ABSTRACT
The Hulunbuir region, known for its diverse terrain and rich wildlife, is a hotspot for various natural epidemic diseases. Between 2021 and 2023, we collected 885 wild rodent samples from this area, representing three families, seven genera, and eleven species. Metagenomic analysis identified three complete nucleic acid sequences from the S, M, and L segments of the Hantaviridae family, which were closely related to the Khabarovsk virus. The nucleotide coding sequences for S, M, and L (1392 nt, 3465 nt, and 6491 nt, respectively) exhibited similarities of 82.34%, 81.68%, and 81.94% to known sequences, respectively, while protein-level analysis indicated higher similarities of 94.92%, 94.41%, and 95.87%, respectively. Phylogenetic analysis placed these sequences within the same clade as the Khabarovsk, Puumala, Muju, Hokkaido, Topografov, and Tatenalense viruses, all of which are known to cause febrile diseases in humans. Immunofluorescence detection of nucleic acid-positive rodent kidney samples using sera from patients with hemorrhagic fever and renal syndrome confirmed the presence of viral particles. Based on these findings, we propose that this virus represents a new member of the Hantaviridae family, tentatively named the Amugulang virus, after its primary distribution area.
KEYWORDS: Hantavirus, Amugulang virus, Hulunbuir, Amugulang Port, emerging infectious diseases
Introduction
The rapid expansion of the diversity of Bunyaviricetes in recent years, their significant clinical importance, and recent changes in the advanced classification structure of Bunyaviruses have prompted continuous adjustments to their classifications. In 2017, the International Committee on the Taxonomy of Viruses (ICTV) officially reclassified Bunyaviridae as Bunyavirales, elevating the former genus Hantavirus to the family Hantaviridae. On April 26, 2024, the ICTV further upgraded Bunyavirales to the class Bunyaviricetes. Hantaviridae is an important branch of the Bunyaviricetes and Elliovirales. Despite these updates, the terms “hantavirus” and “orthohantavirus” are still commonly used to describe members of Hantaviridae. Hantaviruses are characterized by segmented, linear, single-stranded, negative-sense, or ambisense RNA genomes. This family is the largest among the negative-stranded RNA viruses and can infect a wide range of hosts, including vertebrates, invertebrates, and plants, with some species capable of crossing host barriers [1,2]. Only rodent-borne hantaviruses are associated with human disease [3].
As of November 2023, ICTV records indicated 75 identified hantaviruses, with at least 25 known to cause hantavirus pulmonary syndrome (HPS) or hemorrhagic fever with renal syndrome (HFRS). Key pathogenic hantaviruses include the Hantaan virus (HTNV), Seoul virus (SEOV), Dobrava-Belgrade virus, Tula virus, and Puumala virus (PUUV), primarily associated with HFRS. In contrast, the Andes virus, Chocloense orthohantavirus, and Sin Nombre virus are linked to HPS. Given the overlap in clinical symptoms between HFRS and HPS, some experts have proposed a unified clinical syndrome called “hantavirus fever,” although this terminology has not been universally adopted [4]. Annually, there are an estimated 150,000–200,000 cases of HFRS or HPS worldwide, with fatality rates varying significantly depending on the virus type. For instance, the Andes virus and Sin Nombre virus in the Americas have fatality rates of 30–50%, HTNV and SEOV around 1%, and PUUV in Europe around 0.1–0.4% [5]. Various hantaviruses can asymptomatically infect wild animals and livestock, with rodents, shrews, and bats being common natural hosts.
The ongoing discovery of hantaviruses has underscored their considerable threat to public health. Since Professor Ho Wang Lee identified the first hantavirus in South Korea, six members of the Hantaviridae have been identified: HTNV (1976), SEOV (1982), Soochong virus (2006), Muju virus (2007), Linjin virus (2009), and Jeju virus (2012), with HTNV, SEOV, and Muju virus being pathogenic to humans [6,7].
