Apalutamide is a novel non-steroidal, second-generation, selective competitive inhibitor of the androgen receptor approved for the treatment of non-metastatic castration-resistant and metastatic hormone-sensitive prostate cancer (1). Adverse cutaneous reactions (ACRs) to apalutamide have been described in 23.8–27.1% of patients in clinical trials, with 5.2–6.3% grade-3 ACR according to the Common Terminology Criteria for Adverse Events (CTCAE v5.0) (1–4). However, the precise clinical presentation, the histopathological correlation, and the underlying mechanism of ACRS to apalutamide are poorly understood.
Here, we aimed to describe the clinicopathological features of ACRS to apalutamide in a case series of 9 patients.
MATERIALS AND METHODS (see Appendix S1)
RESULTS
The study was conducted according to guidelines from the local institutional Review Board and the Declaration of Helsinki.
Of 121 PCP treated with apalutamide in our institution, 24 patients (19.8%) presented an ACR to the treatment. Due to the extension and/or clinical impact (non-tolerable grade 2 or superior), 11n patients were referred to the dermatology department. Median age of presentation was 77.7 years (range, 65–90). One patient had a personal history of mild psoriasis, without flares since youth. The median latency of onset was 112.9 days (range, 43–223 days). The clinicopathological features are summarized in Table SI.
All presented a maculopapular rash (Grade 2–3) involving a range of 20–90% of total body surface area; none presented mucosal lesions. Four patients had an eczematous rash, 3 a lichenoid aspect (Fig. 1B) and 2 were non-specific. One patient with an exfoliative erythroderma required admission, monitoring, and systemic steroids, with complete recovery (Fig. 1C). Patients reported moderate to severe pruritus but did not present any other systemic symptom (pain, fever, hypotension).
Fig. 1.
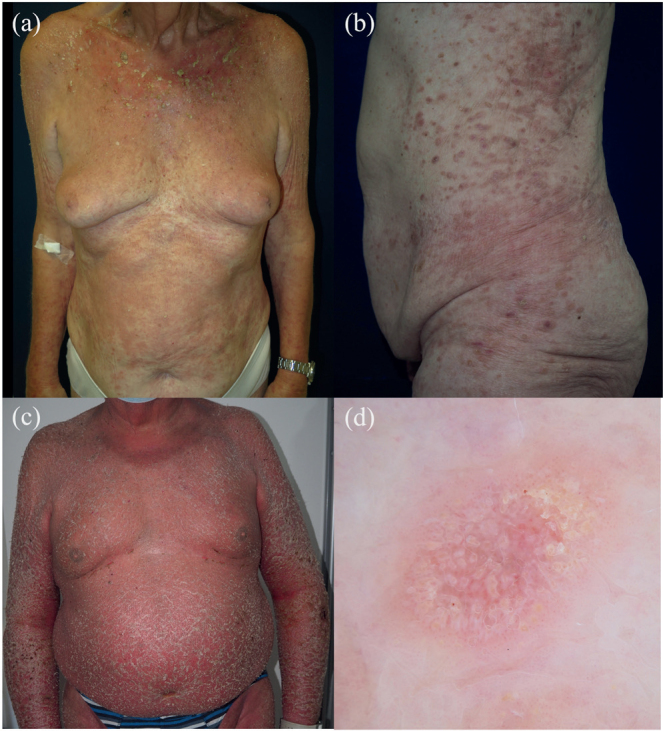
Clinical spectrum of cutaneous adverse reactions to apalutamide treatment in prostate cancer patients. (A) Maculopapular confluent exanthema, with crusting and marked erythema in the neckline. (B) Maculopapular non-confluent lichenoid-like exanthema, affecting predominantly the lateral regions of the trunk. (C) Exfoliative erythroderma with marked desquamation. On dermoscopy (D), a white pseudo-reticular structure, corresponding to Wickham-like striae, with dotted vessels could be observed.
