Abstract
Background
Patients with extensive lymph node metastases have a poor prognosis. Clinical staging of lymph node metastases poses significant challenges given the limited sensitivity and specificity of imaging techniques. The aim of this study was to investigate the overall survival (OS) of patients with N3 disease in a real-world Dutch population and the added value of surgery in these patients.
Methods
Patients with cN3M0 esophageal or gastroesophageal cancer were identified from the Netherlands Cancer Registry (2012–2019). Treatment consisted of neoadjuvant chemo(radio)therapy followed by resection or chemo(radio)therapy, radiotherapy, or esophagectomy alone. OS was calculated using the Kaplan-Meier method.
Results
Some 21,566 patients were diagnosed with esophageal cancer of whom 359 (1.7%) had cN3M0 disease. Median OS of these patients was 12.5 months (95% CI: 10.7–14.3). Median OS following chemoradiotherapy alone and neoadjuvant therapy plus surgery was 13.3 months (95% CI: 10.7–15.9) and 23.7 months (95% CI: 18.3–29.2), respectively. Of all patients who underwent esophagectomy, 391 (2.8%) had (y)pN3 disease, and median OS was 16.1 months (95% CI: 14.8–17.4). Twenty-one patients (5.4%) were correctly classified as cN3, and 3-year OS was 21%.
Conclusion(s)
Clinical staging appears to be difficult, apparently in patients with N3 esophageal cancer. Surgery seems to be of benefit to these patients. More research is required to address the ongoing challenges in clinical staging and the best neoadjuvant therapy.
Keywords: Esophageal cancer, Extensive lymph node metastases, Esophagectomy, Survival, Netherlands Cancer Registry
Introduction
With worldwide over 600,000 people newly diagnosed and 544,000 deaths each year, esophageal cancer is the seventh most common cancer in the world [1]. Approximately 50% of patients present with disseminated disease, and only palliative treatment options can be offered [2]. In the West, standard of care for patients with curable and resectable esophageal or junctional cancer consists of neoadjuvant chemoradiotherapy followed by esophagectomy, and the estimated 5-year overall survival (OS) of patient after trimodality therapy is about 50% [3–5].
There are several factors which influence survival, such as comorbidity, clinical staging, and response to systemic treatment. Patients with extensive lymph node metastases (N3 disease) have a poor prognosis compared to patients with no lymph node metastases (cN0), with a median OS of less than 12 months [6]. It is questionable whether surgery can cure this group of patients. Esophagectomy is associated with postoperative mortality rates of 1–5% and severe postoperative morbidity with a substantial impact on quality of life [7–12].
On the other hand, it is difficult to stage these patients correct preoperatively, and clinical staging of lymph node metastases poses significant challenges. Endoscopic ultrasonography can be used for nodal staging and has an accuracy of 74% when used alone, which increases to nearly 90% when combined with fine-needle aspiration (FNA) [13]. However, endoscopic ultrasonography performs better with advanced tumors (i.e., T4) than early disease (i.e., T1) [14, 15]. In addition, 18F-FDG positron emission tomography/computed tomography (FDG-PET/CT) is used to rule out distant metastases but has a poor spatial resolution for nodal staging [16, 17]. The limited sensitivity and specificity of current imaging techniques contribute to diagnostic uncertainties. These challenges can result in under- or overstaging of esophageal cancer, impacting treatment decisions and prognostic accuracy.
The aim of this study was to investigate the OS of Dutch patients with clinical and/or pathological N3 esophageal cancer, to investigate the degree of under-/overstaging and the added value of surgery in these patients as we hypothesize that patients with cN3 or (y)pN3 disease have significantly worse outcomes.
