Abstract
Background
Cysteine desulfurase (NFS1) is closely associated with the occurrence and development of human tumors, but its relationship with the prognosis and immunity of gastric cancer (GC) patients remains unclear.
Methods
To study the relationship between NFS1 and GC, GC-related data of TCGA were downloaded and analyzed. At the same time, Tumor Immune Estimation Resource (TIMER) and Kaplan‒Meier Plotter were used for relevant online analysis. Clinical samples were collected for immunohistochemical testing to validate the results.
Results
The mRNA and protein levels of NFS1 in GC tissues were significantly higher than those in normal tissues. In terms of the operating characteristic curve (ROC), the area under the curve (AUC) was 0.793, indicating that NFS1 had a high diagnostic value for GC. Further analysis showed that NFS1 expression was highly correlated with the depth of tumor invasion, lymph node metastasis, and tumor stage. Survival analysis showed that patients with high expression of NFS1 had a poorer prognosis, and NFS1 was an independent risk factor. Enrichment analysis by GO, KEGG, and GSEA showed that NFS1 was enriched in immune-related pathways. The expression of NFS1 was significantly positively correlated with the proportion of macrophages M0 and plasma cells but negatively correlated with the proportion of B cells memory, monocytes, and mast cells resting. In addition, NFS1 expression was significantly correlated with TMB levels and responses to immunotherapy.
Conclusion
Our results suggest that NFS1 may be a potential biomarker for the diagnosis and prediction of prognosis and immunotherapy efficacy in GC.
Keywords: NFS1, gastric cancer, prognostic biomarker, immune infiltration
Introduction
Gastric cancer (GC) is one of the most common malignant tumors in the human digestive tract. According to the global cancer statistical analysis data, among all malignant tumors, the incidence of GC ranks fifth and the fatality rate ranks third. In 2020, the number of new cases of GC in the world was about 1.03 million, accounting for 5.7% of the new cases of cancer, and the death rate of GC is about 780,000, accounting for 8.2%.1 With insidious onset, GC is prone to distal metastasis and recurrence, and more than 60% of patients are in the middle and late stages when diagnosed.2,3 Clinically, treatments such as surgical resection, chemotherapy, and radiotherapy are used to combat GC. However, these methods often come with severe toxic side effects, and the 5-year survival rate of patients is as low as 25%-30%.4 Many genes have been found to participate in the occurrence and development of GC. However, more research is needed to identify new diagnostic and prognostic markers as well as therapeutic targets to guide individualized treatment for GC patients.
In recent years, immunotherapy, based on the human immune system and using immune regulation to play an anti-tumor role, has shown remarkable clinical effects and provided a new treatment model for tumor patients.5 Among different types of tumor immunotherapy, anti-programmed death receptor 1(PD-1/PD-L1) therapy is the most widely used,6 and has achieved significant efficacy in a variety of tumors. However, only 20% to 30% of patients show a clinical response to the treatments, and many develop either primary or acquired resistance to drugs,7 so it is very important and promising to find other novel immune targets for GC treatment. Iron-sulfur clusters are play a crucial role in various cellular metabolic activities such as energy metabolism, iron homeostasis, and lipid biosynthesis.8,9 Abnormal metabolism of iron-sulfur clusters can lead to metabolic diseases and the occurrence and development of various human tumors.10,11 As a rate-limiting enzyme, cysteine desulfurase (NFS1) plays a key role in the formation of iron-sulfur clusters by obtaining sulfur from cysteine. Studies have shown that NFS1 may be an oncogene, which is highly expressed in colorectal cancer, lung cancer, and breast cancer, and is involved in tumor cell proliferation and migration, and affects patient prognosis.8,12,13 However, the relationship between NFS1 and GC progression has not been elucidated by research.
This study first evaluated the relationship between NFS1 and GC by analyzing The Cancer Genome Atlas (TCGA) database. The expression of NFS1 in GC tissues and adjacent tissues was validated by immunohistochemistry, and the prognostic relationship between NFS1 and patients with GC was evaluated simultaneously. TCGA data were further used to assess the association of NFS1 with immune invasion and immunotherapy of GC. The results showed that the high expression of NFS1 in GC tissues was correlated with the clinicopathological manifestations of patients, and the survival of patients with high expression was poor. In addition, high expression of NFS1 was significantly associated with immune infiltration and immunotherapy. In conclusion, NFS1 may be a promising biomarker and target for evaluating the prognosis and treatment of GC.
Materials and Methods
Data Collection and Tissue Samples
The UCSC XENA database (https://xenabrowser.net/datapages/) was used to download RNA sequencing data in FPKM (Fragments Per Kilobase Per Million) format for 33 tumors and adjacent normal tissues, which contained RNAseq data for 375 samples of gastric cancer tissues and 32 samples of adjacent normal tissues. The FPKM was converted into TPM (Transcripts Per Million reads) format using R language, and then the TPM data was converted by log2 for subsequent analysis. In this study, tumor tissues of 206 patients who underwent radical surgery for GC in the Second People’s Hospital of Hefei City from January 2014 to May 2018 and corresponding para-cancerous tissues of 35 patients were obtained for immunohistochemical detection, and their clinical data and follow-up data were collected. Inclusion criteria were a primary tumor, pathological confirmation, and no preoperative antitumor therapy. Exclusion criteria were patients with other tumors, incomplete clinical information, and failure to follow up. Informed consent was obtained from all patients included in this study, and the study protocol was conducted in accordance with the Helsinki Declaration and approved by the Ethics Committee of Hefei Second People’s Hospital.
Immunohistochemical Staining
The tissue specimens were fixed in 4% paraformaldehyde, embedded in paraffin wax, and sectioned into 4 µm slices. The sections were then dewaxed in xylene and rehydrated through a series of graded ethanol solutions. To block endogenous peroxidase activity, the sections were treated with 3% hydrogen peroxide solution, and non-specific binding was prevented using 3% goat serum. Following this, the tissue slices were incubated overnight at 4°C with the primary NFS1 antibody (Affinity Biosciences P08498, 1:100 dilution). Sections incubated with phosphate-buffered saline served as negative controls. The sections were then incubated with a peroxidase-conjugated secondary antibody. Diaminobenzidine was used as the chromogen, and the sections were counterstained with hematoxylin. The stained sections were observed under a microscope at 200x magnification and photographed. The immunohistochemical score was determined by assessing the proportion of positive cells and the intensity of staining. The scoring for the proportion of positive cells was as follows: 0% to 25% scored 1 point, 26% to 50% scored 2 points, 51% to 75% scored 3 points, and 76% to 100% scored 4 points. The staining intensity was scored as follows: no color (0 points), light yellow (1 point), yellow-brown (2 points), and dark brown (3 points). The final immunohistochemical score was obtained by multiplying the positive cell ratio by the staining intensity. Scores of 0 to 4 were considered negative for NFS1 expression, while scores of 5 to 12 indicated positive expression.
