Abstract
Optimal non-invasive biomarkers for metabolic dysfunction-associated steatotic liver disease (MASLD) remain elusive, especially in the detection of early stages. This study tested in an asymptomatic cohort of 171 men (49.2 ± 8.6 years) and 131 women (51.8 ± 8.5 years) whether waist circumference (WC) and circulating levels of insulin-like growth factor-binding protein 2 (IGFBP-2) could identify individuals with liver fat >5% as assessed by magnetic resonance spectroscopy. Participants with high WC (> 85 or 90 cm for women and men, respectively) and low IGFBP-2 (< 260 or 230 ng/mL for women and men, respectively) were characterized by a higher risk of having MASLD (46.3%, p < 0.0001). Among the 68 individuals with MASLD, 73.5% fell into the subgroup with high WC and low IGFBP-2 concentrations (p < 0.0001). When combined, these markers reached a sensitivity of 73.5% and specificity of 75.2% for MASLD. Thus, WC and plasma IGFBP-2 levels might be useful as a novel, simple, and non-invasive index to support existing tools in the identification of individuals at risk of early-stage MASLD.
Keywords: humans, insulin-like growth factor-binding protein 2, waist circumference, hepatic fat, metabolic dysfunction-associated steatotic liver disease, biomarker, plasma, non-invasive
Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) is the most frequent liver disease with a global prevalence estimated at 30%.1 MASLD is defined when the hepatic fat fraction (HFF) is higher than 5% of liver weight,1 and can progress to metabolic dysfunction-associated steatohepatitis (MASH), liver cirrhosis and hepatocellular carcinoma.1 It has been suggested that 4 to 22% of hepatocellular carcinoma cases initiate from early MASLD.2 Although magnetic resonance imaging (MRI) and proton magnetic resonance spectroscopy (1H-MRS) are the most precise and sensitive non-invasive techniques to clinically evaluate MASLD, abdominal ultrasonography remains the primary test to diagnose fatty liver, albeit with lower sensitivity.3 In many cases, confirmation occurs through histopathological analyses after invasive biopsies.3 Because these methods are expensive and labor-intensive, there is a need to develop additional simple and inexpensive screening tools to improve the identification of individuals at risk of MASLD, especially in the early stages of pathology when sensitivity is crucial.3,4
MASLD is closely associated with an altered cardiometabolic risk profile, notably with visceral obesity, metabolic syndrome, insulin resistance and type 2 diabetes.4,5 Consistently, in addition to body mass index (BMI), previous marker panels of MASLD have been directed at insulin resistance,6 including homeostatic model assessment of insulin resistance (HOMA-IR), a simple index integrating fasting plasma glucose and insulin levels, and liver fat score (LFS), which is calculated based on the presence of the metabolic syndrome (agglomerate of waist circumference, triglyceridemia, HDL-cholesterol, blood pressure and fasting glycemia), type 2 diabetes, fasting levels of aspartate and alanine aminotransferases (AST/ALT) and insulinemia.7 In addition, a combination of ALT and gamma-glutamyl transpeptidase (GGT) has been shown to predict the changes in MASLD stage in a pediatric cohort (8–17 years old) over the course of one year.8 However, the sensitivity and specificity of these markers in the general adult population with low or early HFF is not optimal.6
Insulin-like growth factor-binding protein 2 (IGFBP-2) is a circulating factor mainly produced by the liver and involved in the modulation of energy metabolism.9 Plasma IGFBP-2 levels are higher in women and lowered in individuals with obesity independently of sex.10 Recently, plasma IGFBP-2 levels have been inversely correlated with steatosis grade and liver fat in mice and humans.11–14 Our group recently further demonstrated that plasma IGFBP-2 concentrations are significantly and negatively correlated with HFF in an age-independent manner in non-obese men and women.15 Based on these observations, we tested the hypothesis that waist circumference (as a marker of abdominal adiposity) and circulating IGFBP-2 levels may represent novel and minimally invasive tools to discriminate for MASLD in a sample of asymptomatic adults.