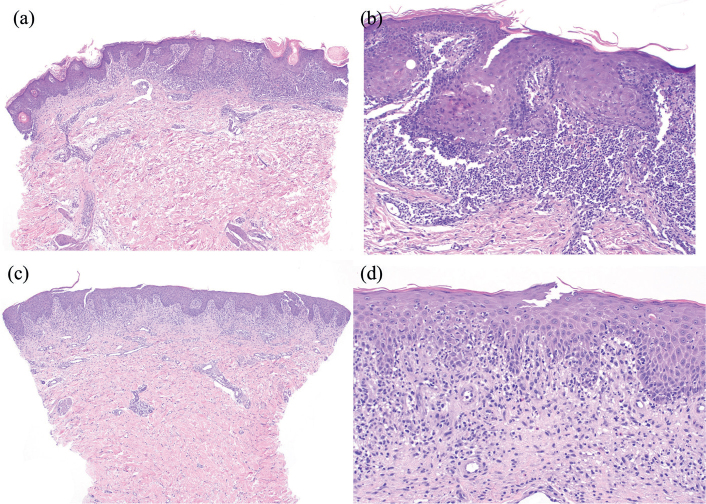
Cutaneous biopsy was obtained from 8 patients. Two patients had a lichenoid interface dermatitis (Fig. 2A–B), one of which was accompanied by abundant eosinophils. Four patients had a spongiotic dermatitis and 2 patients had a mixed spongiotic and lichenoid dermatitis (Fig. 2C–D).
Fig. 2.
Histological spectrum of cutaneous adverse reactions to apalutamide treatment in prostate cancer patients. (A, B) Lichenoid interface dermatitis with a dense dermal-band infiltrate with colloid bodies and without eosinophils. (C, D) Subacute spongiotic dermatitis with marked spongiosis with lymphocytic exocytosis. In the papillary dermis, a mixed infiltrate composed of lymphocytes, neutrophils, and eosinophils could be observed.
A blood sample was obtained from 6 patients: 4 patients exhibited eosinophilia (700–2,000x109 eosinophils/L). In the characterization of the ACRs, in vitro lymphocyte proliferation test (LPT) to apalutamide was positive in 3 patients (3/9). Apalutamide patch tests readings at day 2 and day 4 were negative in the 7 patients tested.
All patients received topical corticosteroids and oral antihistamines, with 6 requiring oral corticosteroids. The ACR led to the discontinuation of treatment in 6 patients. In 3 patients in whom apalutamide was reintroduced, no subsequent ACR appeared. All patients had a complete resolution. Despite this, 2 patients had a post-inflammatory hyperpigmentation and 1 patient marked xerosis.
DISCUSSION
ACR to apalutamide are common, although the pathogenesis remains elusive. This study provides significant novel data regarding the clinicopathological presentation and potential delayed hypersensitivity as the underlying mechanism.
The incidence of ACR in our cohort was 19.8%, similar to SPARTAN and TITAN clinical trials (1, 3, 4), as well as grade-3 ACR incidence (5%). In previous studies, low bodyweight has been associated as a predictive factor with the incidence of ACR (5), without other significant clinical factors for the incidence, worsening, and recurrence of ACR (2, 3, 6). Median latency of onset in our study was longer than in previous studies (112.9 vs 80–82 days) (2, 3). Katsuta et al. (7) described in a recent review of the literature that no SCARS to apalutamide appeared ≥8 weeks. Despite that, in this study, 1 patient had an exfoliative erythroderma 108 days after apalutamide initiation.
ACRs in apalutamide clinical trials were predominantly reported as nonspecific dermatological presentation, such as “rash”, “urticaria”, or “blisters” (2). In our study, the patients presented either a violaceous lichenoid exanthema or an acute eczematous dermatitis. The kind of dermatitis, along with the extension, led to different dermatological management. One patient presented a more severe exfoliative erythroderma (DRESS Regiscar=2, possible). In the literature, isolated case reports of DRESS (8, 9), toxic epidermal necrolysis (TEN) (10), and acute generalized exanthematous pustulosis (AGEP) (11) have been described.
Histologically, spongiotic, lichenoid, or mixed histological patterns were observed, which shows its diversity. Except for 1 patient, eosinophils were abundant in most skin biopsies, which supports a sort of hypersensitivity mechanism as pathogenesis. Histological descriptions of this ACR in the literature are scarce, except for reports of SCARS, with 2 previous report of 2 patients presenting a spongiotic dermatitis (12) and 1 reporting an ACR with lichenoid features (13).
