ABSTRACT
Introduction:
Clinical heterogeneity is one of the biggest challenges for researchers studying underlying neurobiological mechanisms in Autism Spectrum Disorder (ASD). We aimed to use polyinosinic-polycytidylic acid [Poly (I:C)] induced maternal immune activation mice model to investigate the behavioral variation and the role of brain circuits related to symptom clusters in ASD. For this purpose, behavioral tests were applied to offsprings and regional thickness was measured from histological brain sections in medial prefrontal cortex, hippocampus and striatum.
Methods:
Pups of intraperitoneal Poly (I:C)-applied mothers (n: 14) and phosphate buffered saline-applied mothers (n: 6) were used for this study. We used three chamber socialization test and social memory test to evaluate social behavior deficit in mice. Marble burying test was used for assessing stereotypic behavior and new object recognition test for learning and cognitive flexibility. Three subgroups (n: 4 for each) were determined according to behavioral test parameters. Regional thickness was measured in medial prefrontal cortex, hippocampus and striatum and compared between subgroups.
Results:
We detected that the behavioral differences were distributed in a spectrum as expected in the clinic and also detected increased hippocampus thickness in the stereotypic behavior dominant Poly (I:C) subgroup.
Conclusion:
Poly (I:C) induced maternal immune activation model creates the behavioral variation and cortical development differences that are seen in relation with symptom groups in ASD.
Keywords: ASD, regional thickness, social deficiency, stereotypic behaviour
INTRODUCTION
The heterogeneity in clinical presentation is a major challenge in Autism Spectrum Disorder (ASD) etiology studies. On one end of ASD, there are patients with intellectual disability and serious behavioral problems who need continuous supervision, on the other hand, there are patients with high academic achievements who can achieve an independent life. Autism spectrum disorder diagnosis includes different symptom clusters, which suggests involvement of different brain circuits in the pathophysiology of ASD. Social deficit is the main core symptom cluster with varying severity in different patients which includes deficit in reciprocity, in nonverbal and verbal communication. Another symptom cluster is restricted area of interest and repeated behavior, which present as stereotypical movements, insistence on sameness, restricted areas of interest, and hypo/hypersensitivity to sensory aspects of the environment. Intellectual disability and related learning problems may also be a frequent comorbidity. The categorical approach for symptoms remained inadequate for understanding the underlying brain circuits of the disorders and the mechanisms behind it.
Highlights
Clinical heterogeneity is one the biggest challenges for ASD etiology studies.
MIA is an appropriate model for simulating the clinical variation in symptoms.
Symptom clusters may be associated with regional morphological changes in brain.
Human studies indicate morphological and functional differences between ASD patients and healthy controls. Autism spectrum disorder patients were shown to have increased head circumference, brain volume, and cortical neuron numbers (1,2). In postmortem studies, brain weight of ASD patients were found to be higher than controls, and there is a significant increase in neuronal numbers and cortical thickness especially in the medial prefrontal cortex and dorsolateral prefrontal cortex compared to controls (2). The neocortex, entorhinal cortex, cornu ammonis, and dentate gyrus were associated with impairments in social interactions and communication, while the cerebellum was found related to motor deficits and coordination difficulties. The presence of abnormal neuronal migration in subcortical, periventricular, hippocampal, and cerebellar regions was associated with a broad spectrum of clinical manifestations in individuals with ASD (3). In analyses that reviewed structural and functional neuroimaging studies in individuals with ASD, basal ganglia was found to be associated with stereotypic behavior (4) whereas the anterior cingulate cortex was involved in self-referential processing, and medial prefrontal cortex (mPFC) and posterior cingulate cortex were involved in theory of mind processes in individuals with ASD (5).
Animal studies also support the association between autistic-like behaviors and the regional cortical thickness. BTBRT+tf/J mice, which is accepted as a natural ASD model, have been shown to have increased cortical thickness on the 7th postnatal day in the medial prefrontal cortex and insula compared to controls (6). These results suggested that increases in the number of cortical neurons may impair the formation of circuits, which regulate social and language skills.
Etiological studies show the role of multiplex interaction of genetic and environmental factors underlying the disorder (7). Prenatal viral infection is one of the most replicated findings from etiological studies (8). Maternal immune activation (MIA) is one of the most studied models in ASD studies which has advantages to model environmental effect with dosage, in the precise timing (9,10). Also polyinosinic-polycytidylic acid [Poly (I:C)] has the chance to mimic viral infection and the cortical development which is very similar between mice and human (11). Poly (I:C) MIA mice models have been previously shown to be associated with autistic behavior, immune response, and cortical development change (12–15).
Therefore, we chose to use Poly (I:C) MIA mice model to investigate the behavioral variation and the role of brain circuits related to symptom clusters in ASD. After we grouped animals based on behavioral test parameters, we calculated the regional thickness in hippocampus, medial prefrontal cortex and striatum as a correlate of different symptom clusters.