Methods
The present study reports additional IGFBP-2 analyses conducted on a previously described cohort of 312 individuals, including details about recruitment of participants, informed written consent, approval by the review board of the Institut universitaire de cardiologie et de pneumologie de Québec (IUCPQ) (#20769), and deposition of plasma samples in a biobank for future analyses.16 The present specific study also received ethics approval from the IUCPQ review committee (#21433). This study complies with the Declaration of Helsinki.
Briefly, men and women, between 35 and 66 years old, were enrolled from the Québec metropolitan area for voluntary participation in the visceral obesity/ectopic fat and non-invasive markers of atherosclerosis: a cardiometabolic-cardiovascular imaging study (CMCV imaging study). Data reported herein were obtained at study baseline. Data for waist circumference and plasma IGFBP-2 levels were missing for 6 and 4 individuals, respectively, resulting in a cohort of 302 men and women.
Inclusion criteria were BMI < 40 kg/m2 and being nonsmoker for at least 12 months before enrollment. Participants presenting symptoms or being treated for diabetes, hypertension, dyslipidemia and cardiovascular disease were excluded. Were also excluded individuals undergoing hormonal or corticosteroid therapy, presenting a cancer not in remission or with a contraindication for MRI, having received medication in the past 3 months, as well as women in post-menopause for less than 12 months before enrollment. Detailed dietary questionnaires were obtained from participants, notably to obtain information about alcohol and nutrient intake.16
Waist circumference was measured according to standardized procedures.17 Body composition was quantified by dual-energy X-ray absorptiometry on a Lunar Prodigy system (GE, Healthcare, WI, USA). Visceral adipose tissue volume was assessed by MRI as described.16 HFF was evaluated using MRS by breath-hold single‐shot stimulated echo acquisition mode (STEAM) sequence as described.16 HFF levels > 5% indicated early MASLD.4 Blood samples were collected from the antecubital vein after a 12-h overnight fast. Plasma glucose, insulin and lipid profiles were measured as described.16 HOMA-IR18 and LFS7 were calculated as described. Plasma IGFBP-2 levels were quantified by ELISA (kit #22-BP2HU-E01, Alpco, Salem, NH, USA) according to the manufacturer’s instructions. The detection limit was 0.2 ng/mL. The inter- and intra-assay coefficient of variability was < 10%.
Differences between men and women were assessed by unpaired t-test. Differences between quantiles were assessed by Bonferonni’s multiple comparison test. Comparison of MASLD prevalence among subgroups was performed by χ2 analysis. Differences between groups were considered statistically different at p < 0.05. Statistical analyses were performed using GraphPad Prism software v8.1, except for sensitivity/specificity analyses which were performed using SAS v9.4 (SAS Institute Inc., Cary, NC).
Results
Participants were 50.3 ± 8.7 years old and had a mean BMI of 25.9 ± 3.7 kg/m2, with overall normal glucose and lipid profiles. Compared to women, men had a significantly higher BMI, fasting glucose, insulin and HOMA-IR as well as higher triglyceride and LDL-cholesterol levels, and lower HDL-cholesterol concentrations (p < 0.05) (Supplementary Table 1). Plasma IGFBP-2 levels were significantly higher in women (279.8 ± 117.0 ng/mL) than in men (236.1 ± 103.4 ng/mL, p = 0.0007). No difference in IGFBP-2 levels was found (p = 0.29) between the 61 pre-menopausal (268.1 ± 109.6 ng/mL; age 44.0 ± 5.0 years old) and 70 post-menopausal women (290.1 ± 123.1 ng/mL; age 58.5 ± 3.8 years old). Among the participants, MRS imaging revealed that 68 participants were characterized by MASLD (HFF > 5%). Mean HFF was higher in men (5.3 ± 6.3%) than in women (2.9 ± 4.2%, p = 0.0002, Supplementary Table 1). HFF was associated with daily alcohol consumption in men (r = 0.19, p = 0.02) but not in women (r = 0.08, p = 0.38). However, alcohol consumption was not correlated with plasma IGFBP-2 concentrations in either men (r = 0.04, p = 0.62) or women (r = −0.14, p = 0.14). In addition, plasma IGFBP-2 concentrations were negatively correlated with circulating ALT in men only (r = −0.25; p = 0.0008), whereas no association was found with AST or GGT levels.
