed to exclude CAD before establishing the diagnosis of TS. CAG usually demonstrates normal epicardial coronary arteries or nonobstructive atherosclerotic stenoses (< 50%) in TS patients [15]. Our patient had a confusing echocardiographic picture with apical ballooning of the left ventricle and the mid-ventricular segment had more pronounced RWMAs in the LAD territory, which spared the mid-lateral, mid-posterior, mid-inferior, and mid-septal walls. However, these changes reversed a few days after medical management in the hospital, thus a diagnosis of Takotsubo syndrome was made and the unusual RWMA over the LAD territory was attributed to superimposed myocardial stunning from the MB.
There are no established guidelines for treating patients with TS or MB to date. Both are managed similarly to those used for AMI. Management focuses on supportive therapy to maintain vital functions and careful monitoring in the intensive care unit in the acute phase. Pharmacological management of TS includes the use of beta-blockers, angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs), and mineralocorticoid inhibitors are used if the LV ejection fraction is ≤ 40% [2]. Beta-blockers are thought to play a key role because of their ability to antagonize excess catecholamines and prevent LV-free wall rupture. ACEIs provide cardio-protection via their ability to inhibit the renin-angiotensin aldosterone system, block the central sympathetic system, and mediate the vasodilatory effect of bradykinin [2]. For severe presentations like cardiogenic shock, extracorporeal membrane oxygenation (ECMO) and left ventricular assist devices (LVADs) can be used in TS [20]. Several factors influence the outcome for patients with myocardial bridges, making treatment often challenging. These factors include but are not limited to, individual patient symptoms, coronary and cardiac anatomy, ischemia, and comorbid conditions (particularly, the presence of CAD, hypertrophic cardiomyopathy, and valvular heart disease) [17]. Beta-blockers increase diastolic filling time because of their negative effect on heart rate (chronotropy) and contractility (inotropy) [17]. These agents also increase the maximal systolic and diastolic diameters and normalize flow velocities leading to the disappearance of symptoms and ST changes in the ECG [21]. Once atherosclerosis is detected, antiplatelet therapy should be commenced along with cardiovascular risk factor modification [22]. Percutaneous intervention works by protecting the stented arterial segment from systolic compression during exertion and physiological stress, and it is reserved for patients whose symptoms are unmanageable despite optimal anti-anginal therapy. Although research has shown hemodynamic and symptomatic improvements in the MB after PCI, no studies have demonstrated a complete reversal of perfusion defects after stent implantation [22]. Furthermore, preliminary data suggest a greater rate of in-stent restenosis in patients with MBs than those with de-novo atherosclerotic lesions. This high rate might be due to a greater use of bare metal stents and first-generation drug-eluting stents (DES) [17, 23]. Stent fracture and stent perforation are unusual but potentially dreadful complications of PCI, as demonstrated in a few case reports/series 24–26. There is potential for minimizing outcomes in PCI for MBs by the use of second-generation DES, which are stronger, and using intravascular imaging to estimate stent sizing. The strength of the second-generation DES could be attributed to the fact that these newer platinum-chromium stents have greater radial strength compared with previous stainless steel stents and thus can tolerate the radial forces from the cyclical systolic compression better, thus, demonstrating a higher safety profile [17]. Taken together, the use of PCI over medical management or vice-versa is still debatable because of a lack of randomized data relating to its use in patients with symptomatic MB. PCI was not performed in our patient because of the high rates of ISR and the risk of complications such as stent fracture/perforation associated with MBs. Other options for MB surgeries include coronary artery bypass graft (CABG) or supra-arterial myotomy, particularly for patients with an MB length < 2.5 cm and an MB width < 0.5 cm. [22, 24]
Here, we report a unique case of a patient admitted for acute exacerbation of bronchiectasis (see Additional file 4) who later presented with features of AMI, with echocardiography revealing features of TS and angiography revealing MB. It was a challenge to differentiate TS from AMI due to MB. Reversal of echocardiographic features later confirmed TS. Our patient received pharmacological management with beta-blockers, an ARNI, aspirin, and clopidogrel, and for the management of bronchiectasis, nebulized ipratropium, and fluticasone, antibiotics, MgSo4, and oxygen were given. The patient gradually improved, and we plan to continue pharmacological management by optimizing the drugs and scheduling routine follow-up for the patient to monitor therapy.