METHODS
Animals
Adult male THY-Ch2-YFP transgenic (12 weeks) animals were used from our laboratory strain Hacettepe University Neurological and Psychiatric Sciences Institute which was previously purchased from Jackson Lab. Adult C57BL/6 female mice were purchased from Hacettepe University Animal Research Laboratory. This study utilizes 14 Poly (I:C) and 6 PBS mice pups. They showed no difference in terms of their health condition, age and weight. The mice were raised under standard conditions (25±2 °C, 12-h light–dark cycle, free access to water and food). All the procedures related to animal use were approved by the Ethics Committee of Hacettepe University (2019/07–02) and followed the National Research Council’s Guide for the Care and Use of Laboratory Animals (8th edition) (16). All efforts were made to minimize the number of animals used and their suffering.
Study Design and the Maternal Immune Activation (MIA) procedure
Prenatal viral infection is a known environmental risk factor for developing ASD in the offspring. Thus administration of Poly (I:C), a synthetic double stranded RNA to pregnant mice induce ASD-like behaviors in the offsprings by imitating prenatal viral infections (13). Families of one male Thy-Ch2-YFP mouse and two female C57BL/6 mice were formed by grouping them together in a cage. Females were checked daily for the presence of vaginal plug early in the morning. The day when the vaginal plug was detected is considered as embryonic day 0.5 (E0.5). After the confirmation of the vaginal plug in all female mice, the male mice were removed from the cage. Two pregnant mice were kept in a cage to prevent isolation stress. On the 11th and 12th days of pregnancy, pregnant mice either received intraperitoneal Poly (I:C) (5 mg/kg) or phosphate buffered saline (PBS) (17). Phosphate buffered saline was applied to some of the pregnant mice to create a control pup group. Pregnant mice groups did not show a difference after a follow up of weight, physical examination, activity, nutrition and water consumption in the same room room with stabile temperature and humidity. After weaning (between 24–28 days), behavioral tests of the offsprings (n: 20) were performed and compared between the Poly (I:C) (n: 14) and PBS (n: 6) groups to validate the MIA model. Poly (I:C) (n: 14) and PBS (n: 6) groups were offsprings of different mothers treated with Poly (I:C) or PBS. Both sexes were evaluated, as it is hard to differentiate sex at this age. We chose this time range for reflection of the childhood period as ASD symptoms present in early childhood. Figure 1 shows the procedural scheme through our experiments. After the behavioral test analysis, we grouped the animals in the Poly (I:C) group according to behavioral parameters explained in section: The Grouping Based On Behavioral Test Parameters in the Poly I:C Group. Poly (I:C) group 1 represented stereotypic behavior dominant (StD) subgroup (n: 4) and Poly (I:C) group 2 represented social deficiency dominant (SDD) subgroup (n: 4).
Figure 1.
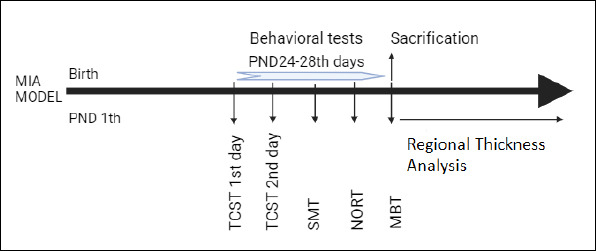
The scheme of the experimental procedures (MIA: maternal immune activation; PND: postnatal day; TCST: three chamber socialization test; SMT: social memory test; NORT: novel object recognition test; MBT: marble burying test.)
Behavioral Experiments
We chose the behavioral tests to evaluate different symptoms of ASD: Social deficits, stereotypic behaviors and cognitive performance. To evaluate social behaviors, we conducted three chamber socialization test, to evaluate stereotypic behaviors, we performed marble burying test, and to evaluate cognitive performance, we performed novel object recognition test. An observer blind to the experimental groups analyzed the results using Ethovision XT8.
Three Chamber Socialization Test (TCST)
Male and female mice were housed in a clean transport cage for habituation for 1 hour, and then released into an empty 3-chamber space for a total of 10 minutes and allowed to freely explore all three chambers. The next day, a plastic toy mouse in a cage was placed on one chamber and another live mouse was placed in a cage on the other chamber; then mice were placed in the middle chamber for 5 minutes with doors to other chambers closed. After 5 minutes, the doors were opened and mice were allowed to explore all the chambers freely. In each session, total time spent in the chambers containing live mice and the toy mouse were recorded and social index scores (time spent in the chamber containing live mice / time spent in the chamber containing live mice + time spent in the chamber containing the toy mice) were calculated for each animal. The frequency of entrances into each chamber and latency to first enter each chamber were recorded. The frequency index was calculated as the duration index. Latency was recorded as seconds (18).
Social Memory Test (SMT)
This test was performed after three-chamber socialization test to determine deficits in social memory. The day after the 3-chamber socialization test, an unfamiliar mouse was placed in the chamber which was previously containing the plastic mouse toy. A familiar mouse was placed in the opposite chamber. Then, the mouse to be tested was placed in the middle chamber and the doors were left open to allow the mouse examine the environment for 10 minutes. Under normal conditions, mice spent more time with the unfamiliar mice. In each session, total time spent in the chambers containing live mice and the toy mouse were recorded and social index scores (time spent in the chamber containing unfamiliar mice / time spent in the chamber containing unfamiliar mice + time spent in the chamber containing the familiar mice) were calculated for each animal. The frequency of entrances into each chamber and latency to first enter each chamber were recorded. The frequency index was calculated as the duration index. Latency was recorded as seconds (19).