Materials and Methods
Study Design
This study included patients with cN3M0 or (y)pN3 esophageal cancer identified from the Netherlands Cancer Registry (NCR) between 2012 and 2019. The NCR is a nationwide population-based cancer registry that covers the entire Dutch population of more than 17 million people. The NCR is based on notification of all newly diagnosed malignancies in the Netherlands by the national automated pathology archive (PALGA). Specially trained employees of the NCR routinely extract additional information on diagnosis, tumor stage, and treatment from the medical records. As the NCR is a nationwide registry, no informed consent or approval of a Medical Ethics Committee was required.
Patients
This retrospective cohort study included patients with squamous cell carcinoma or adenocarcinoma of the esophagus or esophagogastric junction with extensive lymph node metastases (cN3M0 and/or (y)pN3), according to the UICC 7th and 8th TNM staging manual [18, 19]. Patients were identified from the NCR between 2012 and 2019. The NCR is a nationwide population-based cancer registry that covers the entire Dutch population of more than 17 million people. The NCR is based on the notification of all newly diagnosed malignancies in the Netherlands by the national automated pathology archive (PALGA). Specially trained employees of the NCR routinely extract additional information on diagnosis, tumor stage (determined by the local multidisciplinary tumor board), and treatment from the medical records.
Treatment options for these patients are neoadjuvant therapy (i.e., chemo(radio)therapy) plus esophagectomy, chemotherapy alone, radiotherapy alone, chemoradiotherapy alone, or esophagectomy alone. Details about chemo(radio)therapy regimens without surgery were out of interest for this manuscript.
Outcomes
The primary outcome was the OS of patients with cN3M0 disease. Secondary outcomes were the proportion of patients with (y)pN3 disease out of all patients with cN3M0 disease, the proportion of patients with (y)pN3 disease out of all patients who underwent surgery in the NCR, and their corresponding OS.
Statistical Analyses
Patient and tumor characteristics were analyzed using descriptive statistics and were presented as median with interquartile range or frequencies (%). Survival was reported in months and was calculated from the date of diagnosis until the date of death or last day of follow-up, using the Kaplan-Meier method. The proportion of patients with (y)pN3 disease was calculated relative to all patients with cN3M0 disease or relative to all patients who underwent surgery in the NCR. Statistical analysis was performed with the use of R version 4.0.0 (R: A language and environment for statistical computing; R Foundation for Statistical Computing, Vienna, Austria).
Results
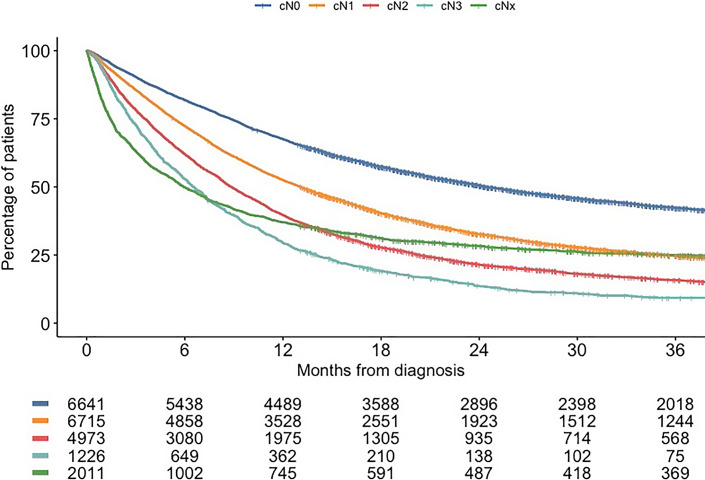
Between 2012 and 2019, a total of 21,566 patients were diagnosed with cT1-4b/TxN0-3/NxM0-1/Mx esophageal cancer in the Netherlands. Of those, 6,641 patients (30.8%) had cN0 disease, 6,715 (31.1%) had cN1 disease, 4,973 (23.1%) had cN2 disease, 1,226 (5.7%) had cN3 disease, and cNx disease was seen in 2,011 patients (9.3%). OS outcomes according to cN category are shown in Figure 1.