Conclusion
Myocardial bridging is a rare condition considered especially in patients at low risk for coronary atherosclerosis but with angina-like chest pain or established myocardial ischemia. However, the low rate of clinical manifestations and the large variability of morphological, functional, and clinical presentations provide a strong barrier for proper diagnosis and therapy. The presence of MI mimickers such as Takotsubo syndrome can impede appropriate management. Additionally, it is unclear whether TS and MB could predispose the patients to one another. Despite the diagnostic dilemma, the treatment was successful. Based on this case report, we highlight the importance of expanding the differential diagnosis to MB in the work-up for the cause of acute MI and the diagnostic dilemma imposed by mimickers of AMI, such as Takotsubo syndrome.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Additional file 1.mp4. Title. Four chamber echocardiography of the patient with demonstrable apical ballooning of the left ventricle. Description. An additional movie file that shows the regional wall motion abnormality with apical ballooning of the left ventricle; apical and mid-ventricular akinesia and basal hyperkinesia in the left ventricle. LV: left ventricle, RV: right ventricle, LA: left atrium, RA: right atrium, LAT: Lateral, SEPT: Septal.
Supplementary Material 2: Additional file 2.mp4. Title. Parasternal short axis view of the left ventricle patient with demonstrable RWMA over the LAD territory. Description. An additional movie file that shows the mid ventricular segment of the left ventricle with RWMA being more pronounced in the LAD territory i.e. the mid-anterior and mid-anteroseptal walls were akinetic but the mid-lateral, mid-posterior, mid-inferior, and mid-septal walls were hyperkinetic. ANT: Anterior, POST: Posterior, LAT: Lateral, INF: Inferior, SEPT: Septal.
Supplementary Material 3: Additional file 3.mp4. Title. Coronary angiography of the patient. Description. Coronary angiography of the patient revealing complete myocardial briding on the distal portion of Left Anterior descending artery (LAD) territory (orange arrow).
Abbreviations
- AMI
Acute Myocardial Infarction
- MB
Myocardial Bridging
- TS
Takotsubo Syndrome
- MMRC
Modified Medical Research Council
- RWMA
Regional Wall Motion Abnormalities
- CAG
Coronary Angiography
- LAD
Left Anterior Descending Artery
- IVUS
Intravascular Ultrasound
- ACS
Acute Coronary Syndrome
- CAD
Coronary Artery Disease
- ECG
Electrocardiogram
- TTE
Transthoracic Echocardiography
- TIMI
Thrombolysis in Myocardial Infarction
- OCT
Optical Coherence Tomography
- ACEI
Angiotensin Convertase Enzyme Inhibitor
- ARNI
Angiotensin Receptor/Neprilysin Inhibitor
- ECMO
Extracorporeal Membrane Oxygenation
- LVAD
Left Ventricular Assist Device
- ISR
In-Stent Restenosis
- PCI
Percutaneous Coronary Intervention
- CABG
Coronary Artery Bypass Graft
- DES
Drug-Eluting Stents
Author contributions
K.B: Conceptualization, design and coordination of the study, Data collection, wrote original draft and manuscript, review of the manuscript, prepared Figs. 1, 2, 3, 4, 5 and 6 and additional files. K.B.S : Patient diagnosis and management, reviewed ECG, performed echocardiography, performed coronary angiography, monitoring of the patient, review of the manuscript Ke. Bo. : Performed and reviewed ECG, Monitoring of the patient, review of the manuscript. O.P.S. : Performed and reviewed ECG, Monitoring of the patient, review of the manuscript. R.S. : Performed and reviewed ECG, Monitoring of the patient, review of the manuscript. K.S. : review of the manuscript. All authors read and approved the final manuscript.
Funding
None.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval
Not applicable.