Using the entire cohort, individuals divided into octiles based on their progressively increasing waist circumference values showed incrementally higher levels of visceral adipose tissue (Supplementary Figure 1A). Using a similar approach, octiles of increasing plasma IGFBP-2 levels showed progressively decreasing HOMA-IR (Supplementary Figure 1B), an established marker of insulin resistance.19 Based on these findings, analyses of sensitivity and specificity of plasma IGFBP-2 levels to detect HFF > 5% were performed as function of waist circumference values. An intercept of at least 70% was targeted in relation to the established cut-offs of 90 and 85 cm for men and women, respectively.20–22 This criterion corresponded to a value of 230 ng/mL in men (Supplementary Figure 2A) and 260 ng/mL in women (Supplementary Figure 2B), consistent with the observation that women had higher IGFBP-2 levels than men on average.
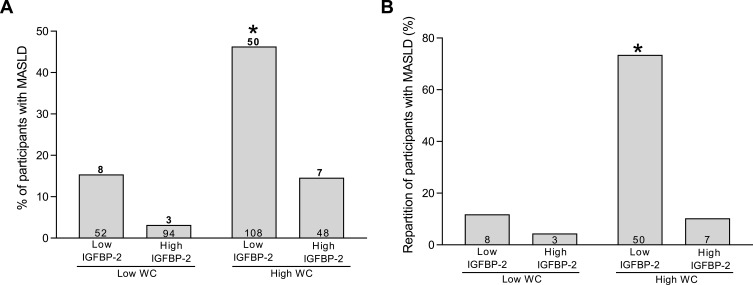
Using these values as cut-offs to classify individuals as having either high or low plasma IGFBP-2 levels, the percentage of participants with MASLD was significantly higher (46.3%, p < 0.0001) in the group characterized by high waist circumference and low IGFBP-2 levels than any other groups (Figure 1A). Among the 68 individuals with MASLD, 50 (73.5%) were characterized by a high waist circumference and low IGFBP-2 levels (Figure 1B, p < 0.0001) compared with other groups. These results were consistent with findings obtained when the cohort was rather divided into octiles (Supplementary Figure 3), indicating that participants with low IGFBP-2 levels and high waist circumference had a higher probability of presenting MASLD.
Figure 1.
(A) Percentage of individuals with metabolic dysfunction-associated steatotic liver disease (MASLD) as defined by hepatic fat fraction > 5%, among the 302 participants classified on basis of waist circumference (WC) and circulating insulin-like growth factor-binding protein 2 (IGFBP-2) levels with a cut-off of 90 (men) and 85 cm (women) and 230 (men) and 260 ng/mL (women), respectively. Above bars are the number of individuals with MASLD. Within bars are the number of participants within the group. *Different from other groups, χ2 = 54.8, p < 0.0001. (B) Repartition of the 68 participants affected by MASLD classified on the basis of WC and circulating IGFBP-2 levels using the same cut-offs as panel A. Within bars are the number of individuals within the group. *Different from other groups, χ2 = 68.0, p < 0.0001.
Finally, using the same cut-offs, the combination of plasma IGFBP-2 and waist circumference to detect HFF > 5% in this cohort of asymptomatic individuals was associated with a sensitivity of 73% (Table 1). This was markedly better than results obtained with HOMA-IR (using the cut-off of 2.03) and LFS (using the cut-off of −0.647), the latter being misleading for more than half of the participants with MASLD (Supplementary Figure 4), and slightly above a combination of GGT and ALT (Supplementary Table 2), albeit both being more narrowly distributed compared to IGFBP-2 (Supplementary Figure 5). In addition, combining LFS with either waist circumference or IGFBP-2 did not improve sensitivity (Table 1). The use of LFS (alone or in combination) and HOMA-IR was associated with a much higher specificity to discriminate for individuals with HFF > 5% compared with waist circumference or IGFBP-2 used in isolation (Table 1). However, combining waist circumference and IGFBP-2 yielded a specificity of 75.2%, resulting in 16.6% of true positive cases (compared to 9.3% and 9.6% for LFS and HOMA-IR, respectively) and 6.0% false negative cases (compared to 13.2% and 12.9% for LFS and HOMA-IR, respectively, Table 1). In contrast, combining waist circumference and HOMA-IR resulted in a lower sensitivity/specificity ratio, with 8.9% of true positive and 13.6% false negative cases (Table 1).