Fig. 1.
OS stratified by cN category.
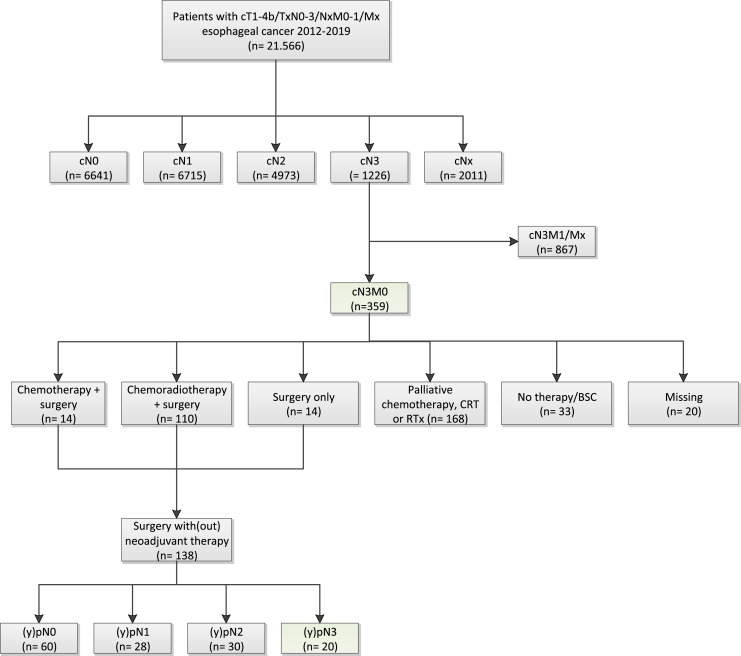
Out of all patients with esophageal cancer, 359 had cN3M0 disease at presentation, and baseline characteristics of these patients are shown in Table 1. Treatment consisted of chemotherapy plus surgery in 14 patients (3.9%), chemoradiotherapy plus surgery in 110 patients (30.6%), and surgery only in 14 patients (3.9%) (Fig. 2). Another 168 patients (46.8%) only underwent palliative chemotherapy, chemoradiotherapy, or palliative radiotherapy, and 33 patients (9%) did not undergo any treatment (i.e., best supportive care). Treatment was missing for the remaining 20 patients (6%).
Table 1.
Baseline characteristics of patients with cN3M0 esophageal cancer
| All patients (n = 359), n (%) | |
|---|---|
| Sex | |
| Male | 266 (74.1) |
| Age, median (IQR) | 67 (60–73) |
| Tumor type | |
| Adenocarcinoma | 235 (65.5) |
| Squamous cell carcinoma | 104 (29) |
| Missing | 20 (5.6) |
| Tumor differentiation grade | |
| Well differentiated (G1) | 11 (3.1) |
| Moderately differentiated (G2) | 86 (24) |
| Poor differentiated (G3) | 124 (34.5) |
| Missing | 138 (38.4) |
| Clinical T category | |
| cTx | 17 (4.7) |
| cT1 | 3 (0.8) |
| cT2 | 44 (12.3) |
| cT3 | 250 (69.6) |
| cT4 | 45 (12.5) |
| Treatment | |
| None | 33 (9.2) |
| Chemotherapy | 34 (9.5) |
| Chemoradiotherapy | 90 (25.1) |
| Radiotherapy | 44 (12.3) |
| Chemotherapy and surgery | 14 (3.9) |
| Chemoradiotherapy and surgery | 110 (30.6) |
| Surgery alone | 14 (3.9) |
| Missing | 20 (5.6) |
IQR, interquartile range.
Fig. 2.
Flowchart of patients included in the study.