Consent for publication
Written consent was obtained from the patient for publication of the patient’s details and any identifying images in the study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mechanic OJ, Gavin M, Grossman SA. Acute myocardial infarction [Internet]. StatPearls - NCBI Bookshelf. 2023. https://www.ncbi.nlm.nih.gov/books/NBK459269/
- 2.Scafa-Udriste A, Horodinschi RN, Babos M, Dinu B. Diagnostic challenges between takotsubo cardiomyopathy and acute myocardial infarction—where is the emergency? a literature review. International Journal of Emergency Medicine [Internet]. 2024;17(1). https://intjem.biomedcentral.com/articles/10.1186/s12245-024-00595-4 [DOI] [PMC free article] [PubMed]
- 3.Yuan SM. Myocardial bridging. Brazilian Journal of Cardiovascular Surgery [Internet]. 2015;31(1). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5062697/ [DOI] [PMC free article] [PubMed]
- 4.Alegria JR, Herrmann J, Holmes DR, Lerman A, Rihal CS. Myocardial bridging. European Heart Journal [Internet]. 2005;26(12):1159–68. https://pubmed.ncbi.nlm.nih.gov/15764618/ [DOI] [PubMed]
- 5.Migliore F, Maffei E, Marra MP, Bilato C, Napodano M, Corbetti F et al. LAD Coronary artery Myocardial bridging and Apical Ballooning Syndrome. JACC Cardiovascular Imaging [Internet]. 2013;6(1):32–41. https://pubmed.ncbi.nlm.nih.gov/23328559/ [DOI] [PubMed]
- 6.Stiermaier T, Desch S, Blazek S, Schuler G, Thiele H, Eitel I. Frequency and significance of myocardial bridging and recurrent segment of the left anterior descending coronary artery in patients with takotsubo cardiomyopathy. the American Journal of Cardiology [Internet]. 2014;114(8):1204–9. https://pubmed.ncbi.nlm.nih.gov/25175156/ [DOI] [PubMed]
- 7.Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M et al. Clinical features and outcomes of Takotsubo (Stress) cardiomyopathy. New England Journal of Medicine/the New England Journal of Medicine [Internet]. 2015;373(10):929–38. https://pubmed.ncbi.nlm.nih.gov/26332547/ [DOI] [PubMed]
- 8.Matta A, Delmas C, Campelo-Parada F, Lhermusier T, Bouisset F, Elbaz M et al. Takotsubo cardiomyopathy. Reviews in Cardiovascular Medicine [Internet]. 2022;23(1):1. https://pubmed.ncbi.nlm.nih.gov/35092230/ [DOI] [PubMed]
- 9.Amin HZ, Amin LZ, Pradipta A. Takotsubo Cardiomyopathy: A Brief Review. Journal of Medicine and Life [Internet]. 2020;13(1):3–7. https://pubmed.ncbi.nlm.nih.gov/32341693/ [DOI] [PMC free article] [PubMed]
- 10.Sharkey SW, Lesser JR, Maron BJ. Takotsubo (Stress) cardiomyopathy. Circulation [Internet]. 2011;124(18). https://www.ahajournals.org/doi/full/10.1161/CIRCULATIONAHA.111.052662 [DOI] [PubMed]
- 11.The effect of myocardial bridging of the coronary artery on vasoactive agents and atherosclerosis localization. PubMed [Internet]. 2001; 10.1002/1096-9896(2000)9999:9999 [DOI] [PubMed]
- 12.Ge J, Erbel R, Gorge G, Haude M, Meyer J. High wall shear stress proximal to myocardial bridging and atherosclerosis: intracoronary ultrasound and pressure measurements. Heart [Internet]. 1995;73(5):462–5. https://pubmed.ncbi.nlm.nih.gov/7786662/ [DOI] [PMC free article] [PubMed]
- 13.Irvin RG. The angiographic prevalence of myocardial bridging in man. Chest [Internet]. 1982;81(2):198–202. https://pubmed.ncbi.nlm.nih.gov/7056084/ [DOI] [PubMed]
- 14.Carrascosa P, López EM, Capunay C, Deviggiano A, Vallejos J, Carrascosa J. Prevalence and characteristics of myocardial bridges in multidetector row computed tomography coronary angiography. Rev Argent Cardiol. 2009;77:268–73. [Google Scholar]
- 15.Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ et al. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical characteristics, diagnostic criteria, and Pathophysiology. European Heart Journal [Internet]. 2018;39(22):2032–46. https://pubmed.ncbi.nlm.nih.gov/29850871/ [DOI] [PMC free article] [PubMed]
- 16.Ki YJ. Myocardial bridging presenting as myocardial ischaemia induced cardiac arrest: a case report. BMC Cardiovascular Disorders [Internet]. 2021;21(1). https://bmccardiovascdisord.biomedcentral.com/articles/10.1186/s12872-021-01975-x [DOI] [PMC free article] [PubMed]