PUUV, identified in Finland in 1980, is prevalent in Central, Northern, and Eastern Europe, causing approximately 3,000 cases annually between 2010 and 2020, typically with mild symptoms and less than 1% mortality [8]. The primary host of PUUV is Myodes rufocanus. In 2019, a novel PUUV strain was identified in small rodents in northwestern Ukraine [9]. To date, eight PUUV subtypes have been identified in Europe and Russia, with PUUV-like viruses being found in Japan, South Korea, and Jilin Province, China. PUUV does not affect host animal growth or reproduction but can cause mild HFRS in humans [10]. Of the 75 Hantaviridae members, 50 have not been confirmed to infect humans [11–15].
Hantaviruses are enveloped, segmented RNA viruses approximately 120–160 nm in diameter, comprising three genome segments: S (1.8 k nt), M (3.7 k nt), and L (6.5 k nt), which encode nucleoproteins, glycoproteins, and polymerase proteins, respectively [16,17]. Classification methods for Hantaviridae remain unstandardized, with some researchers suggesting amino acid sequence similarity thresholds for the S and M segments [14,18]. However, the ICTV emphasizes genome differences, host characteristics, and pathogenicity as classification criteria. Scholars advocate for continuous classification updates in line with new viral discoveries and methodologies [2].
In this study, we identified a novel member of the Hantaviridae family through metagenomic sequencing, named the Amugulang virus, based on its distribution area. Preliminary investigations of its prevalence in various regions and rodent species were conducted using electron microscopy and genetic evolutionary analyses.
Materials and methods
Sample collection and processing
Wild rodents were collected between 2021 and 2023 from various locations in the Hulunbuir border region of Inner Mongolia, including Arihasate Port, Manzhouli City (Shibali Village and Chagan Lake), Ebuduge Port, Heishantou Port, and Aershan Port. The rodents were trapped in cages, morphologically identified, and euthanized under ether anesthesia. The liver, spleen, lungs, and kidneys were aseptically collected and stored in liquid nitrogen. Species identification was performed as previously described [19]. The study was approved by Shenyang Agricultural University (Letter Number: 2021040701).
Metagenomic sequencing and sequence assembly
Lung and kidney samples from wild rodents were thoroughly ground, and total RNA was extracted and quantified using a Qubit 4. Libraries were constructed and analyzed using a Qubit 4 and Qseq100, and were considered qualified if the RNA concentration was >5 ng/μL with peak segments in the 300–500 bp range. High-throughput sequencing was performed using an MGISEQ-2000 sequencer. Sequencing data were processed using fastp (version 0.23.4) to remove adapters and low-quality sequences, resulting in clean data. HISAT2 (version 2.2.1) was used to align the clean data with the host genome, resulting in Rmhost data. MEGAHIT (v1.2.9) was employed for de novo assembly of Rmhost data to obtain contigs. The contigs were compared to the nucleotide database using BLASTN (2.14.0+), and annotation was based on the taxonomy database of the National Center for Biotechnology Information. BWA (version 0.7.17-r1188) was used to index the assembled contig sequences, and the mapped reads were aligned to these sequences to obtain the assembly results.
Epidemiological investigation
cDNA was synthesized from 100 mg of lung and kidney tissues using the EasyPure® Simple Viral DNA/RNA Kit and HiScript® III 1st Strand cDNA Synthesis Kit. Nested PCR primers based on the metagenomic sequencing results were used to detect viral nucleic acids in all samples. Quantitative fluorescence PCR (qPCR) primers and probes were designed for detection in inoculated cells and BALB/c and Kunming mice. Primer and probe sequences are listed in Supplementary Table S1. Positive plasmid standards were synthesized using the detailed sequences provided in Attachment 1.