Novel Object Recognition Test (NORT)
This test is used to evaluate the short-term and long-term object memories of mice (20). In our experiments, this test was chosen to evaluate the learning and mental flexibility that differed within the ASD group. After the mice were placed in the box with dimensions of 22.5×22.5×30 cm, we waited for 10 minutes for the mice to get used to the box. The next day, two objects were placed in the corners of the box with a 6 cm gap, and the mice were placed in the box for 10 minutes. In this box, the time they spent with each object and the frequency of entry to the areas (where the objects were located) were recorded. In order to evaluate short-term memory, one of the objects were replaced with a new object after 6 hours, and the time spent around each object and the frequency of entry into the regions (where the objects were recorded) for 5 minutes. Since mice with good memory were expected to learn and remember old objects, they were expected to spend more time around the new object and/or enter more in the new object area. Novel object recognition test score (NORT index) was calculated by dividing the time or frequency spent around the new object by the sum of the time or frequency spent around the “new+old” objects.
Marble Burying Test (MBT)
There is a relationship between the number of embedded marbles and stereotypical behavior (17). After the mice were accustomed to a clean cage with 3–4 cm of sawdust for 30–60 minutes, 20 marbles were placed in the cage symmetrically in the form of a grid. After the 15-minute inspection opportunity, mice were returned to their cages and the embedded marbles were counted. Marbles buried completely and more than 3/4 were taken into consideration.
Morphological Analysis
After completion of behavioral testing, animals were sacrificed under anesthesia by cardiac perfusion with 4% PFA (n: 20 pups). Brains were fixed with formaldehyde and embedded in paraffin. Four micrometer sagittal sections were taken with microtome. After deparaffinization, sections were rehydrated with decreasing alcohol series. Hematoxylin and eosin (H&E) staining was performed (21). Regional thickness was measured in 3 sections (100 micrometer apart) and averaged for each animal (n=4 mice/group, total 12 mice). Poly (I:C) subgroups were determined based on behavioral test parameters as described in 3.1.5. Accordingly, 4 mice were selected for Poly (I:C) group 1 (StD) and 4 mice were selected for Poly (I:C) group 2 (SDD) and 4 mice were selected for PBS group.
Regional Thickness Measurement
We measured thickness from H&E stained images taken from each sagittal brain section with Slide viewer program. We chose to study medial prefrontal cortex, and hippocampus, striatum and referred to Allen Brain Atlas (https: //developingmouse. brain-map. org/static/atlas) for coordinates of each brain region (Figure 2).
Figure 2.
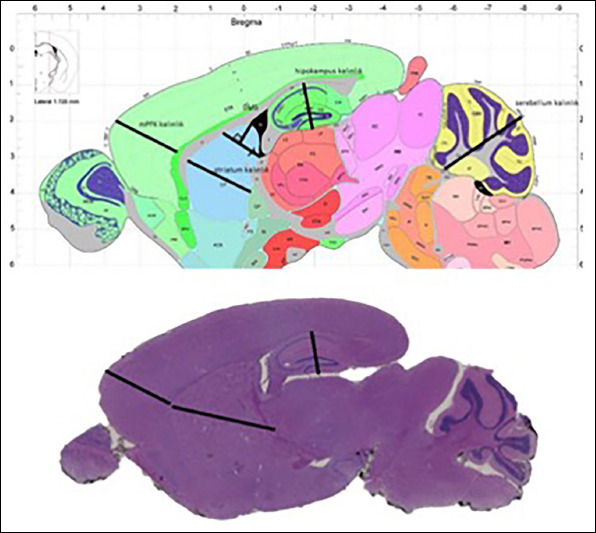
Regional thickness measurement in medial prefrontal cortex, hippocampus and striatum based on Allen Brain Atlas coordinates.
Statistics
IBM Statistical Package for Social Sciences program version 25 was used for statistical analysis. It was checked whether the data were normally distributed or not with the Kolmogorov-Smirnov test. Mann-Whitney U test was used to compare two data groups for non-normally distributed data. When more than two independent groups were compared, Kruskal Wallis test was used. In all tests, a p value of less than 0.05 was considered significant.
RESULTS
Behavioral Test Results
Three Chamber Socialization Test
Social preference index was not significantly different in the social chamber in the Poly (I:C) group when compared to controls (p=0.216). Since social target was placed in one half of the chamber, we then tested whether the mice prefer to spend time in the half that contains the social target. In parallel with the previous observation, social preference index for the half of the social chamber that contains the mice was similar in the Poly (I:C) group with the controls (p=0.804). The index of entrance frequency into the social chamber and social half of the chamber did not differ when analyzed with Mann-Whitney U test (p=0.934). The latency to first enter the social chamber and the social half of the chamber was longer in the Poly (I:C) group, however, there was no statistically significant difference between the Poly (I:C) and control group (p=0.364) (Table 1).
Table 1.