- 17.Sternheim D, Power DA, Samtani R, Kini A, Fuster V, Sharma S. Myocardial bridging: diagnosis, functional assessment, and management. Journal of the American College of Cardiology [Internet]. 2021;78(22):2196–212. https://pubmed.ncbi.nlm.nih.gov/34823663/ [DOI] [PubMed]
- 18.18, Frangieh AH, Obeid S, Ghadri J, Imori Y, D’Ascenzo F, Kovac M et al. ECG criteria to differentiate between takotsubo (Stress) cardiomyopathy and myocardial infarction. Journal of the American Heart Association Cardiovascular and Cerebrovascular Disease [Internet]. 2016;5(6). https://www.ahajournals.org/doi/10.1161/JAHA.116.003418#jah31575-note-0021 [DOI] [PMC free article] [PubMed]
- 19.19, MöHlenkamp S, Hort W, Ge J, Erbel R. Update on myocardial bridging. Circulation [Internet]. 2002;106(20):2616–22. https://pubmed.ncbi.nlm.nih.gov/12427660/ [DOI] [PubMed]
- 20.Madias JE. Takotsubo Cardiomyopathy: current treatment. Journal of Clinical Medicine [Internet]. 2021;10(15):3440. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8347171/ [DOI] [PMC free article] [PubMed]
- 21.Klues HG, Dahl JV, Klein I, Krebs W, Hanrath P. Functional, angiographic and intracoronary doppler flow characteristics in symptomatic patients with myocardial bridging: Effect of short-term intravenous beta-blocker medication. Journal of the American College of Cardiology [Internet]. 1996;27(7):1637–45. https://www.sciencedirect.com/science/article/pii/0735109796000629?via%3Dihub [DOI] [PubMed]
- 22.Tarantini G, Migliore F, Cademartiri F, Fraccaro C, Iliceto S. Left anterior descending artery myocardial bridging. Journal of the American College of Cardiology [Internet]. 2016;68(25):2887–99. https://pubmed.ncbi.nlm.nih.gov/28007148/ [DOI] [PubMed]
- 23.Kursaklioglu H, Barcin C, Iyisoy A, Kose S, Amasyali B, Isik E. Angiographic restenosis after myocardial bridge stenting: A Comparative study with direct stenting of De-Novo atherosclerotic lesions. Japanese Heart Journal [Internet]. 2004;45(4):581–9. https://www.jstage.jst.go.jp/article/jhj/45/4/45_4_581/_article [DOI] [PubMed]
- 24.Li W, Li Y, Sheng L, Gong Y. Myocardial bridge: Is the risk of perforation increased? Canadian Journal of Cardiology [Internet]. 2008;24(11):e80–1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2644544/ [DOI] [PMC free article] [PubMed]
- 25.Tandar A, Whisenant BK, Michaels AD. Stent fracture following stenting of a myocardial bridge: Report of two cases. Catheterization and Cardiovascular Interventions [Internet]. 2008;71(2):191–6. https://pubmed.ncbi.nlm.nih.gov/18327836/ [DOI] [PubMed]
- 26.Landrum EB, Schussler JM. Recurrent stent fracture due to myocardial bridging: A brief report and review of published cases. the American Journal of Cardiology [Internet]. 2023;200:75–7. https://www.ajconline.org/article/S0002-9149(23)00304-1/abstract [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Additional file 1.mp4. Title. Four chamber echocardiography of the patient with demonstrable apical ballooning of the left ventricle. Description. An additional movie file that shows the regional wall motion abnormality with apical ballooning of the left ventricle; apical and mid-ventricular akinesia and basal hyperkinesia in the left ventricle. LV: left ventricle, RV: right ventricle, LA: left atrium, RA: right atrium, LAT: Lateral, SEPT: Septal.
Supplementary Material 2: Additional file 2.mp4. Title. Parasternal short axis view of the left ventricle patient with demonstrable RWMA over the LAD territory. Description. An additional movie file that shows the mid ventricular segment of the left ventricle with RWMA being more pronounced in the LAD territory i.e. the mid-anterior and mid-anteroseptal walls were akinetic but the mid-lateral, mid-posterior, mid-inferior, and mid-septal walls were hyperkinetic. ANT: Anterior, POST: Posterior, LAT: Lateral, INF: Inferior, SEPT: Septal.
Supplementary Material 3: Additional file 3.mp4. Title. Coronary angiography of the patient. Description. Coronary angiography of the patient revealing complete myocardial briding on the distal portion of Left Anterior descending artery (LAD) territory (orange arrow).
Data Availability Statement
No datasets were generated or analysed during the current study.