In patients with cN3M0 disease, median OS was 12.5 months (95% CI: 10.7–14.3) with 1- and 3-year survival rates of 51.8% and 22.5%. Median OS for patients who were selected for surgery after neoadjuvant therapy was 23.7 months (95% CI: 18.3–29.2), with 1- and 3-year survival rates of 76% and 38%. Palliative treatment with chemotherapy or radiotherapy alone resulted in a median OS of 7.1 months (95% CI: 5.7–8.6) and 1- and 3-year survival of 24% and 4%. Median OS of patients treated with chemoradiotherapy alone was 13.3 months (95% CI: 10.7–15.9), with 1- and 3-year survival rates of 57% and 26%.
Out of the 138 patients with cN3M0 disease who underwent surgery (with or without neoadjuvant therapy), 60 patients (43.5%) were (y)pN0, 28 (20.3%) were (y)pN1, and 30 (21.7%) were (y)pN2 (Fig. 2; Table 2). The associated 1- and 3-year OS for (y)pN0 disease was 88% and 58%, while this was 68% and 46% for (y)pN1 disease and 74% and 32% for (y)pN2 disease.
Table 2.
Pathological N-stage after therapy in patients with cN3M0 esophageal cancer
| Neoadjuvant chemotherapy + surgery (n = 14) | Neoadjuvant chemoradiotherapy + surgery (n = 110) | Surgery only (n = 14) | |
|---|---|---|---|
| (y)pN0 | 5 (35.7) | 53 (48.2) | 2 (14.3) |
| (y)pN1 | 1 (7.1) | 22 (20.0) | 5 (35.7) |
| (y)pN2 | 6 (42.9) | 21 (19.1) | 3 (21.4) |
| (y)pN3 | 2 (14.3) | 14 (12.7) | 4 (28.6) |
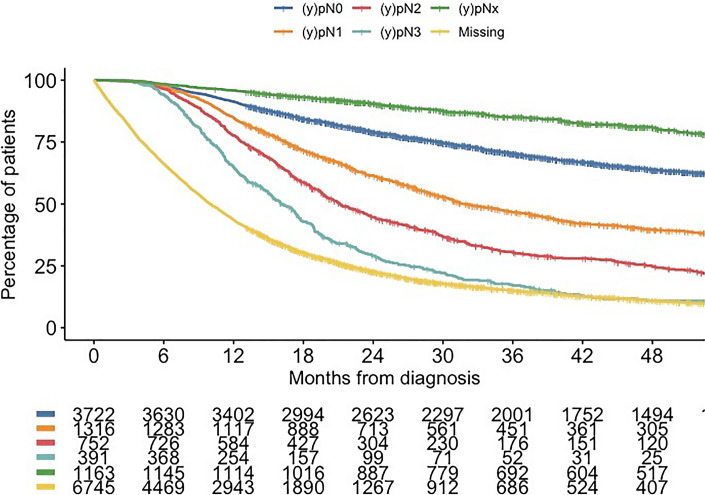
Only 20 patients (14.5%) had (y)pN3 disease, and the associated median OS of these patients was 17.4 months (95% CI: 14.5–20.4), with 1- and 3-year survival of 65% and 15%. Some 14,093 cM0 patients underwent surgery in the NCR, and corresponding survival for each (y)pN category is shown in Figure 3.
Fig. 3.
OS of cM0 patients who underwent surgery stratified by (y)pN category.
Of those 14,093 patients, 391 (2.8%) had (y)pN3 disease, and the associated median OS of these patients was 16.1 months (95% CI: 14.8–17.4), with 1- and 3-year survival of 65% and 21%. Of those, 107 patients (27.4%) were classified upfront as cN0, 145 (37.1%) as cN1, 113 (28.9%) as cN2, and only 21 patients (5.4%) with (y)pN3 disease were classified as cN3 and 5 (1.3%) as cNx.