Comparison of behavioral test results of PBS and Poly (I:C) groups
| Test | PBS n=6 mean ± sd | Poly (I:C) n=14 mean ± sd | Statistics (Mann-Whitney U-test) |
|---|---|---|---|
| TCST–Duration index in the social chamber | 0.60±0.12 | 0.50±0.08 | p=0.216, z=-1.237 |
| TCST–Frequency index for entrance to the social chamber | 0.5±0.24 | 0.46±0.19 | p=0.934, z=0.082 |
| TCST–Latency to first enter the social chamber (min) | 0.96±0.46 | 2.04±1.18 | p=0.364, z=-0.907 |
| TCST–Duration index in the social half of the chamber | 0.61±0.12 | 0.52±0.10 | p=0.284, z=-1.072 |
| TCST–Frequency index for entrance to the social half of the chamber | 0.56±0.12 | 0.52±0.20 | p=0.805, z=-0.247 |
| TCST–Frequency index for entrance to the social half of the chamber | 0.96±0.46 | 2.04±1.86 | p=0.364, z=-0.907 |
| SMT–Duration index in the social target zone | 0.52±0.06 | 0.47±0.02 | p=0.804, z=-0.248 |
| SMT–Frequency index for entrance to the social target zone | 0.55±0.09 | 0.60±0.05 | p=0.620, z=-0.495 |
| SMT–Latency to first enter the social target zone | 3.00±0.30 | 1.7±0.48 | p=0.160, z=-1.404 |
| NORT–Duration index in the new object area | 0.48±0.04 | 0.47±0.06 | p=0.869, z=-0.165 |
| NORT–Frequency to enter the new object area | 0.45±0.06 | 0.42±0.05 | p: 0.869, z=-0.165 |
| NORT–Latency to enter the new object area | 36.6±14.9 | 38.6±8.0 | p=0.741, z=-0.330 |
| NORT–Distance moved in the locomotor test (cm) | 1477.53±69.8 | 1304.61±117.2 | p=0.216, z=-1.237 |
| MBT-Total marble burying score | 3.6±1.1 | 5.00±0.6 | p=0.611, z=-0.509 |
| MBT–3/4 marble burying score | 7.50±0.7 | 8.7±0.6 | p=0.424, z=-0.800 |
MBT: marble burying test; NORT: novel object recognition test; PBS: phosphate buffered saline; Poly (I:C): Polyinosinic: polycytidylic acid; SMT: social memory test; TCST: three chamber socialization test.
Social Memory Test
The preference index for interacting with a stranger mouse over a familiar mice was similar between Poly (I:C) and PBS groups (p: 0.804). Latency to first enter the chamber containing the stranger mouse as well as the frequency of entrance into the chamber containing the stranger mouse were not different between groups (p=0.160; p=0.620, respectively) (Table 1).
Locomotor activity and NORT results
Locomotor activity was not statistically significant between the two groups (p=0.216). The ratio of the duration spent exploring the novel object over total time spent exploring both objects for the PBS and Poly (I:C) groups were 48% and 47%, respectively. There was no statistically significant difference in novel object recognition index (p=0.869). The frequency index of the entrance into the novel object area and the latency to first enter the novel object area were similar between PBS and Poly (I:C) groups (p=0.869; p=0.41, respectively) (Table 1).
Marble Burying Test
The numbers of totally buried marbles and 3/4 buried marbles were not different between Poly (I:C) and PBS groups (p: 0.611; p: 0.424, respectively) (Table 1).
The Grouping Based On Behavioral Test Parameters in the Poly (I:C) Group
Unlike previous studies, we did not find statistically significant effects of prenatal Poly (I:C) exposure on ASD-like behaviors at postnatal week-4. However, the variability in the social preference index was high, suggesting that only a subgroup of mice subjected to Poly (I:C) may have developed ASD-like behavior. This is in line with the multifactorial etiologic hypothesis of ASD. Although prenatal exposure to viral infections is a risk factor for ASD, not all of the children prenatally exposed to viral infection show ASD symptoms. The contribution of both genetic and environmental factors is necessary for the development of ASD.
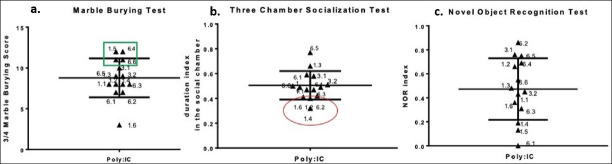
We, therefore investigated the behavioral data further for possible subgroups. Intriguingly, two groups were distinguishable in the Poly (I:C) group: First Poly (I:C) group mainly showing stereotypic behaviors, second Poly (I:C) group mainly showing lack of social interest (Figure 3a and 3b).
Figure 3.
The determination of subgroups in the Poly (I:C) group is shown according to the distribution of the selected behavioral test results. 3a shows the first Poly (I:C) group, where stereotypic behavior predominates, containing animals with the highest 3/4 marble burying scores within the Poly (I:C) group. 3b shows the second Poly (I:C) group, whose social deficit is dominant, containing animals with the lowest duration index in the social compartment within the Poly (I:C) group. 3c shows the high variation in the distribution of the NOR index within the Poly ( I:C) group.
The social deficit dominant (SDD) group showed variability in stereotypic behavior, i. e. mice in this group may have high or low stereotypic behavior. Similarly, the social behaviors of the stereotypic behavior dominant (StD) group showed high variability, including mice with high and poor socialization index. Variability in cognitive performance was also observed in both SDD and StD groups. (Figure 3, Table 2).