Transmission electron microscopy and indirect immunofluorescence observation
Kidneys from positive wild rodents were sectioned, and viral particles were observed by electron microscopy after negative staining (detailed methods are provided in Attachment 2). Positive sera from clinically diagnosed patients with HFRS (from the Sixth People’s Hospital of Dandong City) were used as the primary antibody, and FITC-labeled goat anti-human Fab segment antibody was used as the secondary antibody. Fluorescent staining of frozen sections was performed, and specific fluorescence in the cytoplasm indicated positive staining (detailed methods are presented in Attachment 3).
Viral isolation
Viral isolation was performed as previously described [20]. Lungs and kidneys from positive rodent samples were ground, suspended in sterile phosphate-buffered saline (PBS), and centrifuged at 1000 ×g to obtain the supernatant, which was filtered through a 0.22 μm filter and stored. Vero and BHK-21 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. The cells were washed with PBS, digested with trypsin, centrifuged, mixed with the filtrate, centrifuged again at 1000 ×g for 1 h, and transferred to culture flasks. The supernatant was collected at each passage for nucleic acid extraction and reverse transcription. The filtrate was inoculated into 21-day-old BALB/c and Kunming mice via the abdominal and nasal cavities. Cell morphology was observed, and nucleic acids were extracted from the mouse lungs and kidneys on days 3, 7, 14, and 21 for viral detection.
Phylogenetic analyses
The sequences of the latest members of the Hantaviridae family were retrieved from the ICTV website. The S, M, and L gene segments and corresponding protein sequences were downloaded and combined with the Amugulang virus nucleic acid sequences to create a FASTA file. Molecular phylogenetic trees were constructed using the neighbor-joining (NJ) method in MEGA11 software.
Results
Metagenomic identification of Amugulang virus
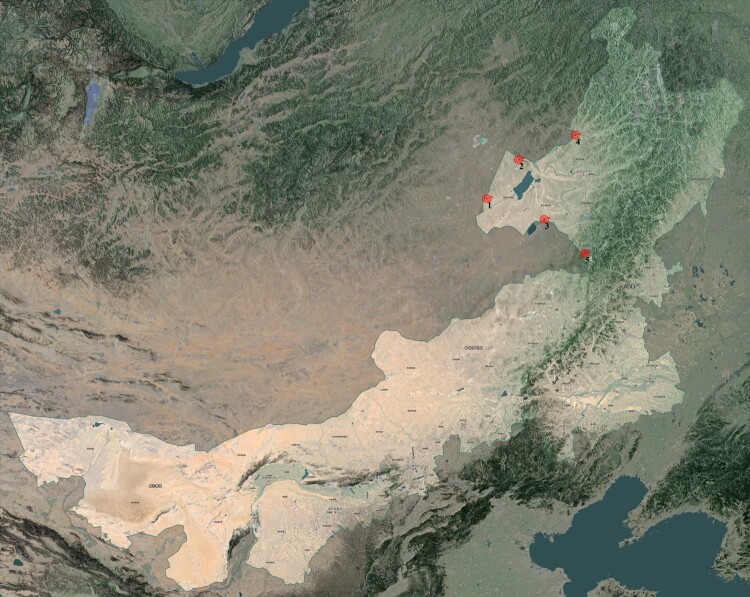
Between 2021 and 2023, 885 wild rodent samples were collected from the Hulunbuir region, including Meriones unguiculatus, Lasiopodomys brandtii, Allactaga sibirica, Microtus gregalis, Spermophilus dauricus, Apodemus agrarius, Apodemus peninsulae, Phodopus sungorus, and Mus musculus. L. brandtii was the dominant species, accounting for 56.3% (498) of the total collected samples. Detailed information on the samples is provided in Attachment 4. Nucleic acid was extracted from pooled samples and subjected to metagenomic sequencing. BLAST analysis of assembled contigs revealed several sequence fragments highly homologous to those of the family Hantaviridae. Positive samples were further confirmed using RT–PCR and Sanger sequencing. The CDS region sequences of the S, M, and L segments, with lengths of 1392, 3465, and 6491 nt respectively, exhibited only 82.34%, 81.68%, and 81.94% similarity to known sequences. Amino acid sequence analysis indicated higher similarities of 94.92%, 94.41%, and 95.87%. These results suggest that these sequences may derive from a new member of the Hantaviridae family. Based on virus classification, we tentatively named this virus the Amugulang virus, after its primary distribution area (Figure 1).