Discussion
This study demonstrated limited median survival of 12.5 months and 3-year survival of only 22.5% in a real-world Dutch population of patients with cN3M0 esophageal cancer, which demonstrates the poor prognosis associated with extensive lymph node involvement. In addition, 15% of patients with cN3M0 esophageal cancer actually had (y)pN3 disease, and conversely, only 5.4% of patients with (y)pN3 disease after resection were staged as cN3. So, clinical staging remains an ongoing challenge in esophageal cancer. Patients who underwent esophagectomy had a better OS (23.7 months) compared to those receiving chemoradiotherapy alone (13.3 months) in patients with cN3M0 disease. Median survival for patients with (y)pN3 disease was 16.1 months.
Upper endoscopy with biopsies, endoscopic ultrasound with FNA of suspected lymph nodes, and FDG-PET/CT are mainstays in clinical staging of esophageal cancer [20]. However, the results of our study show that the quality of these diagnostic tests is limited as only 15% of patients have (y)pN3 out of all patients who were staged as cN3. This implies that clinical staging of lymph node involvement is still challenging. Hence, it would be advantageous to determine the effectiveness of neoadjuvant therapy in treating lymph node metastases (ycN stage). Unfortunately, our dataset lacks this information. The clinical overstaging of the majority of patients in the current study may be explained by the limited resolution of FDG-PET/CT scan in detecting lymph node metastases [21]. For example, a sarcoid-like reaction on the PET/CT scan could have been wrongly reported as cN3 disease. Therefore, a possible advice may be to puncture all suspected lymph nodes with FNA anyway since survival is considerably poor if (y)pN3 disease is actually present. However, this will not always be feasible in clinical practice. On the other hand, understaging is evident, with just 5.4% of patients diagnosed with (y)pN3 initially classified as cN3. This discrepancy might be attributed to sampling errors in endoscopic ultrasound or the tumor’s resistance to treatment, leading to disease progression into lymph nodes during neoadjuvant therapy.
In recent years, numerous studies have concentrated on novel imaging methods to improve the staging of esophageal cancer and identify any remaining disease or metastases after neoadjuvant therapy. Recent studies examining the performance of MRI in assessing lymph nodes have reported a pooled sensitivity of 71% (95% CI: 60–80%) and specificity of 72% (95% CI: 64–79%) [22]. Additionally, more recent techniques like Fibroblast Activation Protein Specific Enzyme Inhibitor (FAPI)-PET/CT show potential for clinical staging, although further research is necessary to determine their usefulness and added value [23]. These findings offer promise for future applications in the routine clinical staging and follow-up of esophageal cancer.
According to the CROSS regimen, the standard radiation field compasses the tumor area with precision and limit exposure to surrounding healthy tissues. In particular, the tumor length is restricted to not exceed 8 cm, and its width should not surpass 5 cm [3]. Adhering to these specific size limitations ensures that the radiation is concentrated on the affected region, effectively eradicating cancerous cells, while minimizing potential damage to adjacent healthy structures. Patients with extensive lymph node metastases located in the abdomen or thorax face the challenge of requiring a large radiation field when chemoradiotherapy is the preferred therapy. Unfortunately, a large radiation field is associated with increased toxicity. Moreover, as the number of lymph node metastases rises, the risk of developing metastatic disease becomes higher. Therefore, in patients with cN3 disease, induction chemotherapy could be considered as alternative therapy instead of only locoregional chemoradiotherapy as it reduces tumor volume and therefore select good responders who may benefit from additional surgery or additional chemoradiotherapy followed by surgery. A study performed in the USA in 2018 showed an increased OS in patients with cN3M0 disease who underwent induction therapy followed by surgery compared to induction therapy alone (20.0 vs. 12.2 months, p < 0.0001) [24]. However, the absolute survival benefit of surgery was significantly lower in cN3 disease compared to other nodal stages (cN1 or cN2).