Mechanism of apalutamide ACR is not well defined. Previous studies have postulated that these reactions may be dose-dependent (14) and that dose reduction could potentially lead to clinical improvement. In fact, apalutamide plasma concentration had been found to be numerically higher in patients with an ACR, but this did not reach statistical significance (2). This would explain why low bodyweight is a risk factor for ACR development. We could demonstrate that, at least in part, some cases could be explained by development of delayed hypersensitivity to apalutamide. However, the reason why most cases do not present positive LPT, and none of them positive patch testing, remains elusive. The metabolism of apalutamide results in the formation of different metabolites. N-desmethyl apalutamide (M3) is the major one, which contributes to one-third of the clinical activity of apalutamide. The role of these metabolites in the development of a drug reaction is unknown and could be the reason for the negativity in patch testing. Overall, non-standardization of LPT and no use of controls for patch testing, as well as metabolism transformation of apalutamide, may limit the use of LPT and patch testing to prove a delayed immune response to apalutamide.
As the frequency of ACR is much higher with apalutamide than with any other antiandrogens, some authors have postulated it is related to a structure-specific non-related to the mechanism of action. In fact, despite enzalutamide and apalutamide sharing up to 70% of chemical structure, as opposed to flutamide and bicalutamide, the prevalence of ACR to enzalutamide is much lower (2.4%) (15). Ji et al. (16) proved in animals models that the 2-cyanopyridine moiety in apalutamide, not present in enzalutamide, may react with cysteine in proteins forming haptens and triggering a T-cell mediated immune response. These findings would support a delayed-onset hypersensitivity reaction as the main mechanism of action, along with the clinical presentation (delayed onset, responsiveness to corticosteroids, and recurrence after drug exposure) (16). In our study, LPT was positive in only 3 out of 9 patients. Interestingly, a previous study showed that LTT was positive in 4 out of 4 severe cases (3 TEN, 1 AGEP) and only positive in 2 of 7 (28.6%) in milder cases (7). Therefore, the mechanism of ACR to apalutamide might be multifactorial and not only explained by a delayed hypersensitivity reaction, especially in milder cases.
In this study, all patients had ad integrum evolution without significant sequelae. Despite this, ACR led to the complete discontinuation of treatment in 5 out of 9 patients. In apalutamide clinical trials, treatment discontinuation due to cutaneous toxicity was present in 8.5–9.9% of patients (1, 4). Such differences might be explained by the fact that most grade 1–2 ACR resolved with topical therapy and were managed by the urology department without dose interruption or reduction, and that the patients in this series were highly selected due to the severity of the clinical presentation. In fact, in our whole cohort, no other patient discontinued apalutamide due to skin toxicity, having an overall prevalence of treatment discontinuation due to ACR of 4.1%. Dose reduction was a frequent therapeutic option in other studies (11.5–19.7% [1, 4]), although we used it in only 2 patients, and has been proved to be an effective preventive measure, especially in small body sizes, allowing lower rates of overall ACR and grade-3 ACR, without impacting on overall survival of PCP (17, 18). PCP response to apalutamide can be monitored through clinical exploration, PSA levels, and imaging studies, while apalutamide therapeutic drug monitoring is not used in clinical practice.
Of the 3 patients in whom apalutamide was not discontinued, none presented further ACR. In previous studies, recurrence of apalutamide ACR has been described in up to 45.2% of patients who resumed apalutamide (6) with a potential worsening in posterior flares (3).
In conlcusion, apalutamide is associated with a variable spectrum of severity and type of ACRs, mainly spongiotic and/or lichenoid dermatosis, that can appear after months of treatment. In our experience, grade-3 ACR frequently implied treatment discontinuation. Despite supporting evidence in the literature of a structural-specific induced hypersensitivity response in animal models, in our series LPT suggested this mechanism in one-third of patients. The risk of maintaining the treatment and the available therapeutic oncological alternatives must be agreed on an individual basis to avoid a detrimental oncological impact on survival. Dose reduction or titration might be a good preventive or therapeutic alternative in mild cases (grade 1–2 ACR) without impacting the oncological control, but the risk–benefit ratio should be considered in severe cases as SCAR cannot be ruled out.