Table 1.
Comparison of Sensitivity and Specificity Between the Indicated Putative Markers (Alone or in Combination) to Detect Metabolic Dysfunction-Associated Steatotic Liver Disease (Hepatic Fat Fraction > 5%) Within the Cohort
| WC + IGFBP-2 |
WC | IGFBP-2 | LFS | WC +LFS | IGFBP-2 +LFS | WC + IGFBP-2 +LFS | HOMA-IR | WC + HOMA-IR | |
|---|---|---|---|---|---|---|---|---|---|
| Sensitivity (%) | 73.5 | 83.8 | 85.3 | 41.3 | 39.7 | 38.2 | 36.8 | 42.7 | 39.7 |
| Specificity (%) | 75.2 | 57.7 | 56.4 | 94.2 | 94.0 | 95.7 | 95.7 | 91.6 | 91.5 |
| True positive (%) | 16.6 | 18.9 | 19.2 | 9.3 | 8.9 | 8.6 | 8.3 | 9.6 | 8.9 |
| False positive (%) | 19.2 | 32.8 | 33.8 | 4.6 | 4.6 | 3.3 | 3.3 | 6.6 | 6.6 |
| False negative (%) | 6.0 | 3.6 | 3.3 | 13.2 | 13.6 | 13.9 | 14.2 | 12.9 | 13.6 |
| True negative (%) (%) | 58.2 | 44.7 | 43.7 | 72.9 | 72.9 | 74.2 | 74.2 | 70.9 | 70.9 |
Notes: HOMA-IR: homeostatic model assessment of insulin resistance; IGFBP-2: insulin-like growth factor-binding protein-2; LFS: liver fat score; WC: waist circumference. Sensitivity was calculated as true positive cases/(true positive + false negative cases). Specificity was calculated as true negative cases/(true negative + false positive cases). In each column, the sum of true positive and false negative cases is 22.5%, which is 68 individuals with MASLD over the 302 participants in the entire cohort. Cut-offs: Plasma IGFBP-2: 230 ng/mL (men), 260 ng/mL (women) (based on Supplementary Figure 2). WC: 90 cm (men), 85 cm (women) (based on references20–22). LFS: −0.64 (based on reference18). HOMA-IR: 2.0 (based on reference7).
Discussion
The gold-standard method for the diagnosis of MASLD with best sensitivity is 1H-MRS.4 However, this imaging technology is expensive and may be impractical to implement in large-scale clinical routine. Furthermore, several non-invasive biomarkers described to identify people at risk of developing MASLD, such as LFS, use complicated algorithms comprising many analyses and measurements, which also limit their use in clinical practice. In addition, their sensitivity and specificity in the general population are not optimal.6 Given the high prevalence of MASLD and the diagnostic challenges faced by clinicians to screen the general population, additional non- or minimally invasive tools are needed.4,23
Because of the significant number of individuals with obesity who do not have MASLD,5,24 waist circumference alone cannot be an optimal maker of fatty liver as this anthropometric variable cannot distinguish between subcutaneous and visceral abdominal adiposity. Insulin resistance is another important component of MASLD pathogenesis independent of visceral adiposity.5 Based on the robust associations of IGFBP-2 and insulin sensitivity in mice and humans,9 and the reported correlations between IGFBP-2 and MASLD,11–14 plasma IGFBP-2 levels were added to waist circumference in our assessment model. Our model was based on a similar approach that had revealed that hypertriglyceridemic waist was an inexpensive screening tool to identify individuals with visceral obesity at increased cardiovascular risk.22 Findings of the present study support the concept that the combination of waist circumference and circulating IGFBP-2 levels could be used as an additional simple index to the existing screening tools for MASLD. In this cohort of asymptomatic individuals, a waist circumference above 90 or 85 cm and IGFBP-2 concentration below 230 or 260 ng/mL (for men and women, respectively) allowed to discriminate participants affected by hepatic steatosis (HFF > 5%) with much higher sensitivity than LFS. Individuals identified in this subgroup represented 73.5% of the total participants affected by MASLD, with a specificity of 75.2%. In that regard, combining waist circumference with IGFBP-2 also provided better sensitivity/specificity than with HOMA-IR, suggesting an added informative value of IGFBP-2 beyond its mere association with insulin resistance. The combination of waist circumference with plasma IGFBP-2 to assess specificity was also slightly superior to that of GGT and ALT combined, which could be attributed to a larger IGFBP-2 data distribution within this asymptomatic cohort. Thus, although plasma IGFBP-2 is quantified by ELISA and not part of routine clinical biochemistry (contrary to insulin, glucose and liver enzymes), its combination with waist circumference appears to detect the majority of participants with MASLD with promising efficacy and better large-scale screening capability compared to imaging.