The limited accuracy of preoperative clinical staging and unavailable data on ycN stage limit the conclusions on the benefit of surgery in patients with cN3 esophageal cancer. Esophagectomy after neoadjuvant treatment almost doubles the OS of patients with cN3 disease, but the impact of surgery should be weighed against its associated possible risks and complications. Additionally, no adjustments were made for performance status, which should also be considered when interpreting the data. Esophagectomy is a major survival procedure, with major morbidity and mortality rates of 1–5% [7, 11]. Recovery after surgery is estimated to take up to 6–12 months and have a significant impact on quality of life [9, 25, 26]. Whether impact of surgery is justified by the negative prognosis of cN3 disease remains to be established.
This study possesses several limitations. Since it was conducted retrospectively, certain data were unavailable, leading to the inability to conduct comprehensive analyses. First, no data were available on which diagnostic tests were performed upon diagnosis which creates uncertainty about the reliability of the clinical N-stage. Notably, there was lack of data on nodal stage after neoadjuvant therapy, limiting the comparison to patients with cN3 disease who underwent surgical resection. There were also no data available about performance status and comorbidities, which limits the possibility to perform multivariable Cox regression analysis of survival outcomes. However, it is important to acknowledge the strengths of this study as well. First, it stands out as one of the largest retrospective studies on cN3 disease in esophageal cancer to date. Furthermore, prospective tracking of follow-up data was conducted to guarantee comprehensive and current details on survival and subsequent monitoring.
In summary, this nationwide study demonstrates the limited OS among patients with cN3 and (y)pN3 esophageal cancer, highlighting the challenges of accurate clinical staging of (y)pN3 disease. It also shows that surgery could improve survival in these patients. Further research is needed to optimize clinical staging, to identify which factors are important in making a treatment decisions and to explore optimal treatment approaches for this specific population.
Statement of Ethics
As the NCR is a nationwide registry, no informed consent or approval of a Medical Ethics Committee was required.
Conflict of Interest Statement
All the authors declared to have no conflicts of interest.
Funding Sources
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contributions
Study concepts and study design: C.J.Z., P.B.O., S.E., J.W.T.D., and S.M.L. Data acquisition, quality control of data and algorithms, data analysis and interpretation, and manuscript preparation: C.J.Z. and P.B.O. Statistical analysis: C.J.Z. Manuscript editing and review: all the authors.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2. Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. 2013;19(34):5598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–84. [DOI] [PubMed] [Google Scholar]
- 4. Shapiro J, van Lanschot JJB, Hulshof M, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–8. [DOI] [PubMed] [Google Scholar]
- 5. Eyck BM, van Lanschot JJB, Hulshof M, van der Wilk BJ, Shapiro J, van Hagen P, et al. Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial. J Clin Oncol. 2021;39(18):1995–2004. [DOI] [PubMed] [Google Scholar]
- 6. Kamarajah SK, Newton N, Navidi M, Wahed S, Immanuel A, Hayes N, et al. Long-term outcomes of clinical and pathological-staged T3 N3 esophageal cancer. Dis Esophagus. 2020;33(8):doz109. [DOI] [PubMed] [Google Scholar]
- 7. Bailey SH, Bull DA, Harpole DH, Rentz JJ, Neumayer LA, Pappas TN, et al. Outcomes after esophagectomy: a ten-year prospective cohort. Ann Thorac Surg. 2003;75(1):217–22.; discussion 22. [DOI] [PubMed] [Google Scholar]