Kaplan–Meier Plotter Database Analysis
Kaplan and Meier Plotter (http://kmplot.com/analysis/) online database was used to evaluate different genes in different clinical prognostic values of tumors.14 GC patients were divided into high expression group and low expression group according to the median expression of NFS1, and the correlation between the expression of NFS1 and overall survival (OS) and relapse-free survival (RFS), progression-free survival (PFS), and disease-specific survival (DSS) was analyzed.
TIMER Database Analysis
The TIMER online database (https://cistrome.shinyapps.io/timer/), an interactive web portal, enables comprehensive analysis of the infiltration levels of various immune cells. In this study, The TIMER was used to analyze and evaluate the relationship between NFS1 expression and tumor-infiltrating immune cells including CD4+ T cells, macrophages, CD8+ T cells, dendritic cells, neutrophils, and B cells.
Functional Enrichment Analysis of Differential Genes
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis were performed to identify potential functional categories and pathways. Among them, GO enrichment analysis includes BP (biological processes), CC (cell components), and MF (molecular function). We used GSEA analysis and set the permutation test to 1000 times to search for NFS1-related pathways and phenotypes by comparing biological functional pathways between patients with low and high NFS1 levels. The “clusterProfiler” package is adopted to perform statistical analysis and create graphs. The threshold of significant difference was set as P < 0.05 and FDR < 0.2.
NFS1 Expression and Immune-Related Analysis
The “estimate” package is used to calculate the tumor microenvironment (TME) score of the GC sample, including the stromal score and the immune score. Then, the difference in TME score was compared between the two groups according to the level of NFS1 expression. We used CIBERSORT (http://cibersort.stanford.edu/) to calculate the percentage of immune cells, aiming to explore the relationship between NFS1 and immune cell infiltration. The packages “reshape2” and “ggpubr” were adopted to visualize the analysis results, including scatter diagrams, box plots, and violin plots. Finally, the correlation of immune checkpoints with NFS1 expression was also evaluated. In addition, we explored the relationship between NFS1 expression and immunotherapy scores, which were downloaded from the TCIA website (https://tcia.at/).
Statistical Analysis
Statistical analysis and plot visualization were performed in SPSS 26.0, R software 4.3.5, and GraphPad Prism 8. To determine the best cut point values for grouping, survival, survminer, and dplyr packages are applied and Kaplan-Meier curve analysis is also carried out. Measurement data and counting data were compared using t-tests and χ2 tests or Fisher’s exact test, respectively. The prognostic value of NFS1 in patients with GC was evaluated by univariate and multivariate Cox regression. Spearman’s test was performed for immune cell infiltration analysis, while the immune checkpoint blockade (ICB) response was evaluated using the TIDE algorithm. Two-tailed P< 0.05 was considered as the threshold of statistical significance.
Results
The Expression Level of NFS1 is Up-Regulated in GC
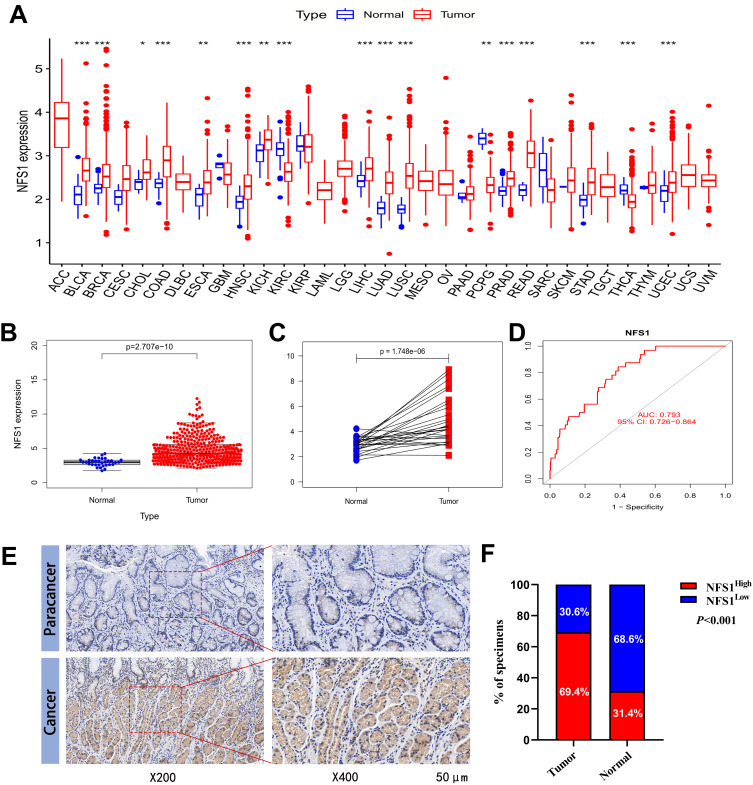
We first used the data of the TCGA database to evaluate and compare the expression level of NFS1 in different human cancer tissues and corresponding normal tissues. NFS1 mRNA is significantly upregulated in a variety of tumor tissues, including GC, compared to normal tissues (Figure 1A). For GC, unpaired and paired analyses by TCGA data showed that NFS1 mRNA expression was significantly higher in tumor samples than in adjacent normal samples (Figure 1B and C). The result of receiver operating characteristic curves (ROC) shows the area under the curve (AUC) is 0.793, indicating that NFS1 had a high predictive ability for GC (Figure 1D). Clinical tissue samples were collected for IHC staining to further verify the expression of NFS1 protein. The representative images are shown in Figure 1E, which shows that NFS1 is mainly expressed in the nucleus. The results showed that the expression of NFS1 protein in cancer tissues was significantly higher than that in adjacent tissues (143/206 vs.11/35, P<0.001, Figure 1F).