Table 2.
Behavioral summary of Poly (I:C) subgroups
| Behavior test parameter | Poly (I:C) StD ( first group) (stereotypic behavior dominant) | Poly (I:C) SDD ( second group) (socialization deficit dominant) |
|---|---|---|
| Duration index in the social chamber | high/low | low |
| ¾ marble burying score | high | high/low |
| NORT duration index | high/low | high/low |
NORT: novel object recognition test; Poly (I:C): Polyinosinic: polycytidylic acid.
We also confirmed that the social deficits and stereotypic behaviors in the selected groups were different than PBS treated controls with Kruskal Wallis analysis. Total marble burying score (p=0.034), ¾ marble burying score (p=0.022), time index in the social chamber (p=0.018),frequency index for social chamber entry (p=0.021), time index in social half of the chamber(p=0.012) and frequency index for the social half of the chamber(p=0.018)were found to be significantly different among the three groups.
To control for the effects of transgene introduced by use of both wild type and Thy-YFP-1 mice, we further analyzed the behavioral data of control group, which includes 3 Thy+ mice and 3 wild type mice. We did not find any statistifically significant difference in ASD-like behaviors between groups.
Regional Thickness Measurement Results
To test whether these differences in behaviors were reflected in brain morphometry, we measured the thickness of brain regions shown to be involved in the pathophysiology of ASD. From the selected subjects (n=4/group) we compared differences in thickness across 3 brain regions: hippocampus, striatum and mPFC.
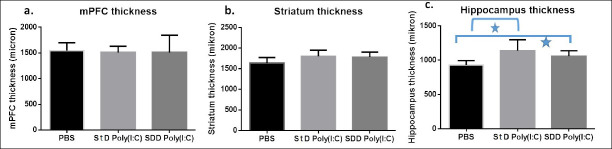
When Kruskal Wallis was conducted between the three groups, mPFC thickness and striatum thickness did not differ among groups. However, the hippocampus thickness was found significantly different among three groups (p=0,021). Moreover, in the comparison of hippocampus thickness between the two groups made by Mann Whitney U test, there was a significant difference between the PBS-StD Poly(I:C) group and the PBS-SDD group (p=0.021) (Figure 4, Table 3).
Figure 4.
Analysis of regional thickness in three groups by Kruskal Wallis test is shown. There was no statistically significant change in cortical thickness between the groups in the medial prefrontal cortex in 4a and in the striatum in 4b. It is seen that the StD and SDD Poly (I:C) groups shown in 4c, has higher hippocampus thickness (p: 0.021) compared to the control PBS group.
Table 3.
Comparison of the measurement of regional thicknesses according to behavioral subgroups
| Regional thickness (micron) | PBS n=4 mean ± sd | StD Group Poly (I:C) Group 1 n=4 mean ± sd | SDD Poly (I:C) Group 2 n=4 mean ± sd | Statistics (Kruskal Wallis) |
|---|---|---|---|---|
| mPFC thickness | 1543.64±154.75 | 1511.54±118.64 | 1510.48±335.19 | p=0,926 |
| Striatum thickness | 1636.88±133.46 | 1800.67±148.48 | 1780.34±121.13 | p:0.227 |
| Hippocampus thickness | 932.40±62.67 | 1138.77±162.44 | 1059.93±77.99 | p=0,021 |
mPFC: Medial prefrontal cortex; NORT: Novel Object Recognition Test; PBS: Phosphate buffered saline; Poly (I:C): Polyinosinic: polycytidylic acid; SDD: socialization deficit dominant; StD: stereotypic behavior dominant.
The Correlation of Regional Thickness and Behavioral Test Results
The mean thicknesses from different mediolateral coordinates in all brain regions of interest were further analyzed for correlations. The hippocampal thickness was positively correlated with marble burying scores (p:0.040,r:0.554). Novel object recognition duration index was positively correlated with striatum thickness (p:0.029, r:0.582). Hippocampus thickness was found to show a strong correlation with striatum thickness (p=0,003, r=0,723).
DISCUSSION
In this study, we showed that midgestational Poly (I:C)-induced MIA model did not create statistically significant difference in socialization behavior in the postnatal 24–28th days. On the other hand, we saw high variation in behavioral results mimicking real life situation and grouped the animals depending on our behavioral test parameters. Duration index for the social chamber in three social chamber test and marble burying scores were found useful for determining our subgroups. One favorable explanation may be that only a subgroup of mice with prenatal exposure to Poly (I:C) develop ASD-like behavior, because similar subgroups can also be seen in ASD patients in clinical settings. Our stereotypic behavior dominant Poly (I:C) group traits resembled ASD patients with prominent stereotypic behaviors but normal/higher IQ levels, and mild socialization deficits who are categorized as high functioning ASD. Our second Poly (I:C) group showed social deficits mainly similar to ASD patients who have serious deficit in socialization, low/normal IQ scores and variable stereotypic behaviors who are categorized as low functioning ASD (22).