Supplementary Material
ACKNOWLEDGEMENTS
The study has been approved by the institutional review board guided by the STROBE checklist.
The patients have given written consent for publication and use of clinical photographs and medical information.
Funding Statement
Funding sources The research at the Dermatology Department in Hospital Clinic Barcelona is partially funded by Instituto de Salud Carlos III (ISCIII) through the projects PI22/01457 and PI23/0692 and co-funded by the European Union, by the CIBER de Enfermedades Raras of the Instituto de Salud Carlos III, Spain, co-funded by ISCIII-Subdireccion General de Evaluacion and European Regional Development Fund (ERDF), “a way to make Europe”; AGAUR 2017_SGR_1134 and CERCA Programme by Generalitat de Catalunya, Spain; European Commission under the 6th Framework Programme, Contract No. LSHC-CT-2006-018702 (GenoMEL), by the European Commission under the 7th Framework Programme, Diagnostics, and the European Union’s Horizon 2020 research and innovation programme under grant agreements 875171 Qualitop (Horizon 2020), 965221 iTOBOS (Horizon 2020), and 101096667 MELCAYA (HORIZON-RIA- Call: HORIZON-MISS-2021-CANCER-02) within the framework of the Horizon Europe research and innovation programme; the National Cancer Institute (NCI) of the US National Institute of Health (NIH) (CA83115); a grant from “Fundació La Marató de TV3” 201331-30, Catalonia, Spain; and a grant from “Fundación Científica de la Asociación Española Contra el Cáncer” GCB-15152978SOEN, Spain and “Fundación Leo Messi”.
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Saad F, Cella D, Basch E, Hadaschik BA, Mainwaring PN, Oudard S, et al. Effect of apalutamide on health-related quality of life in patients with non-metastatic castration-resistant prostate cancer: an analysis of the SPARTAN randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2018; 19: 1404–1416. 10.1016/S1470-2045(18)30456-X [DOI] [PubMed] [Google Scholar]
- 2.Uemura H, Arai G, Uemura H, Suzuki H, Aoyama J, Hatayama T, et al. Safety and efficacy of apalutamide in Japanese patients with metastatic castration-sensitive prostate cancer receiving androgen deprivation therapy: final report for the Japanese subpopulation analysis of the randomized, placebo-controlled, phase III TITAN study. Int J Urol 2022; 29: 533–540. 10.1111/iju.14843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uemura H, Koroki Y, Iwaki Y, Imanaka K, Kambara T, Lopez–Gitlitz A, et al. Skin rash following administration of apalutamide in Japanese patients with advanced prostate cancer: an integrated analysis of the phase 3 SPARTAN and TITAN studies and a phase 1 open-label study. BMC Urol 2020; 20: 139. 10.1186/s12894-020-00689-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal N, McQuarrie K, Bjartell A, Chowdhury S, Pereira de Santana Gomes AJ, Chung BH, et al. Health-related quality of life after apalutamide treatment in patients with metastatic castration-sensitive prostate cancer (TITAN): a randomised, placebo-controlled, phase 3 study. Lancet Oncol 2019; 20: 1518–1530. 10.1016/S1470-2045(19)30620-5 [DOI] [PubMed] [Google Scholar]
- 5.Katsuta M, Kimura T, Tashiro K, Murakami M, Hata K, Yanagisawa T, et al. Low body weight as a risk factor for apalutamide-related cutaneous adverse events. Anticancer Res 2022; 42: 2023–2028. 10.21873/anticanres.15682 [DOI] [PubMed] [Google Scholar]
- 6.Tohi Y, Kato T, Fukuhara H, Kobayashi K, Ohira S, Ikeda K, et al. Real-world analysis of apalutamide-associated skin adverse events in Japanese patients with advanced prostate cancer: a multi-institutional study in the Chu-shikoku Japan Urological Consortium. Int J Clin Oncol 2022; 27: 1348–1355. 10.1007/s10147-022-02183-z [DOI] [PubMed] [Google Scholar]