Figure 1.
Expression level of NFS1 gene in GC. (A) NFS1 expression in pan-cancer was analyzed in TCGA and GTEx. (B) NFS1 expression in tumor and normal tissues in TCGA GC data. (C) NFS1 mRNA expression in GC tissues and paired adjacent normal tissues including 27 tissues from TCGA. (D) Diagnostic value of NFS1 in GC. (E) Representative NFS1 IHC staining images in GC tissue and paracancerous tissue. (F)The statistical analysis of NFS1 expression in GC and adjacent normal tissues of the clinical cohort by the IHC scoring. P-value significant codes: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Overexpression of NFS1 is Related to Adverse Clinicopathological Parameters of GC
Clinical samples were divided into high-expression groups and low-expression groups according to immunohistochemical scores, and the correlation between NFS1 expression level and clinicopathological features of GC was investigated. Our study showed that NFS1 was highly expressed in 143 tissue samples and was lowly expressed in 63 tissue samples. The high expression level of NFS1 was not correlated with gender (P = 0.339), age (P = 0.115), tumor location (P= 0.428), tumor size (P = 0.310), M stage (P = 0.221), and differentiation (P = 0.868), but was significantly correlated with the depth of invasion (P = 0.017), lymph node metastasis (P = 0.002), and TNM stage (P = 0.001) (Table 1). It is associated with deep tumor invasion, lymph node metastasis, and high TNM stage, suggesting that high expression of NFS1 can promote the occurrence and development of GC.
Table 1.
Clinicopathologic Variables and NFS1 Level in 206 GC Patients
| Variables | No. | NFS1 Expression | χ2 | P-value | |
|---|---|---|---|---|---|
| High n=143 | Low n=63 | ||||
| Gender | 0.913 | 0.339 | |||
| Male | 156 | 111 | 45 | ||
| Female | 50 | 32 | 18 | ||
| Age (years) | 2.478 | 0.115 | |||
| <65 | 91 | 58 | 33 | ||
| ≥65 | 115 | 85 | 30 | ||
| Tumor location | 0.629 | 0.428 | |||
| Upper | 64 | 42 | 22 | ||
| Middle + lower | 142 | 101 | 41 | ||
| Tumor size (cm) | 1.033 | 0.310 | |||
| <5 | 130 | 87 | 43 | ||
| ≥5 | 76 | 56 | 20 | ||
| Depth of invasion | 5.689 | 0.017 | |||
| T1 + T2 | 36 | 19 | 17 | ||
| T3 + T4 | 170 | 124 | 46 | ||
| Lymph node metastasis | 10.018 | 0.002 | |||
| Absent | 72 | 40 | 32 | ||
| Present | 134 | 103 | 31 | ||
| M stage | 1.501 | 0.221 | |||
| M0 | 181 | 123 | 58 | ||
| M1 | 25 | 20 | 5 | ||
| Differentiation | 0.027 | 0.868 | |||
| Well + moderate | 80 | 55 | 25 | ||
| Poor + undifferentiated | 126 | 88 | 38 | ||
| TNM stage | 11.365 | 0.001 | |||
| I + II | 76 | 42 | 34 | ||
| III + IV | 130 | 101 | 29 | ||
NFS1 High Expression Correlates with Poor Prognosis in GC Patients
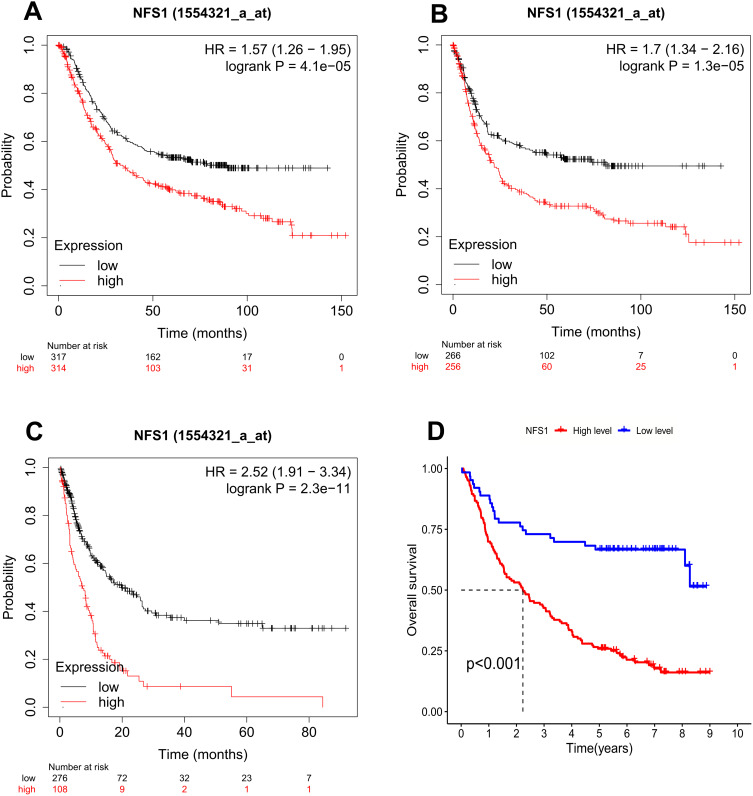
First of all, the Kaplan and Meier Plotter (http://kmplot.com/analysis/) database is used to evaluate the correlation between NFS1 and the prognosis of GC. The results showed that overall survival (OS), first progression survival (FP), and post-progression survival (PPS) in patients with high expression of NFS1 were significantly worse than those with low expression (Figure 2A–C). Consistent with the above results, our cohort Kaplan-Meier curve showed shorter OS in the NFS1 high expression group (Figure 2D). It was further confirmed that the prognosis of GC patients with high expression of NFS1 was poor. Univariate and multivariate Cox regressions were performed to analyze our cohort. Tumor size, depth of invasion, lymph node metastasis, M stage, differentiation, TNM stage, and NFS1 were risk factors for OS of GC in univariate analysis. However, only lymph node metastasis, M stage, differentiation, and NFS1 remained risk factors for OS of GC after multivariate analysis (Table 2). In summary, NFS1 is an independent risk factor for the prognosis of GC and can be used as a biomarker for prognostic prediction.