Figure 1.
Site of sample collection.
Spatial and host distribution of Amugulang virus
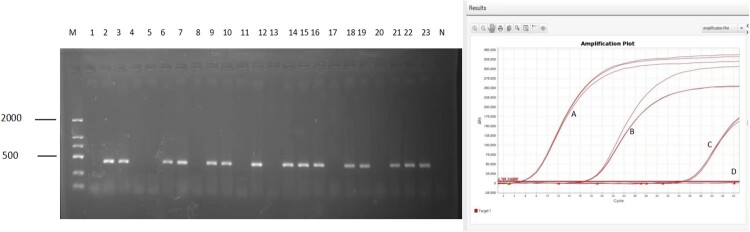
Nucleic acid extracts from all samples were tested by RT–PCR for the presence of the virus sequence. Of the 885 samples tested, 66 were positive, yielding a positivity rate of 7.5% (Table 1 and Figure 2). The distribution of positive samples varied by sampling site, with higher rates at Ebuduge Port (14.5%), Arihasate Port (4.1%), and Aershan Port (9.9%). Notably, Shibali Village in Manzhouli City had the highest positive rate of 18.4%, despite having fewer samples (Table 1). Host species analysis showed that only L. brandtii samples were positive for the Amugulang virus, consistent with this species being the most abundant in the region (56.3% of all samples) (Table 2). Attachment 4 presents the details of the detection results.
Table 1.
Detection of Amugulang virus in rodent samples from different sampling sites.
| Sampling position | East longitude/ north latitude | Landforms | Total number of rodents | Total number of positive rodents | Positive rate (%) |
|---|---|---|---|---|---|
| Ebuduge Port | 50.2424/120.1909 | Grassland | 172 | 25 | 14.5 |
| Chagan Lake, Manzhouli City | 49.58/117.45 | Grassland | 34 | 0 | 0 |
| Heishantou Port | 40.234/122.0775 | Grassland | 75 | 2 | 2.7 |
| Arihasate Port | 48.5989/115.8815 | Grassland | 414 | 17 | 4.1 |
| Aershan Port | 47.1771/119.9431 | Forest | 152 | 15 | 9.9 |
| Shibali Village, Manzhouli City | 49.58/117.45 | Grassland | 38 | 7 | 18.4 |
Figure 2.
Virus detection results. Left: Amugulang virus detection results
(Note: M: DNA marker-DL2000; N, negative control). Right: qPCR results for inoculated cells, BALB/c mice, and Kunming mice (Note: A: Positive plasmid, B: Positive rodent kidney sample, C: Cell culture supernatant (at day 3), D: Cells washed with PBS (at day 7), and negative control).
Table 2.
Amugulang virus detection in rodent samples.