- 7.Katsuta M, Nobeyama Y, Hirafuku K, Tashiro K, Kimura T, Asahina A. Characteristics of mild and severe apalutamide-related cutaneous adverse events in patients with prostate cancer: s review of the literature. J Dermatol 2024; 51: 110–114. 10.1111/1346-8138.16972 [DOI] [PubMed] [Google Scholar]
- 8.Ducharme O, Sanchez-Pena P, Pham-Ledard A, Beylot-Barry M, Milpied B. The first case of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome caused by apalutamide, a novel oral androgen receptor antagonist. Contact Dermatitis 2022; 86: 313–315. 10.1111/cod.14024 [DOI] [PubMed] [Google Scholar]
- 9.Hsu YSO, Hsieh TS, Huang PW, Chu CY. Drug reaction with eosinophilia and systemic symptoms with features resembling Stevens-Johnson syndrome/toxic epidermal necrolysis related to apalutamide. J Eur Acad Dermatol Venereol 2023; 37: 246–248. 10.1111/jdv.18660 [DOI] [PubMed] [Google Scholar]
- 10.Huang Y, Luo J, Luo J, Leng Y, Han Q, Wen J, et al. Toxic epidermal necrolysis associated with apalutamide: a case report and brief review of the literature. 2022. [cited 2023 Mar 22]. Available from: https://doi.org/10.22541/au.165392104.40579095/v1 https://doi.org/10.22541/au.165392104.40579095/v1
- 11.Honda T, Tohi Y, Kaku Y, Kimura N, Kato T, Haba R, et al. Acute generalized exanthematous pustulosis during apalutamide treatment in a patient with prostate cancer. IJU Case Rep 2022; 5: 497–500. 10.1002/iju5.12525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawakami Y, Mitsui M, Takamoto H, Yamamoto Y. Apalutamide-induced exanthematous drug eruption displaying spongiotic dermatitis successfully treated with dose reduction. Int J Dermatol 2021; 60: 315–317. 10.1111/ijd.15420 [DOI] [PubMed] [Google Scholar]
- 13.Cremante M, Puglisi S, Gandini A, Guadagno A, Catalano F, Damassi A, et al. Apalutamide-induced lichenoid reaction in a patient with non-metastatic castrate-resistant prostate cancer. J Oncol Pharm Pract 2023; 29: 1748–1753. 10.1177/10781552231180598 [DOI] [PubMed] [Google Scholar]
- 14.T’jollyn H, Ackaert O, Chien C, Lopez-Gitlitz A, McCarthy S, Ruixo CP, et al. Efficacy and safety exposure-response relationships of apalutamide in patients with metastatic castration-sensitive prostate cancer: results from the phase 3 TITAN study. Cancer Chemother Pharmacol 2022; 89: 629–641. 10.1007/s00280-022-04427-1 [DOI] [PubMed] [Google Scholar]
- 15.Saito-Sasaki N, Sawada Y, Okada E, Nakamura M. Drug eruption caused by enzalutamide: a case and literature review of androgen receptor inhibitor-related drug eruptions. Australas J Dermatol 2018; 59: 133–134. 10.1111/ajd.12694 [DOI] [PubMed] [Google Scholar]
- 16.Ji C, Guha M, Zhu X, Whritenour J, Hemkens M, Tse S, et al. Enzalutamide and apalutamide: in vitro chemical reactivity studies and activity in a mouse drug allergy model. Chem Res Toxicol 2020; 33: 211–222. 10.1021/acs.chemrestox.9b00247 [DOI] [PubMed] [Google Scholar]
- 17.Oishi T, Hatakeyama S, Tabata R, Fujimori D, Kawashima Y, Tanaka R, et al. Effects of apalutamide dose reduction on skin-related adverse events in patients with advanced prostate cancer: a multicenter retrospective study. Prostate 2023; 83: 198–203. 10.1002/pros.24453 [DOI] [PubMed] [Google Scholar]
- 18.Yu Z, Yu K, Lv S, Wen, H, Fang W. Titration as an effective strategy to reduce apalutamide induced skin rash: a multi-institutional real-world experience. J Clin Oncol 2023; 41: 197–197. 10.1200/JCO.2023.41.6_suppl.197 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.