One of the advantages of our study to the previous studies is investigating the behavioral and morphometric changes in preadolescent mice, corresponding to the age ASD is first diagnosed. Although MIA in mice has been linked to changes in gene expression, neuroanatomy, and neurochemistry consistent with ASD in adult offspring (14,15), the studies carried out during adulthood cannot rule out adaptive changes. Further, fetal brain development differences may not be fully captured in adult mice as it has been shown that age created differences in the neuropsychiatric disorder models in some genetic backgrounds including C57BLJ6 (13,23).
The timing and dosage of Poly (I:C) use in MIA models are also factors that have been found to have an effect on behavior and immunologic response (11). We used i.p. 5 mg/kg dosage twice, as we could not risk miscarriage, low number of pups and toxicity due to our preliminary experiments which is also a concern for other researchers in this field (24). Higher dosage was related with behavioral differences before (14,15), however, rats and adult animals were generally used taking these into consideration. This may also be another possible explanation for not finding a difference in behavior in our study between PBS and Poly (I:C) group in total. Also, although the pregnant mice in our study carried C57 background and were from the same source with the same age and weight, they were not siblings from the same mother unlike the offsprings used in this study. Therefore, genetic variability in the mice strain may also have a slight effect on our results.
Environmental factors have been shown to be related to area specificity. As MIA models environmental factors, changes in specific brain areas need to be considered. For example; in an ASD human twin neuroimaging study, grey matter thickness in the orbitofrontal cortex (OFC) and anterior cingulate cortex (ACC) were found to be positively correlated with ASD. Also, the repetitive behavior severity was correlated with OFC thickness (25). mPFC, hippocampus, striatum are the most studied areas in ASD research, which have been found to be related with differences in social behavior, restricted interest area, repetitive behavior and sensory integration problems (26,27). Although increased head circumference, brain volume, and neuron number in cortex at the onset of symptoms in early childhood have been shown in human ASD studies (1,2), it is not clear if the differences in morphology or functionality in specific brain areas found are the results or the causes of the disorder. As a possible explanation, prenatal immune activation is hypothesized to be involved in pathogenesis especially in critical vulnerable periods in neurodevelopment. To further confirm this relationship, MIA animal model study findings indicate a link between maternal immune activation and cortical development (14).
When we checked the morphometric differences and correlations of our ASD subgroups, we found that hippocampi were thicker in StD subgroup and stereotypic behavior correlated positively with hippocampus thickness. Brain volume changes reported in ASD subjects were associated with deficits in synaptic pruning. Microglia are important actors in synaptic pruning and significant changes in microglial activation have been shown between postnatal days 14–28 in the hippocampus (28). This critical timing of microglial activation may be the reason for area specific (hippocampus) increase in thickness in StD subgroup in our study. Pruning deficits may be one of the explanations of the higher hippocampal thickness and social behavior deficit (duration index in the social chamber) found in our study. Lesions in the hippocampus, inactivation of the CA1, CA2, and inhibition of protein synthesis paradigms in animal models in these areas have been shown many times with social recognition memory deficits (29). These findings suggest that the hippocampus is crucial for social memory and recognition, which are important components of social behavior (9). However, we did not use additional immunohistological or molecular method to show if area-specific changes matched with specific differences on cellular level further supporting the link with synaptic pruning and regional thickness measurements.
We did not find any difference in striatum thickness between our groups. Although striatum is generally the brain region found to be correlated with stereotypic behavior, we found a correlation between striatum thickness and NOR index in our study. This may be related to the regulating effect of striatum on memory (30). Hippocampus and striatum thickness showed a strong positive correlation, which may be the result of associations between these areas supplying the crosstalk for regulating social behavior in the context of learning and stereotypical behavior. This may also be a possible explanation for finding marble burying score positively correlated with hippocampus thickness. In addition, higher striatal thickness accompanying higher NOR duration index may also be thought as a presentation of better IQ scores seen in the high functional autistic humans. This may be the result of a compensatory mechanism behind better socialization scores in the first Poly (I:C) group (StD) as social behaviors cannot be basically evaluated without learning.
To our surprise, we found no association between mPFC thickness and autistic-like behavior. This may be related to the timing of our experiments (PN24–28th day) which corresponds to the toddler stage in mice lifetime. As we conducted our experiments in this age interval, which may be before the prefrontal cortex maturation, this may be one of the reasons that we could not find any difference in the medial prefrontal cortex thickness. The prefrontal cortex is the latest area to be organized in the brain development order. Additionally, the small sample size and variation among subjects may have limited the interpretation of our results.
We are proposing that MIA can be used to model clinical heterogeneity. Prenatal exposure to MIA resulted in increased stereotypic behaviors in a subgroup of mice and increased social deficits in another subgroup. Subgroups showed variation in cognitive performance scores similar to clinical phenotypes. Our StD group was found to have thicker hippocampi compared to other subgroups. Difference in hippocampal thickness was found to be associated with stereotypic behavior, whereas striatal thickness was correlated with learning and memory. We conclude that the mechanisms beyond the symptom groups and phenotypes may differ from each other depending on timing and brain areas in ASD. However, we did not study molecular actors behind these differences between subgroups. Future ASD MIA studies in larger samples should address molecular mechanisms considering symptom heterogeneity.
Acknowledgment:
The parafinned brain sections were obtained and stained with H-E procedure in Ankara University at the Department of Pathology. The microscobic evaluation in this study was also done by the digital pathology system obtained with the Project (14A0230003) by Ankara University Scientific Fund (BAP).