| Rodent species (Latin name) |
Family | Genus | Total number of rodents |
Total number of positive rodents |
Positive rate (%) |
|---|---|---|---|---|---|
| Lasiopodomys brandtii | Circetidae | Microtus | 498 | 66 | 13.2 |
| Mus musculus | Muridae | Murine | 56 | 0 | 0 |
| Spermophilus dauricus | Sciuridae | Citellus | 11 | 0 | 0 |
| Microtus gregalis | Circetidae | Microtus | 108 | 0 | 0 |
| Allactaga sibirica | Dipodidae | Allactaga | 14 | 0 | 0 |
| Apodemus agrarius | Muridae | Apodemus | 120 | 0 | 0 |
| Meriones unguiculatus | Circetidae | Meriones | 48 | 0 | 0 |
| Cricetulus barabensis | Circetidae | Cricetulus | 2 | 0 | 0 |
| Rattus norvegicus | Muridae | Rattus | 16 | 0 | 0 |
| Phodopus sungorus | Circetidae | Phodopus | 4 | 0 | 0 |
| Apodemus peninsulae | Muridae | Apodemus | 8 | 0 | 0 |
No evidence of vertical transmission of Amugulang virus in pregnant rodents
Four L. brandtii samples from Ebuduge Port (three samples) and Arihasate Port (one sample) contained fetuses during dissection. Fetuses (9, 10, 9, and 8, respectively) and their mothers were tested simultaneously. Two maternal samples from Ebuduge Port were nucleic acid positive, but their fetus samples were negative. This result suggests that the virus may not be transmitted vertically from mother to fetus.
Viral isolation and detection
Given the wide distribution of the virus, we attempted to isolate it by inoculating the positive samples in cells and mice. However, attempts to isolate the virus using Vero and BHK-21 cells, as well as BALB/c and Kunming mice, were unsuccessful. Viral nucleic acids were detected in the culture supernatant of Vero cells on the third and seventh days post-inoculation; however, subsequent tests on washed cells were negative. Continuous cultivation for 21 days yielded negative results. Similarly, lung and kidney samples from inoculated BALB/c and Kunming mice tested negative at 3, 7, 14, and 21 days post-inoculation. The qPCR results are shown in Figure 2.
Virus confirmation by transmission electron microscopy and indirect immunofluorescence
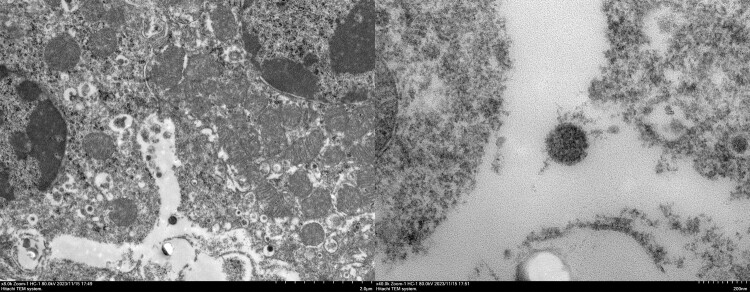
Given the unsuccessful isolation in cell culture and mice, we attempted to observe the virus directly in positive samples. Transmission electron microscopy and immunofluorescence microscopy of kidney samples from infected L. brandtii revealed detailed viral morphology. Electron microscopy showed incomplete Amugulang virus particles within Golgi apparatus vesicles, measuring 120–160 nm, as well as mature spherical particles approximately 160 nm in size with visible capsules, characteristic of hantaviruses [16,21–23] (Figure 3). Notably, some viral particles in the cytoplasm and organelles were not fully assembled and measured approximately 120–160 nm.
Figure 3.
Amugulang virus morphology by electron microscopy. Left: Amugulang virus in the Golgi apparatus and vesicles. Scale bar = 200 nm. Right: Free Amugulang virus. Scale bar = 20 nm. The scale bars are located in the lower-right corner of the images.