Figure 2.
Kaplan–Meier curves were estimated for the survival distributions in GC. (A-C) OS, FP, and PPS were expressed using Kaplan–Meier survival curves. (D) Kaplan– Meier plots of the high- and low-NFS1 expression GC patients from our cohort.
Abbreviations: OS, overall survival; FP, first progression; PPS, post-progression survival.
Table 2.
Univariate/Multivariate Cox Regression Analysis of NFS1 Expression, OS, and Clinicopathological Characteristics in Patients with GC
| Characteristics | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P value | Hazard Ratio (95% CI) | P value | |
| Gender | ||||
| Male vs Female | 0.988 (0.665–1.468) | 0.952 | 0.967 (0.631–1.482) | 0.878 |
| Age (years) | ||||
| ≥65 vs <65 | 1.428 (1.009–2.020) | 0.044 | 1.133 (0.786–1.633) | 0.502 |
| Tumor location | ||||
| Upper vs Middle + lower | 1.063 (0.743–1.521) | 0.739 | 0.854 (0.583–1.251) | 0.418 |
| Tumor size (cm) | ||||
| ≥5 vs <5 | 2.220 (1.584–3.112) | <0.001 | 1.221 (0.850–1.755) | 0.280 |
| Depth of invasion | ||||
| T3 + T4 vs T1 + T2 | 8.559 (3.766–19.450) | <0.001 | 2.248 (0.861–5.868) | 0.098 |
| Lymph node metastasis | ||||
| Present vs Absent | 7.891 (4.819–12.921) | <0.001 | 2.776 (1.060–7.274) | 0.038 |
| M stage | ||||
| M1 vs M0 | 7.811 (4.857–12.561) | <0.001 | 4.169 (2.529–6.872) | <0.001 |
| Differentiation | ||||
| Poor + undifferentiated vs Well + moderate | 2.035 (1.413–2.929) | <0.001 | 1.559 (1.049–2.316) | 0.028 |
| TNM stage | ||||
| III + IV vs I + II | 8.024 (4.988–12.906) | <0.001 | 1.662 (0.624–4.424) | 0.309 |
| NFS1 | ||||
| High vs Low | 3.362 (2.138–5.287) | <0.001 | 2.516 (1.571–4.031) | <0.001 |
GO, KEGG, and GSEA Analysis of DEGs
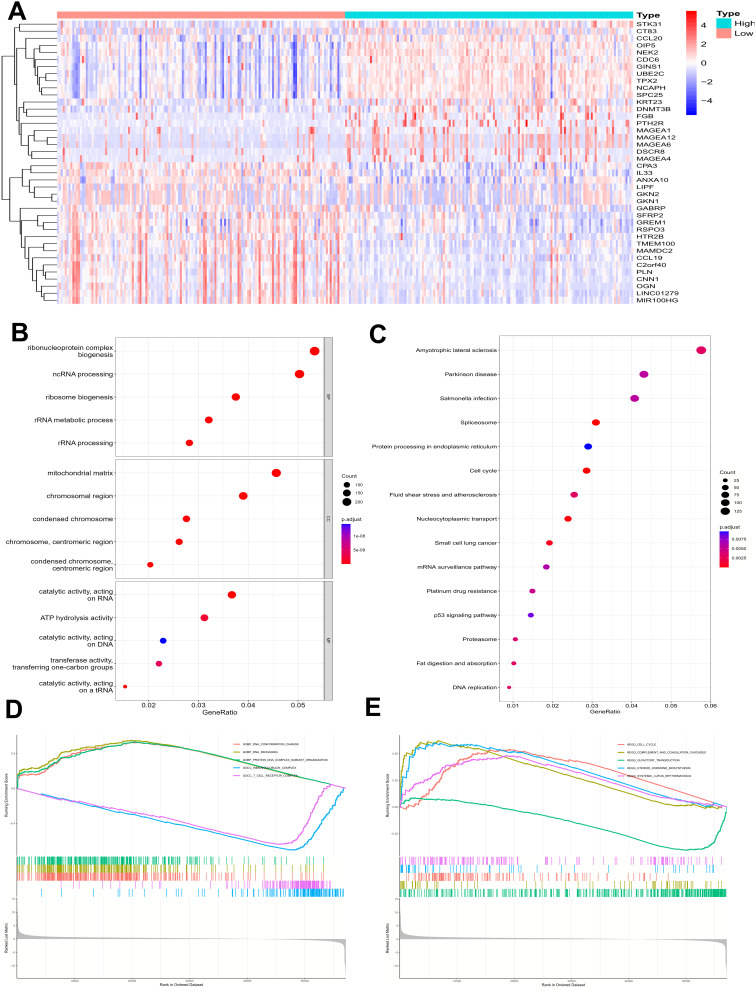
According to the median expression of NFS1, GC patients in TCGA were divided into two groups with high and low expression. The top 20 genes positively correlated with NFS1 expression and the bottom 20 genes negatively correlated with NFS1 expression were shown in a heat map (Figure 3A). GO enrichment showed that NFS1 was related to ribonucleoprotein complex biogenesis, chromosome segregation, cell−substrate junction, focal adhesion, DNA−binding transcription factor binding, ATP hydrolysis activity and cadherin binding (Figure 3B). KEGG pathway analysis suggested that coexpressed genes are mainly enriched in the following pathways, including p53 signaling pathway, small cell lung cancer, mRNA surveillance pathway, platinum drug resistance, Parkinson disease, and Cell cycle (Figure 3C). GSEA analysis revealed that these genes are mainly enriched in a variety of pathways including immune response-related signaling pathways such as T cell receptors and immunoglobulins (Figures 3D and 3E).
Figure 3.
Enrichment analysis of NFS1 expression-correlated DEGs in GC. (A) The heatmap shows the top 20 up- or down-regulated DEGs between high- and low-NFS1 groups. (B, C) GO and KEGG enrichment analysis of NFS1-associated DEGs. (D, E) GSEA analysis of differential genes NFS1.