We are grateful to Prof. Aylin Heper Okçu for her valuable mentoring and guidance through the microscobic evaluation. We give our special thanks for her labor to Kadriye Yücel Aydın in the sectioning to Filiz Yeşilyurt in the staining processes and to Gökhan Özdoğan for his labor in the digital imaging process.
Footnotes
Ethics Committee Approval: All procedures for animal use were approved by the Hacettepe University Animal Experimentation Ethics Committee (2019/07–02), and the procedures followed the National Research Council’s Guide for the Care and Use of Laboratory Animals (8th edition) (16).
Peer-review: Externally peer-reviewed.
Author Contributions: Concept- DÜ, EEK; Design- DÜ, EEK; Supervision- DÜ, EEK, ABY; Resource- DÜ, EEK, ABY; Materials- DÜ, ABV; Data Collection and/or Processing- DÜ, EEK, ABV, TBK; Analysis and/or Interpretation- DÜ, EEK, ABV; Literature Search- DÜ, EEK, AV; Writing- DÜ, EEK, ABV, TBK; Critical Reviews- DÜ, EEK.
Conflict of Interest: The authors declared that there is no conflict of interest.
Financial Disclosure: Hacettepe University Scientific Studies Project Unit (Project ID:18547).
REFERENCES
- 1.Schumann CM, Nordahl CW. Bridging the gap between MRI and postmortem research in autism. Brain Res. 2011;1380:175–186. doi: 10.1016/j.brainres.2010.09.061. https://doi.org/10.1016/j.brainres.2010.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Courchesne E, Mouton PR, Calhoun ME, Semendeferi K, Ahrens-Barbeau C, Hallet MJ, et al. Neuron number and size in prefrontal cortex of children with autism. JAMA. 2011;306(18):2001–2010. doi: 10.1001/jama.2011.1638. https://doi.org/10.1001/jama.2011.1638. [DOI] [PubMed] [Google Scholar]
- 3.Wegiel J, Flory M, Kuchna I, Nowicki K, Ma SY, Imaki H, et al. Stereological study of the neuronal number and volume of 38 brain subdivisions of subjects diagnosed with autism reveals significant alterations restricted to the striatum, amygdala and cerebellum. Acta Neuropathol Commun. 2014;2(1):1–18. doi: 10.1186/s40478-014-0141-7. https://doi.org/10.1186/s40478-014-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nickl-Jockschat T, Habel U, Maria Michel T, Manning J, Laird AR, Fox PT, et al. Brain structure anomalies in autism spectrum disorder-a meta-analysis of VBM studies using anatomic likelihood estimation. Hum Brain Mapp. 2012;33(6):1470–1489. doi: 10.1002/hbm.21299. https://doi.org/10.1002/hbm.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patriquin MA, DeRamus T, Libero LE, Laird A, Kana RK. Neuroanatomical and neurofunctional markers of social cognition in autism spectrum disorder. Hum Brain Mapp. 2016;37(11):3957–3978. doi: 10.1002/hbm.23288. https://doi.org/10.1002/hbm.23288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenlon LR, Liu S, Gobius I, Kurniawan ND, Murphy S, Moldrich RX, et al. Formation of functional areas in the cerebral cortex is disrupted in a mouse model of autism spectrum disorder. Neural Dev. 2015;10(1):1–14. doi: 10.1186/s13064-015-0033-y. https://doi.org/10.1186/s13064-015-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaugler T, Klei L, Sanders SJ, Bodea CA, Goldberg AP, Lee AB, et al. Most genetic risk for autism resides with common variation. Nature Genet. 2014;46(8):881–885. doi: 10.1038/ng.3039. https://doi.org/10.1038/ng.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hornig M, Bresnahan MA, Che X, Schultz AF, Ukaigwe JE, Eddy ML, et al. Prenatal fever and autism risk. Mol Psychiatry. 2018;23(3):759–766. doi: 10.1038/mp.2017.119. https://doi.org/10.1038/mp.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gzielo K, Piotrowska D, Litwa E, Popik P, Nikiforuk A. Maternal immune activation affects socio-communicative behavior in adult rats. Sci Rep. 2023;13(1):1918. doi: 10.1038/s41598-023-28919-z. https://doi.org/10.1038/s41598-023-28919-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haida O, Al Sagheer T, Balbous A, Francheteau M, Matas E, Soria F, et al. Sex-dependent behavioral deficits and neuropathology in a maternal immune activation model of autism. Transl Psychiatry. 2019;9(1):124. doi: 10.1038/s41398-019-0457-y. https://doi.org/10.1038/s41398-019-0457-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haddad FL, Patel SV, Schmid S. Maternal Immune Activation by Poly I:C as a preclinical model for neurodevelopmental disorders:a focus on autism and schizophrenia. Neurosci Biobehav Rev. 2020;113:546–567. doi: 10.1016/j.neubiorev.2020.04.012. https://doi.org/10.1016/j.neubiorev.2020.04.012. [DOI] [PubMed] [Google Scholar]