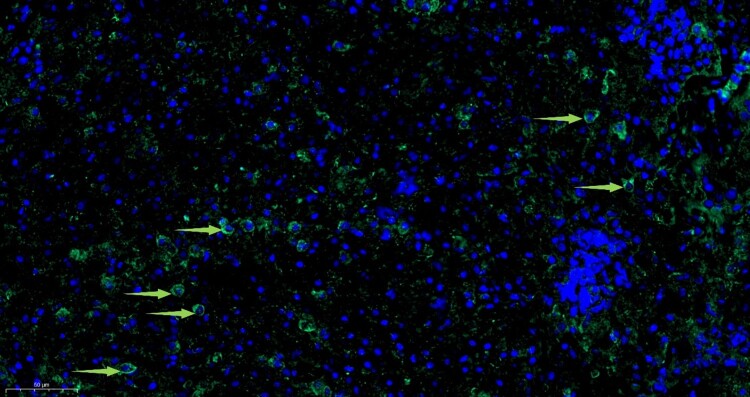
Since the Amugulang virus belongs to the family Hantaviridae, it may be recognized by serum from patients infected with Hantavirus. To confirm this, we collected sera samples from patients with HFRS. RT–PCR positive samples were tested with the serum using indirect immunofluorescence. Immunofluorescence revealed specific green fluorescence in the cytoplasm, indicating the presence of viral particles or proteins, consistent with hantavirus replication patterns (Figure 4). These results suggest cross-reactivity between Hantavirus and Amugulang virus, indicating that the virus could be recognized by antibodies against Hantavirus (Figure 5).
Figure 4.
Amugulang virus in the kidney by immunofluorescence microscopy. Scale bar = 10μm and is located in the lower-left corner of the image.
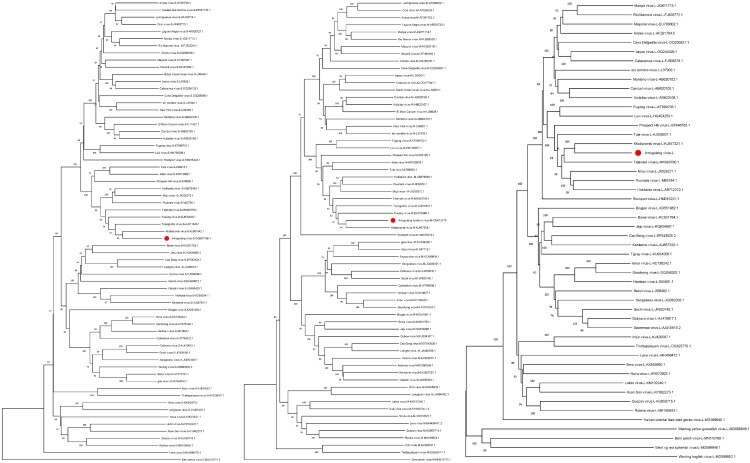
Figure 5.
The NJ tree of the Amugulang virus S (left), M (middle), and L (right) segments.
Note: The system evolution parameters are as follows: 1. Constructed using Neighbour-Joining method; 2. Test of Phylogeny: Bootstrap method; 3. No. of Bootstrap Replications: 1000; 4. Gaps/Missing Data Treatment: Complete deletion.
Phylogeny of the Amugulang virus
The sequences of the S (Accession: OR767834.1), M (Accession: ON012175.1), and L (Accession: OR767833.1) segments of the Amugulang virus were obtained using metagenomics. Phylogenetic analysis included 75 Hantaviridae members from the ICTV directory, with 65 S, 63 M, and 49 L nucleic acid sequences. An NJ tree was constructed using MEGA 11 software (Figure 4), and the results showed that the closest relationship was between the Amugulang and Khabarovsk viruses hosted by Microtus maximowiczii. Other closely related viruses included Fusong, Hokkaido, Muju, Puumala, Tatenale, and Topografov, all of which belong to the family Cricetidae.
Among all the members of the Hantaviridae family, the genetic distances between the S, M, and L nucleotide sequences of the Amugulang and Khabarovsk viruses were the lowest, with values of 15.474, 18.196, and 18.647, respectively (Table 3). Genetic distances between the corresponding amino acid sequences were 6.032, 5.975, and 4.231, respectively. According to the literature [2], the nucleotide and amino acid sequences of the S and M segments were concatenated and analyzed, revealing genetic distances of 17.215 and 5.818, respectively. Notably, the hosts of the eight viruses most similar to the Amugulang virus, although different, belong to the Cricetidae family.
Table 3.