NFS1 Relates to Tumor Microenvironment (TME) and Immune Cell Infiltration
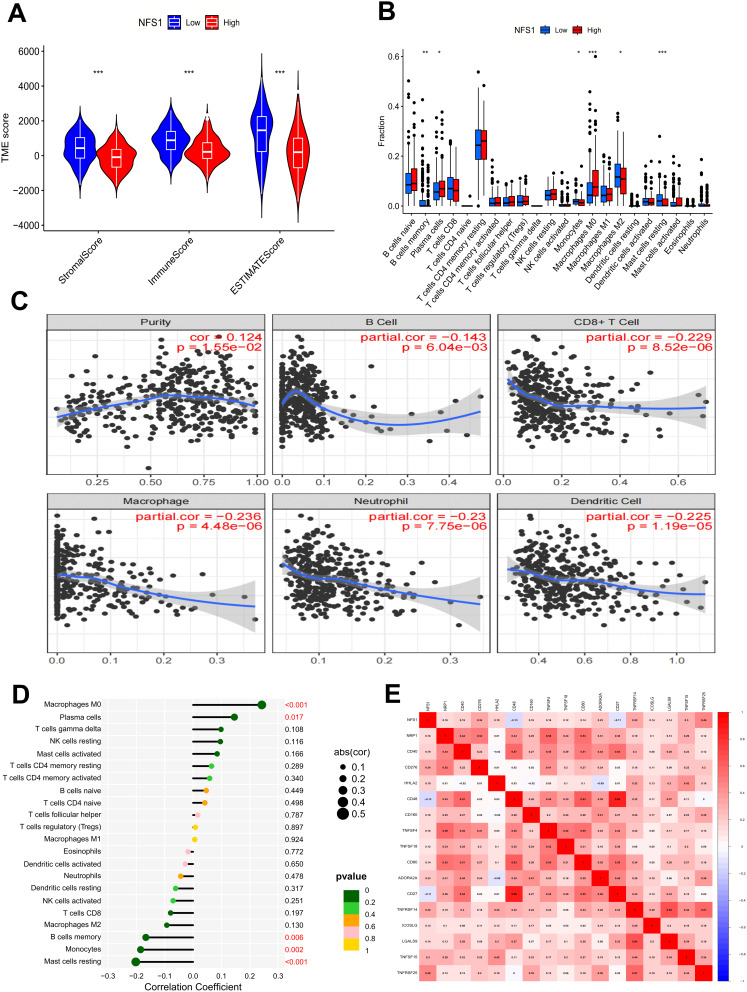
Samples with complete gene expression profiles and clinical information were obtained from the TCGA database to further investigate the role of NFS1 in the TME of GC. Results obtained by the ESTIMATE algorithm showed that the immune score and interstitial score were significantly lower in the group with high NFS1 expression than in the group with low NFS1 expression. Therefore, we speculate that NFS1 may be related to the purity of GC tumors (Figure 4A). Subsequently, we used the CIBERSORT algorithm to analyze and study the relationship between NFS1 expression and the proportion of immune cells, and the results revealed that the proportion of various immune cells was correlated with the expression of NFS1 (Figure 4B). We further investigated the relationship between immune-infiltrating cells and NFS1 expression in GC using the TIMER2.0 database, showing that NFS1 expression was positively correlated with tumor purity (cor=0.124, P=1.55e-02), but negatively correlated with the proportion of B cell (cor=−0.143, P=6.04e-03), CD8+ cell (cor=−0.229, P=8.52e-06), macrophage (cor=−0.236, P=4.48e-06), neutrophil (cor=−0.23, P=7.75e-06), and dendritic cell (cor=−0.225, P=1.19e-05) (Figure 4C). The lollipop chart presents correlation analysis results that also confirm our conclusion (Figure 4D). Overexpression of immunosuppressive checkpoint molecules can inhibit the body’s anti-tumor immune response. Therefore, we investigated the correlation between NFS1 expression and immune checkpoint, and the results are shown in Figure 4E.
Figure 4.
NFS1 relates to TME and immune cell infiltration. (A) Analysis of differences in TME scores. (B) Analysis of differences in immune cells. (C) NFS1 expression is associated with immune cell infiltration in GC using the TIMER2.0 database. (D) Correlation analysis of immune cells and (E) Immune checkpoint *p < 0.05, **p < 0.01, ***p < 0.001.
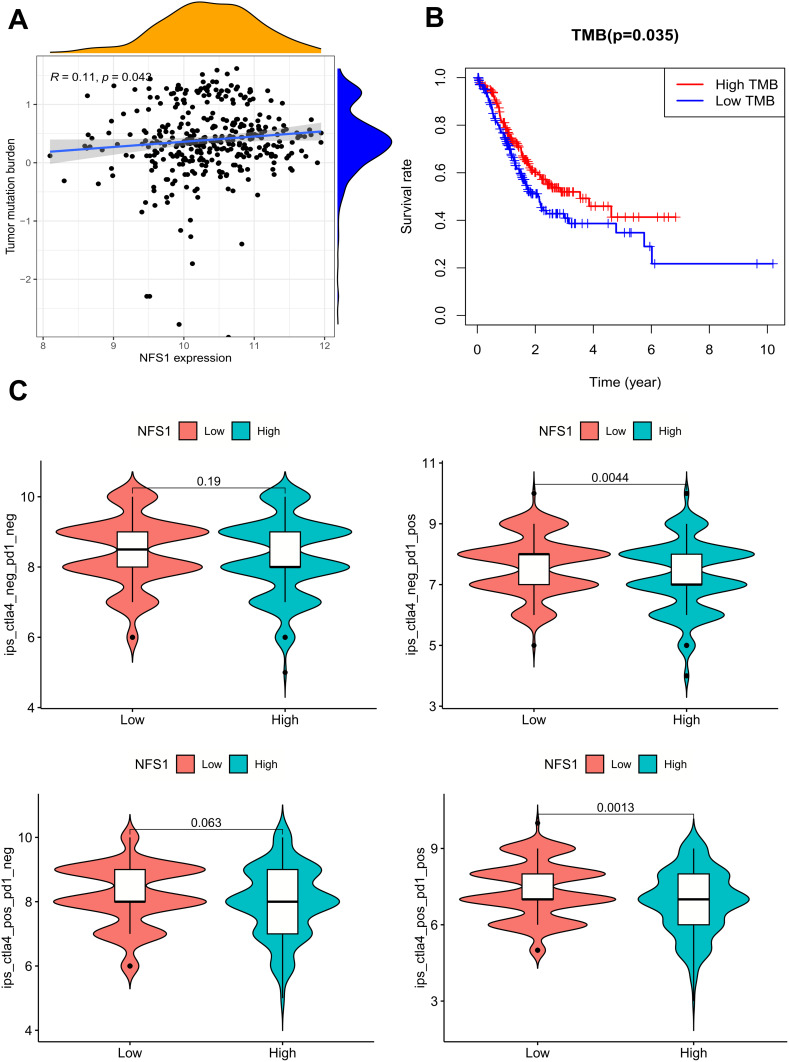
Total Mutational Burden (TMB) and Immunotherapy Analysis
TMB is an index to evaluate the frequency of gene mutation. The more mutations a tumor has, the more antigens it carries on its cell surface. Such tumor cells are more easily recognized by the immune system. Therefore, detecting the tumor mutation load of malignant tumors can predict the patient’s response to immunotherapy. In addition, its stimulation of the immune system can also indirectly improve the survival prognosis of tumor patients. Although we found that TMB levels gradually increased with increased NFS1 expression, the correlation between NFS1 and TMB was low (r= 0.11, p=0.043), (Figure 5A). The survival state was different between the high and low TMB groups, and the survival time of the high TMB group was significantly better than that of the low TMB group (Figure 5B).