- 8. Verhoef C, van de Weyer R, Schaapveld M, Bastiaannet E, Plukker JT. Better survival in patients with esophageal cancer after surgical treatment in university hospitals: a plea for performance by surgical oncologists. Ann Surg Oncol. 2007;14(5):1678–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Djärv T, Lagergren J, Blazeby JM, Lagergren P. Long-term health-related quality of life following surgery for oesophageal cancer. Br J Surg. 2008;95(9):1121–6. [DOI] [PubMed] [Google Scholar]
- 10. Djärv T, Blazeby JM, Lagergren P. Predictors of postoperative quality of life after esophagectomy for cancer. J Clin Oncol. 2009;27(12):1963–8. [DOI] [PubMed] [Google Scholar]
- 11. Seely AJ, Ivanovic J, Threader J, Al-Hussaini A, Al-Shehab D, Ramsay T, et al. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg. 2010;90(3):936–42; discussion 42. [DOI] [PubMed] [Google Scholar]
- 12. Donohoe CL, McGillycuddy E, Reynolds JV. Long-term health-related quality of life for disease-free esophageal cancer patients. World J Surg. 2011;35(8):1853–60. [DOI] [PubMed] [Google Scholar]
- 13. Noordman BJ, Spaander MCW, Valkema R, Wijnhoven BPL, van Berge Henegouwen MI, Shapiro J, et al. Detection of residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer (preSANO): a prospective multicentre, diagnostic cohort study. Lancet Oncol. 2018;19(7):965–74. [DOI] [PubMed] [Google Scholar]
- 14. Puli SR, Reddy JB, Bechtold ML, Antillon D, Ibdah JA, Antillon MR. Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systematic review. World J Gastroenterol. 2008;14(10):1479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krill T, Baliss M, Roark R, Sydor M, Samuel R, Zaibaq J, et al. Accuracy of endoscopic ultrasound in esophageal cancer staging. J Thorac Dis. 2019;11(Suppl 12):S1602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liberale G, Van Laethem JL, Gay F, Goldman S, Nagy N, Coppens E, et al. The role of PET scan in the preoperative management of oesophageal cancer. Eur J Surg Oncol. 2004;30(9):942–7. 2004 2004/11/01/. [DOI] [PubMed] [Google Scholar]
- 17. Jiang C, Chen Y, Zhu Y, Xu Y. Systematic review and meta-analysis of the accuracy of 18F-FDG PET/CT for detection of regional lymph node metastasis in esophageal squamous cell carcinoma. J Thorac Dis. 2018;10(11):6066–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. International Union against Cancer (UICC) . TNM classification of malignant tumours. 7th ed.Chichester: Wiley-Blackwell; 2009. [Google Scholar]
- 19. International Union against Cancer (UICC) . TNM classification of malignant tumours. 8th ed.Chichester: Wiley-Blackwell; 2017. [Google Scholar]
- 20. Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg. 2017;6(2):119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Vliet EP, Heijenbrok-Kal MH, Hunink MG, Kuipers EJ, Siersema PD. Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer. 2008;98(3):547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee SL, Yadav P, Starekova J, Christensen L, Chandereng T, Chappell R, et al. Diagnostic performance of MRI for esophageal carcinoma: a systematic review and meta-analysis. Radiology. 2021;299(3):583–94. [DOI] [PubMed] [Google Scholar]
- 23. Ristau J, Giesel FL, Haefner MF, Staudinger F, Lindner T, Merkel A, et al. Impact of primary staging with Fibroblast Activation Protein specific Enzyme inhibitor (FAPI)-PET/CT on radio-oncologic treatment planning of patients with esophageal cancer. Mol Imaging Biol. 2020;22(6):1495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jalal SI, Ezzeddine F, Kesler K, Birdas TJ. Is esophagectomy indicated after induction therapy in patients with clinical N3 esophageal cancer? J Clin Oncol. 2018;36(15_Suppl l):e16073–73. [Google Scholar]
- 25. Scarpa M, Valente S, Alfieri R, Cagol M, Diamantis G, Ancona E, et al. Systematic review of health-related quality of life after esophagectomy for esophageal cancer. World J Gastroenterol. 2011;17(42):4660–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Noordman BJ, Verdam MGE, Lagarde SM, Shapiro J, Hulshof M, van Berge Henegouwen MI, et al. Impact of neoadjuvant chemoradiotherapy on health-related quality of life in long-term survivors of esophageal or junctional cancer: results from the randomized CROSS trial. Ann Oncol. 2018;29(2):445–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.