There are several limitations to the present study. First, the combination of waist circumference and plasma IGFBP-2 levels to evaluate HFF needs to be more thoroughly compared to other liver fat indices,6 and not only in transversal but also longitudinal designs, and integrated with established MASLD risk factors. In addition, it remains to be experimentally tested whether this combination can distinguish between grades of steatosis or be used to evaluate subsequent treatment response. As it is recognized that MRI might miss stages of fibrosis, the present study was not designed to address this issue. Furthermore, opposite to liver fat, IGFBP-2 levels have been positively correlated with liver fibrosis in several reports,25,26 which might indicate that it is better suited to highlight early deposition of hepatic fat before the onset and progression of fibrosis. Finally, although IGFBP-2 levels were not correlated with alcohol consumption in the present study, a report has suggested that a high intake of alcohol is associated with lower IGFBP-2 concentrations in men when adjusted for smoking habits and BMI,27 a finding that will require further attention.
The present study revealed an IGFBP-2 cut-off of 230 ng/mL for men, which was higher than the concentration of 220 ng/mL suggested as a cut-off value linked with the NCEP ATP III clinical criteria for the diagnosis of metabolic syndrome.10 However, the present cohort was metabolically healthier than the group used to determine the value of 220 ng/mL.10 Thus, it is possible that the suggested combination has better efficacy in specific settings associated with early stages of the pathology without other clinical symptoms of metabolic diseases, which was the case in the present cohort and that cut-off values for IGFBP-2 need to be adjusted depending on MASLD severity or presence of other conditions affecting liver fat, such as muscle wasting.28 Integration of the present observations with ongoing international initiatives such as the FNIH NIMBLE project3 would facilitate comparisons of screening potential and reproducibility value in other independent and larger cohorts, including in individuals with more advanced MASLD.
In summary, our findings suggest that the simultaneous interpretation of waist circumference and plasma IGFBP-2 levels may be an additional and minimally invasive tool to the current screening arsenal for the identification of individuals with MASLD. Use of these markers in clinical practice may prove to represent a simple approach for initial screening of MASLD before diagnostic validation with effective but patient-oriented and labor-expensive abdominal ultrasonography scans or other imaging techniques. More work will be required to test the relevance and validity of the approach in several independent cohorts and ability to distinguish between steatosis grades, ultimately improving risk assessment.
Acknowledgments
We thank Serge Simard for his kind help in the statistical analysis of sensitivity/specificity curves.
Funding Statement
The CMCV study was supported by the Canadian Institutes of Health Research (CIHR) (MOP-114820) and is a component of a research program supported by a Foundation Scheme Grant (FDN-167278) from the CIHR awarded to J.-P. Després. This work was supported by operating grants to F.P. from the CIHR (PJT-148550) as well as from the IUCPQ Foundation. D. J. Chartrand is a recipient of the Frederick Banting & Charles Best Canada Graduate Scholarships-Doctoral Award from the CIHR and of a doctoral training award from the FRQS. J.-P. Després is the co-holder of the Chaire de recherche en santé durable funded by the Fonds de recherche du Québec – Santé (FRQS). P. Pibarot holds the Canada Research Chair in Valvular Heart Disease.
News and Noteworthy
Besides resource-consuming imaging techniques or invasive liver biopsies, no optimal biomarker has yet been established to screen for individuals with fatty liver. In asymptomatic individuals, we found that those with high waist circumference and low blood insulin-like growth factor-binding protein 2 (IGFBP-2) levels have an elevated probability of having a fatty liver. Combined interpretation of waist circumference and of IGFBP-2 levels could therefore be relevant as an additional tool to screen for MASLD in clinical practice.