Figure 5.
TMB and Immunotherapy Analysis. (A) The expression level of NFS1 was correlated with the level of TMB in GC. (B) The survival state was different between the high and low TMB groups. (C) Differential analysis of immune checkpoint inhibitor sensitivity in the high and low NFS1 expression groups.
PD-1 and CTLA-4 are the most commonly used immunotherapy protocols. NFS1 expression and immunotherapy scores were evaluated to determine the effect of NFS1 expression on the efficacy of PD-1 and CTLA-4 in GC. The results showed that in the case of PD-1 positive, patients with low NFS1 expression had higher immune scores, regardless of whether CTLA-4 was positive (Figure 5C). This suggests that PD-1-positive patients with low NFS1 expression in GC may be more effective against CTLA4 and/or PD-1/PD-L1 therapy.
Discussion
GC is a highly heterogeneous disease with significant differences in biological behavior, pathological features, and gene expression profiles. Although progress has been made in diagnosis and treatment, most patients with GC are in the advanced stage of diagnosis, with a high recurrence rate in the later stage of treatment, poor overall prognosis, and high mortality.15 Only 10% of patients diagnosed with advanced GC survive beyond 5 years.16,17 In recent years, the immunological treatment of tumors has attracted great attention due to its rapid progress in lung cancer, liver cancer, and other tumors.18–20 Although the survival of GC patients has been significantly improved by the application of immune checkpoint inhibitors and targeted drugs, this effect is only reflected in a small number of patients due to the complex TME of GC and the high heterogeneity of the tumor, and the overall efficacy is not ideal.21–24 Therefore, it is necessary and urgent to search for novel prognostic indicators and therapeutic targets to improve the prognosis of GC patients.
NFS1, a pyridoxal phospho-dependent enzyme, plays a key role in the biosynthesis of iron-sulfur (FeS) clusters, which are key cofactors for the activity of many cellular proteins.25 Abnormal expression of NFS1 has been reported to play an important role in various tumors such as lung adenocarcinomas, triple-negative breast cancer, and colorectal cancer.8,12,13 However, the correlation between NFS1 expression and GC remains unclear. In this study, we utilized data from public databases and clinical cohorts to investigate the role of NFS1 in GC. We observed that NFS1 expression was significantly elevated in cancerous tissues compared to adjacent normal tissues, which is consistent with previous findings.26,27 In addition, we evaluated the diagnostic ability of NFS1 expression in GC, and the AUC of ROC is 0.793, indicating a high diagnostic ability of NFS1 expression. Further analysis of the correlation between clinicopathological features and NFS1 expression in our cohort showed that high expression of NFS1 was correlated with the depth of tumor invasion, lymph node metastasis, and TNM stage, indicating that the high expression of NFS1 was associated with the adverse biological behavior of GC. Kaplan-Meier analysis showed that high NFS1 expression was associated with poor prognosis in patients with GC, and similar conclusions was found in studies of NFS1 and breast cancer, colorectal cancer, and hepatocellular carcinoma.12,13,27 Both univariate and multivariate analyses showed that high expression of NFS1 was significantly associated with shorter overall survival of GC. Therefore, NFS1 is an independent risk factor for the overall survival of GC and can be used as a potential biomarker for predicting the prognosis of GC.
Mechanistically, Sviderskiy et al reported that NFS1 inhibition can lead to breast cancer cell death by inducing persistent DNA damage.28 A recent study found that NFS1 promotes colorectal cancer progression and is transcriptionally regulated by MYC. NFS1 deficiency can trigger PANoptosis by increasing ROS levels.13 In lung and breast cancer, high expression of NFS1 promotes tumor progression by resisting ferroptosis.8,12 To elucidate the functional mechanism of NFS1 in GC, we performed GO enrichment analysis and KEGG signaling pathway analysis. GO analysis showed that NFS1 was associated with DNA replication, cellular proliferation, and energy metabolism. KEGG signaling pathway analysis showed that NFS1 was mainly associated with the p53 signaling pathway, platinum drug resistance, small cell lung cancer, and mRNA surveillance pathway. As a tumor suppressor gene, P53 loses its function when it is mutated, leading to abnormal cell proliferation and tumor progression.29 P53 is closely related to the occurrence, development, and prognosis of GC.30 As a standard treatment strategy for postoperative adjuvant chemotherapy and patients with advanced GC, 5-FU-based chemotherapy is ineffective for some patients due to primary or acquired drug resistance.31 Lin et al reported that inhibition of NFS1 could enhance the chemotherapy sensitivity of colorectal cancer to oxaliplatin.13 The KEGG analysis suggests that NFS1 is linked to platinum resistance, raising the question as to whether inhibition of NFS1 could reverse resistance to platinum-based chemotherapy in GC. This warrants further investigation. GSEA analysis revealed that these genes are mainly enriched in a variety of pathways including immune-related signaling pathways such as T cell receptors and immunoglobulins.