Genetic distances of nucleotide and amino acid sequences of the tandem sequence of the Amugulang virus S, M, L, and S-M segments.
| Amugulang virus | Seg S | Seg M | Seg L | Seg S-M | ||||
|---|---|---|---|---|---|---|---|---|
| aa | nt | aa | nt | aa | nt | aa | nt | |
| Adler virus | 17.991 | 24.961 | 19.947 | — | — | — | 19.334 | 27.325 |
| Amur virus | 36.066 | 36.456 | 45.053 | 43.383 | 30.279 | 33.282 | 42.342 | 41.180 |
| Andes virus | 27.336 | 30.327 | 32.865 | 38.345 | 21.897 | 28.920 | 31.074 | 34.976 |
| Anjozorobe virus | 40.930 | 38.581 | 45.841 | 44.558 | — | — | — | — |
| Asama virus | — | — | 47.401 | 46.322 | — | — | 45.356 | 43.859 |
| Asikkala virus | 40.376 | 41.484 | 46.208 | 43.598 | — | — | 44.409 | 42.923 |
| Bayou virus | 25.467 | 30.560 | 32.337 | 36.762 | 21.711 | 28.563 | 30.179 | 34.261 |
| Bern perch virus | 82.683 | 64.831 | 89.683 | 66.379 | 75.405 | 60.983 | 87.492 | 64.556 |
| Black Creek Canal virus | 25.234 | 29.860 | 32.601 | 36.611 | — | — | 30.243 | 33.772 |
| Bowe virus | 38.498 | 38.486 | 46.561 | 43.509 | 33.628 | 34.430 | 44.116 | 42.057 |
| Brno virus | 47.518 | 42.484 | 58.090 | 50.407 | 37.962 | 35.500 | 55.110 | 47.700 |
| Bruges virus | 41.121 | 39.019 | 46.032 | 44.270 | 31.349 | 33.919 | 44.544 | 42.774 |
| Cano Delgadito virus | 28.505 | 30.016 | 34.007 | 38.679 | 21.339 | 28.263 | 32.417 | 35.699 |
| Cao Bang virus | 38.967 | 38.407 | 46.296 | 45.071 | 29.767 | 32.413 | 43.987 | 42.457 |
| Carrizal virus | 27.103 | 31.260 | 31.168 | 34.945 | 21.292 | 28.159 | 29.904 | 33.700 |
| Castelo dos Sonhos virus | 28.912 | 33.552 | — | — | — | — | — | — |
| Catacamus virus | 25.935 | 31.260 | 32.513 | 37.216 | 21.804 | 28.666 | 30.499 | 34.445 |
| Choclo virus | 26.636 | 30.949 | 34.446 | 38.225 | — | — | 32.097 | 35.282 |
| Dabieshanense virus | 36.534 | 34.348 | 44.867 | 46.398 | — | — | 42.370 | 41.948 |
| Dobrava virus | 37.705 | 37.315 | 45.318 | 45.376 | 30.326 | 33.750 | 43.050 | 42.747 |
| El Moro Canyon virus | 27.570 | 31.104 | 32.221 | 34.844 | — | — | 30.735 | 33.486 |
| Fugong virus | 24.826 | 28.538 | 27.441 | 30.700 | 20.502 | 27.602 | 26.407 | 29.833 |
| Fusong virus | 9.513 | 19.751 | 12.379 | 23.654 | — | — | 11.438 | 22.192 |
| Gou virus | 36.534 | 35.831 | 44.779 | 44.916 | — | — | 42.332 | 41.979 |
| Hainan oriental lead-toed gecko virus |
— | — | — | — | 47.922 | 43.535 | — | — |
| Hantaan virus | 35.831 | 37.627 | 44.788 | 43.843 | 30.558 | 33.590 | 42.085 | 41.979 |
| Hokkaido virus | 11.601 | 23.717 | 15.189 | 24.157 | 10.414 | 23.732 | 14.121 | 24.079 |