- 12.Amodeo DA, Lai CY, Hassan O, Mukamel EA, Behrens MM, Powell SB. Maternal immune activation impairs cognitive flexibility and alters transcription in frontal cortex. Neurobiol Dis. 2019;125:211–218. doi: 10.1016/j.nbd.2019.01.025. https://doi.org/10.1016/j.nbd.2019.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergdolt L, Dunaevsky A. Brain changes in a maternal immune activation model of neurodevelopmental brain disorders. Prog Neurobiol. 2019;175:1–19. doi: 10.1016/j.pneurobio.2018.12.002. https://doi.org/10.1016/j.pneurobio.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cieslik M, Gassowska-Dobrowolska M, Jesko H, Czapski GA, Wilkaniec A, Zawadzka A, et al. Maternal immune activation induces neuroinflammation and cortical synaptic deficits in the adolescent rat offspring. Int J Mol Sci. 2020;21(11):4097. doi: 10.3390/ijms21114097. https://doi.org/10.3390/ijms2111409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varghese M, Keshav N, Jacot-Descombes S, Warda T, Wicinski B, Dickstein DL, et al. Autism spectrum disorder:neuropathology and animal models. Acta Neuropathol. 2017;134(4):537–566. doi: 10.1007/s00401-017-1736-4. https://doi.org/10.1007/s00401-017-1736-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Institute for Laboratory Animal Research Division on Earth and Life Studies National Research Council. Guide for the care and use of laboratory animals. 8th ed. Washington, DC: National Academies Press; 2011. [Google Scholar]
- 17.Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351(6276):933–939. doi: 10.1126/science.aad0314. https://doi.org/10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Dev Neurosci. 2007;25(8):515–521. doi: 10.1016/j.ijdevneu.2007.09.008. https://doi.org/10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rein B, Ma K, Yan Z. A standardized social preference protocol for measuring social deficits in mouse models of autism. Nat Protoc. 2020;15(10):3464–77. doi: 10.1038/s41596-020-0382-9. https://doi.org/10.1038/s41596-020-0382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zou D, Chen L, Deng D, Jiang D, Dong F, McSweeney C, et al. DREADD in parvalbumin interneurons of the dentate gyrus modulates anxiety, social interaction and memory extinction. Curr Mol Med. 2016;16((1)):91–102. doi: 10.2174/1566524016666151222150024. https://doi.org/10.2174/1566524016666151222150024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onaolapo AY, Onaolapo OJ, Nwoha PU. Alterations in behaviour, cerebral cortical morphology and cerebral oxidative stress markers following aspartame ingestion. J Chem Neuroanat. 2016;78:42–56. doi: 10.1016/j.jchemneu.2016.08.006. https://doi.org/10.1016/j.jchemneu.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Chakrabarti B. Commentary:Critical considerations for studying low-functioning autism. J Child Psychol Psychiatr. 2017;58(4):436–438. doi: 10.1111/jcpp.12720. https://doi.org/10.1111/jcpp.12720. [DOI] [PubMed] [Google Scholar]
- 23.Eltokhi A, Kurpiers B, Pitzer C. Behavioral tests assessing neuropsychiatric phenotypes in adolescent mice reveal strain- and sex-specific effects. Sci Rep. 2020;10(1):11263. doi: 10.1038/s41598-020-67758-0. https://doi.org/10.1038/s41598-020-67758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atanasova E, Arevalo AP, Graf I, Zhang R, Bockmann J, Lutz AK, et al. Immune activation during pregnancy exacerbates ASD-related alterations in Shank3-deficient mice. Mol Autism. 2023;14(1):1. doi: 10.1186/s13229-022-00532-3. https://doi.org/10.1186/s13229-022-00532-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hegarty JP, 2nd, Pegoraro LFL, Lazzeroni LC, Raman MM, Hallmayer JF, Monterrey JC, et al. Genetic and environmental influences on structural brain measures in twins with autism spectrum disorder. Mol Psychiatry. 2020;25(10):2556–2566. doi: 10.1038/s41380-018-0330-z. https://doi.org/10.1038/s41380-018-0330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banker SM, Gu X, Schiller D, Foss-Feig JH. Hippocampal contributions to social and cognitive deficits in autism spectrum disorder. Trends Neurosci. 2021;44(10):793–807. doi: 10.1016/j.tins.2021.08.005. https://doi.org/10.1016/j.tins.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Zhang K, He X, Zhou J, Jin C, Shen L, et al. Structural, functional, and molecular imaging of autism spectrum disorder. Neurosci Bull. 2021;37(7):1051–1071. doi: 10.1007/s12264-021-00673-0. https://doi.org/10.1007/s12264-021-00673-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delpech JC, Wei L, Hao J, Yu X, Madore C, Butovsky O, et al. Early life stress perturbs the maturation of microglia in the developing hippocampus. Brain Behav Immun. 2016;57:79–93. doi: 10.1016/j.bbi.2016.06.006. https://doi.org/10.1016/j.bbi.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Zhan Y. Regulation of social recognition memory in the hippocampal circuits. Front Neural Circuits. 2022;16:839931. doi: 10.3389/fncir.2022.839931. https://doi.org/10.3389/fncir.2022.839931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, et al. Natural neural projection dynamics underlying social behavior. Cell. 2014;157(7):1535–1551. doi: 10.1016/j.cell.2014.05.017. https://doi.org/10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]