Disclosure
Dr Philippe Pibarot reports grants from Novartis, during the conduct of the study. Dr Jean-Pierre Després reports personal fees from Inversago, outside the submitted work. Dr Frédéric Picard reports grants from the Canadian Institutes for Health Research and the IUCPQ Foundation, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- 1.Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77(4):1335–1347. doi: 10.1097/HEP.0000000000000004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michelotti GA, Machado MV, NAFLD DAM. NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10(11):656–665. doi: 10.1038/nrgastro.2013.183 [DOI] [PubMed] [Google Scholar]
- 3.Sanyal AJ, Shankar SS, Calle RA, et al. Non-Invasive Biomarkers of Nonalcoholic Steatohepatitis: the FNIH NIMBLE project. Nat Med. 2022;28(3):430–432. doi: 10.1038/s41591-021-01652-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong VW, Adams LA, de Ledinghen V, Wong GL, Sookoian S. Noninvasive biomarkers in NAFLD and NASH - current progress and future promise. Nat Rev Gastroenterol Hepatol. 2018;15(8):461–478. doi: 10.1038/s41575-018-0014-9 [DOI] [PubMed] [Google Scholar]
- 5.Tejani S, McCoy C, Ayers CR, et al. Cardiometabolic Health Outcomes Associated With Discordant Visceral and Liver Fat Phenotypes: insights From the Dallas Heart Study and UK Biobank. Mayo Clin Proc. 2022;97(2):225–237. doi: 10.1016/j.mayocp.2021.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahl S, Strassburger K, Nowotny B, et al. Comparison of liver fat indices for the diagnosis of hepatic steatosis and insulin resistance. PLoS One. 2014;9(4):e94059. doi: 10.1371/journal.pone.0094059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotronen A, Peltonen M, Hakkarainen A, et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137(3):865–872. doi: 10.1053/j.gastro.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 8.Newton KP, Lavine JE, Wilson L, et al. Alanine Aminotransferase and Gamma-Glutamyl Transpeptidase Predict Histologic Improvement in Pediatric Nonalcoholic Steatohepatitis. Hepatology. 2021;73(3):937–951. doi: 10.1002/hep.31317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boughanem H, Yubero-Serrano EM, Lopez-Miranda J, Tinahones FJ, Macias-Gonzalez M. Potential Role of Insulin Growth-Factor-Binding Protein 2 as Therapeutic Target for Obesity-Related Insulin Resistance. Int J Mol Sci. 2021;22(3):1133. doi: 10.3390/ijms22031133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter S, Li Z, Lemieux I, et al. Circulating IGFBP-2 levels are incrementally linked to correlates of the metabolic syndrome and independently associated with VLDL triglycerides. Atherosclerosis. 2014;237(2):645–651. doi: 10.1016/j.atherosclerosis.2014.09.022 [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Tang Y, Chen S, Ling W, Wang Q. IGFBP-2 as a biomarker in NAFLD improves hepatic steatosis: an integrated bioinformatics and experimental study. Endocr Connect. 2021;10(10):1315–1325. doi: 10.1530/EC-21-0353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanley TL, Fourman LT, Zheng I, et al. Relationship of IGF-1 and IGF-Binding Proteins to Disease Severity and Glycemia in Nonalcoholic Fatty Liver Disease. J Clin Endocrinol Metab. 2021;106(2):e520–e533. doi: 10.1210/clinem/dgaa792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J, Zhou W, Wu Y, et al. Circulating IGFBP-2 levels are inversely associated with the incidence of nonalcoholic fatty liver disease: a cohort study. J Int Med Res. 2020;48(8):300060520935219. doi: 10.1177/0300060520935219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fahlbusch P, Knebel B, Horbelt T, et al. Physiological Disturbance in Fatty Liver Energy Metabolism Converges on IGFBP2 Abundance and Regulation in Mice and Men. Int J Mol Sci. 2020;21(11):4144. doi: 10.3390/ijms21114144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rauzier C, Chartrand DJ, Almras N, et al. Associations of insulin-like growth factor binding protein-2 with metabolic profile and hepatic fat deposition in asymptomatic men and women. Am J Physiol Endocrinol Metab. 2023;325(1):E99–E105. doi: 10.1152/ajpendo.00108.2023 [DOI] [PubMed] [Google Scholar]