Tumor immunity plays a crucial role in the occurrence, development, recurrence, and outcome of tumors, and tumor immunotherapy has been an emerging and important strategy for anti-tumor therapy in recent years.32,33 The TME changes during tumor development, and infiltrated immune cells play an important role in tumor proliferation and metastasis as an important component of TME.24,34 Therefore, we investigated the correlation between NFS1 and immune invasion of GC. The immunoscore results of the TCGA STAD cohort showed that the stromal and immune scores of the group with high expression of NFS1 were lower, suggesting that an immunosuppressive state is present in the group with high expression of NFS1. The results of immunoinfiltration analysis showed that NFS1 expression was positively correlated with the proportion of macrophages M0 and plasma cells, but negatively correlated with the proportion of B cells memory, monocytes, and mast cells resting. Immune checkpoint, including stimulating and inhibiting checkpoint molecules, is a key regulator of the immune system, and its role in autologous tolerance and cellular immunity should not be underestimated. At present, the use of immune checkpoint inhibitors such as PD-L1/PD-1 inhibitors in tumor immunotherapy has made remarkable achievements, which have improved the prognosis of tumor patients.35 However, some patients, including some patients with GC, do not respond well to PD1/PDL1 checkpoint-blocking therapy because PD-1 plays a key role in tumor antigen tolerance.36,37 Therefore, it is essential to improve the response of tumor cells to immune checkpoint inhibitors. In the current study, NFS1 was found to be associated with the expression of multiple immune checkpoint molecules. Notably, our findings also suggest that anti-PD1 or anti-CTLA4 therapy is more favorable for treatment with low NFS1 expression, suggesting that high expression of NFS1 may inhibit the efficacy of immunosuppressive therapy. In brief, these results suggest that NFS1 affects the immune microenvironment and immunotherapy in patients with GC, and the underlying mechanisms need to be further investigated.
In this study, we concluded that NFS1 expression is closely related to the prognosis of GC patients and tumor immune invasion, but some limitations should be pointed out. First, the relationship between NFS1 and GC progression has not been verified in vivo/in vitro, and the reliability of our results needs to be further improved. Second, although we have concluded that NFS1 expression affects immune invasion and immunotherapy in GC, its underlying regulatory mechanisms have not been clarified. Finally, this study did not further explore the potential molecular mechanism of NFS1 expression affecting the occurrence and development of GC, which is also the study we are currently carrying out as a continuation of the present study.
Conclusion
In summary, the results of this study signal that NFS1 is significantly up-regulated in GC and is an independent predictor of poor prognosis, and can be used as a tumor marker to evaluate prognosis. In addition, NFS1 expression in GC is associated with immune cell immersion and immunotherapy. Therefore, NFS1 can be used as a potential diagnostic and prognostic biomarker and therapeutic target for GC.
Acknowledgments
The authors would like to thank the Kanehisa Laboratories for the KEGG copyright permission, and gratefully acknowledge the TCGA databases, which made the data available.
Funding Statement
This study was supported by the Anhui Provincial Natural Science Foundation (2008085MH294).
Data Sharing Statement
The data that support the findings of this study are available when requested from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
Tissue samples were collected from patients who provided informed consent according to the protocol approved by the Ethics Review Committee of Hefei Second People’s Hospital. This study complies with the Declaration of Helsinki. All experiments were conducted following applicable guidelines and regulations.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors have no conflicts of interest to declare in this work.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Katai H, Ishikawa T, Akazawa K, et al. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese gastric cancer association (2001–2007). Gastric Cancer. 2018;21(1):144–154. doi: 10.1007/s10120-017-0716-7 [DOI] [PubMed] [Google Scholar]
- 3.Sawaki K, Kanda M, Kodera Y. Review of recent efforts to discover biomarkers for early detection, monitoring, prognosis, and prediction of treatment responses of patients with gastric cancer. Expert Rev Gastroenterol Hepatol. 2018;12(7):657–670. doi: 10.1080/17474124.2018.1489233 [DOI] [PubMed] [Google Scholar]
- 4.Bonotto M, Garattini SK, Basile D, et al. Immunotherapy for gastric cancers: emerging role and future perspectives. Expert Rev Clin Pharmacol. 2017;10(6):609–619. doi: 10.1080/17512433.2017.1313113 [DOI] [PubMed] [Google Scholar]
- 5.Kruger S, Ilmer M, Kobold S, et al. Advances in cancer immunotherapy 2019 - latest trends. J Exp Clin Cancer Res. 2019;38(1):268. doi: 10.1186/s13046-019-1266-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu X, Gu Z, Chen Y, et al. Application of PD-1 blockade in cancer immunotherapy. Comput Struct Biotechnol J. 2019;17:661–674. doi: 10.1016/j.csbj.2019.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrews LP, Yano H, Vignali DAA. Inhibitory receptors and ligands beyond PD-1, PD-L1, and CTLA-4: breakthroughs or backups. Nat Immunol. 2019;20(11):1425–1434. doi: 10.1038/s41590-019-0512-0 [DOI] [PubMed] [Google Scholar]
- 8.Alvarez SW, Sviderskiy VO, Terzi EM, et al. NFS1 undergoes positive selection in lung tumors and protects cells from ferroptosis [published correction appears in nature. Nature. 2017;551(7682):639–643. doi: 10.1038/nature24637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stehling O, Wilbrecht C, Lill R. Mitochondrial iron-sulfur protein biogenesis and human disease. Biochimie. 2014;100:61–77. doi: 10.1016/j.biochi.2014.01.010 [DOI] [PubMed] [Google Scholar]