- 16.Chartrand DJ, Larose E, Poirier P, et al. Visceral adiposity and liver fat as mediators of the association between cardiorespiratory fitness and plasma glucose-insulin homeostasis. Am J Physiol Endocrinol Metab. 2020;319(3):E548–E556. doi: 10.1152/ajpendo.00251.2020 [DOI] [PubMed] [Google Scholar]
- 17.Gordon CC, Chumlea WC, Roche AF. Stature, recumbent length, and weight. In: Lohman TG, Roche AF, Martorell R, editors. Anthropometric Standardization Reference Manual. Champaign (IL, USA): Human Kinetics Books; 1988:3–8. [Google Scholar]
- 18.Salgado AL, Carvalho L, Oliveira AC, Santos VN, Vieira JG, Parise ER. Insulin resistance index (HOMA-IR) in the differentiation of patients with non-alcoholic fatty liver disease and healthy individuals. Arq Gastroenterol. 2010;47(2):165–169. doi: 10.1590/S0004-28032010000200009 [DOI] [PubMed] [Google Scholar]
- 19.Tahapary DL, Pratisthita LB, Fitri NA, et al. Challenges in the diagnosis of insulin resistance: focusing on the role of HOMA-IR and Tryglyceride/glucose index. Diabetes Metab Syndr. 2022;16(8):102581. doi: 10.1016/j.dsx.2022.102581 [DOI] [PubMed] [Google Scholar]
- 20.Bao Y, Lu J, Wang C, et al. Optimal waist circumference cutoffs for abdominal obesity in Chinese. Atherosclerosis. 2008;201(2):378–384. doi: 10.1016/j.atherosclerosis.2008.03.001 [DOI] [PubMed] [Google Scholar]
- 21.Blackburn P, Lemieux I, Lamarche B, et al. Type 2 diabetes without the atherogenic metabolic triad does not predict angiographically assessed coronary artery disease in women. Diabetes Care. 2008;31(1):170–172. doi: 10.2337/dc07-0272 [DOI] [PubMed] [Google Scholar]
- 22.Lemieux I, Pascot A, Couillard C, et al. Hypertriglyceridemic waist: a marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation. 2000;102(2):179–184. doi: 10.1161/01.CIR.102.2.179 [DOI] [PubMed] [Google Scholar]
- 23.Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109 [DOI] [PubMed] [Google Scholar]
- 24.Albhaisi S, Chowdhury A, Sanyal AJ. Non-alcoholic fatty liver disease in lean individuals. JHEP Rep. 2019;1(4):329–341. doi: 10.1016/j.jhepr.2019.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kratzsch J, Blum WF, Schenker E, Keller E. Regulation of growth hormone (GH), insulin-like growth factor (IGF)I, IGF binding proteins −1, −2, −3 and GH binding protein during progression of liver cirrhosis. Exp Clin Endocrinol Diabetes. 1995;103(5):285–291. doi: 10.1055/s-0029-1211366 [DOI] [PubMed] [Google Scholar]
- 26.Ross RJ, Chew SL, Li L D, et al. Expression of IGF-I and IGF-binding protein genes in cirrhotic liver. J Endocrinol. 1996;149(2):209–216. doi: 10.1677/joe.0.1490209 [DOI] [PubMed] [Google Scholar]
- 27.Watts EL, Perez-Cornago A, Appleby PN, et al. The associations of anthropometric, behavioural and sociodemographic factors with circulating concentrations of IGF-I, IGF-II, IGFBP-1, IGFBP-2 and IGFBP-3 in a pooled analysis of 16,024 men from 22 studies. Int J Cancer. 2019;145(12):3244–3256. doi: 10.1002/ijc.32276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong J, Yu J, Li Z, et al. Serum insulin-like growth factor binding protein 2 levels as biomarker for pancreatic ductal adenocarcinoma-associated malnutrition and muscle wasting. J Cachexia Sarcopenia Muscle. 2021;12(3):704–716. doi: 10.1002/jcsm.12692 [DOI] [PMC free article] [PubMed] [Google Scholar]