- 10.Rouault TA. Biogenesis of iron-sulfur clusters in mammalian cells: new insights and relevance to human disease. Dis Model Mech. 2012;5(2):155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ward NP, Kang YP, Falzone A, Boyle TA, DeNicola GM. Nicotinamide nucleotide transhydrogenase regulates mitochondrial metabolism in NSCLC through the maintenance of Fe-S protein function. J Exp Med. 2020;217(6):e20191689. doi: 10.1084/jem.20191689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chafe SC, Vizeacoumar FS, Venkateswaran G, et al. Genome-wide synthetic lethal screen unveils novel CAIX-NFS1/xCT axis as a targetable vulnerability in hypoxic solid tumors. Sci Adv. 2021;7(35):eabj0364. doi: 10.1126/sciadv.abj0364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin JF, Hu PS, Wang YY, et al. Phosphorylated NFS1 weakens oxaliplatin-based chemosensitivity of colorectal cancer by preventing PANoptosis. Signal Transduct Target Ther. 2022;7(1):54. doi: 10.1038/s41392-022-00889-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lánczky A, Győrffy B. Web-based survival analysis tool tailored for medical research (KMplot): development and implementation. J Med Internet Res. 2021;23(7):e27633. doi: 10.2196/27633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nie K, Shi L, Wen Y, et al. Identification of hub genes correlated with the pathogenesis and prognosis of gastric cancer via bioinformatics methods. Minerva Med. 2020;111(3):213–225. doi: 10.23736/S0026-4806.19.06166-4 [DOI] [PubMed] [Google Scholar]
- 16.Li GZ, Doherty GM, Wang J. Surgical management of gastric cancer: a review. JAMA Surg. 2022;157(5):446–454. doi: 10.1001/jamasurg.2022.0182 [DOI] [PubMed] [Google Scholar]
- 17.Lei ZN, Teng QX, Tian Q, et al. Signaling pathways and therapeutic interventions in gastric cancer. Signal Transduct Target Ther. 2022;7(1):358. doi: 10.1038/s41392-022-01190-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Budhu A, Pehrsson EC, He A, et al. Tumor biology and immune infiltration define primary liver cancer subsets linked to overall survival after immunotherapy. Cell Rep Med. 2023;4(6):101052. doi: 10.1016/j.xcrm.2023.101052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Servetto A, Di Maio M, Salomone F, et al. Analysis of Phase III clinical trials in metastatic NSCLC to assess the correlation between QoL results and survival outcomes. BMC Med. 2023;21(1):234. doi: 10.1186/s12916-023-02953-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Wang X, Wu Y, et al. A novel microenvironment regulated system CAR-T (MRS.CAR-T) for immunotherapeutic treatment of esophageal squamous carcinoma. Cancer Lett. 2023;568:216303. doi: 10.1016/j.canlet.2023.216303 [DOI] [PubMed] [Google Scholar]
- 21.Fridman WH, Zitvogel L, Sautès-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14(12):717–734. doi: 10.1038/nrclinonc.2017.101 [DOI] [PubMed] [Google Scholar]
- 22.Zeng D, Wu J, Luo H, et al. Tumor microenvironment evaluation promotes precise checkpoint immunotherapy of advanced gastric cancer. J Immunother Cancer. 2021;9(8):e002467. doi: 10.1136/jitc-2021-002467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei J, Lu X, Liu Q, et al. Neoadjuvant sintilimab in combination with concurrent chemoradiotherapy for locally advanced gastric or gastroesophageal junction adenocarcinoma: a single-arm Phase 2 trial. Nat Commun. 2023;14(1):4904. doi: 10.1038/s41467-023-40480-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang Y, Zhou K, Sun Z, et al. Non-invasive tumor microenvironment evaluation and treatment response prediction in gastric cancer using deep learning radionics. Cell Rep Med. 2023;4(8):101146. doi: 10.1016/j.xcrm.2023.101146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uzarska MA, Grochowina I, Soldek J, et al. During FeS cluster biogenesis, ferredoxin and frataxin use overlapping binding sites on yeast cysteine desulfurase Nfs1. J Biol Chem. 2022;298(2):101570. doi: 10.1016/j.jbc.2022.101570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yun D, Wang X, Wang W, et al. A novel prognostic signature based on glioma essential ferroptosis-related genes predicts clinical outcomes and indicates treatment in glioma. Front Oncol. 2022;12:897702. doi: 10.3389/fonc.2022.897702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pang Y, Tan G, Yang X, et al. Iron-sulphur cluster biogenesis factor LYRM4 is a novel prognostic biomarker associated with immune infiltrates in hepatocellular carcinoma. Cancer Cell Int. 2021;21(1):463. doi: 10.1186/s12935-021-02131-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sviderskiy VO, Blumenberg L, Gorodetsky E, et al. Hyperactive CDK2 activity in basal-like breast cancer imposes a genome integrity liability that can be exploited by targeting DNA polymerase ε. Mol Cell. 2020;80(4):682–698.e7. doi: 10.1016/j.molcel.2020.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanapathipillai M. Treating p53 mutant aggregation-associated cancer. Cancers. 2018;10(6):154. doi: 10.3390/cancers10060154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X, Wang M, Wang Y, Cheng X, Jiang Y, Xiao H. Clinicopathologic significance of Her-2 and P53 expressions in gastric cancer. Asian J Surg. 2023;46(1):526–531. doi: 10.1016/j.asjsur.2022.06.039 [DOI] [PubMed] [Google Scholar]
- 31.Yuan J, Khan SU, Yan J, Lu J, Yang C, Tong Q. Baicalin enhances the efficacy of 5-Fluorouracil in gastric cancer by promoting ROS-mediated ferroptosis. Biomed Pharmacother. 2023;164:114986. doi: 10.1016/j.biopha.2023.114986 [DOI] [PubMed] [Google Scholar]
- 32.Ravi R, Noonan KA, Pham V, et al. Bifunctional immune checkpoint-targeted antibody-ligand traps that simultaneously disable TGFβ enhance the efficacy of cancer immunotherapy. Nat Commun. 2018;9(1):741. doi: 10.1038/s41467-017-02696-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang P, Gu S, Pan D, et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med. 2018;24(10):1550–1558. doi: 10.1038/s41591-018-0136-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao G, Liao W, Shu P, et al. Targeting sphingosine 1-phosphate receptor 3 inhibits T-cell exhaustion and regulates the recruitment of proinflammatory macrophages to improve the antitumor efficacy of CAR-T cells against solid tumors. J Immunother Cancer. 2023;11(8):e006343. doi: 10.1136/jitc-2022-006343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang Y, Zhang Z, Wang W, et al. Biology-guided deep learning predicts prognosis and cancer immunotherapy response. Nat Commun. 2023;14(1):5135. doi: 10.1038/s41467-023-40890-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomized, double-blind, placebo-controlled, Phase 3 trial. Lancet. 2017;390(10111):2461–2471. doi: 10.1016/S0140-6736(17)31827-5 [DOI] [PubMed] [Google Scholar]
- 37.Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24(9):1449–1458. doi: 10.1038/s41591-018-0101-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available when requested from the corresponding author upon reasonable request.