| Huitzilac virus | 27.336 | 31.960 | 31.343 | 35.801 | 21.339 | 29.116 | 30.032 | 34.495 |
| Imjin virus | 53.412 | 45.027 | 56.810 | 51.301 | 38.472 | 37.496 | 55.974 | 50.207 |
| Jeju virus | 41.080 | 39.032 | 45.944 | 44.095 | 32.512 | 34.305 | 44.437 | 42.351 |
| Kenkeme virus | 40.845 | 39.844 | 47.090 | 45.630 | 30.930 | 33.385 | 45.180 | 43.600 |
| Khabarovsk virus | 6.032 | 15.474 | 5.975 | 18.196 | 4.231 | 18.647 | 5.818 | 17.215 |
| Laguna Negra virus | 26.636 | 31.182 | 33.128 | 38.477 | — | — | 31.138 | 35.710 |
| Laibin virus | 46.370 | 41.810 | 53.072 | 47.299 | 34.967 | 35.336 | 50.973 | 45.356 |
| Lechiguanas virus | 27.336 | 30.793 | 32.777 | 38.326 | — | — | 31.074 | 35.362 |
| Lena virus | 52.941 | 47.105 | 53.778 | 51.552 | 38.293 | 38.264 | 53.562 | 49.676 |
| Lianghe virus | 38.732 | 37.939 | 48.413 | 45.274 | — | — | 45.466 | 42.642 |
| Longquan virus | 45.626 | 41.831 | 58.422 | 50.582 | — | — | 54.828 | 47.309 |
| Luxi virus | 25.290 | 28.305 | 27.001 | 30.750 | 20.419 | 28.191 | 26.215 | 30.046 |
| Maporal virus | 26.869 | 31.571 | 33.949 | 37.879 | 21.339 | 27.974 | 31.734 | 35.364 |
| Maripa virus | 26.869 | 30.793 | 32.337 | 36.762 | 21.525 | 28.931 | 30.563 | 34.455 |
| Montano virus | 26.402 | 30.560 | 33.450 | 35.786 | 21.711 | 28.798 | 31.374 | 34.047 |
| Muju virus | 12.529 | 21.773 | 13.345 | 24.056 | 11.111 | 23.255 | 13.035 | 23.196 |
| Necocli virus | 28.271 | 31.104 | 33.216 | 38.074 | — | — | 31.586 | 35.119 |
| New York virus | 27.336 | 31.260 | 32.397 | 36.606 | — | — | 30.863 | 34.454 |
| Nova virus | 47.196 | 42.980 | 53.695 | 48.904 | 38.064 | 36.936 | 51.427 | 46.712 |
| Oran virus | 27.570 | 30.793 | 32.865 | 39.637 | — | — | 31.138 | 36.097 |
| Oxbow virus | 40.000 | 39.515 | 46.737 | 43.191 | — | — | 44.695 | 42.269 |
| Prospect Hill virus | 17.401 | 23.950 | 21.705 | 30.363 | 15.628 | 26.106 | 20.205 | 27.823 |
| Puumala virus | 12.993 | 23.250 | 16.945 | 24.610 | 11.297 | 23.039 | 15.783 | 24.170 |
| Quezon virus | 42.857 | 38.768 | 54.756 | 50.868 | 36.444 | 37.862 | 51.197 | 45.696 |
| Rio Mamor virus | 27.103 | 30.638 | 32.425 | 37.166 | 21.478 | 28.920 | 30.691 | 34.517 |
| Robina virus | 44.028 | 40.827 | 53.125 | 50.901 | 36.800 | 37.926 | 50.130 | 47.004 |
| Rockport virus | 27.570 | 31.726 | 37.093 | 38.213 | 24.558 | 30.008 | 34.337 | 35.876 |
| Saaremaa virus | — | — | — | — | 30.512 | 33.811 | — | — |
| Sangassou virus | 37.002 | 36.768 | 45.936 | 44.785 | 30.326 | 33.647 | 43.308 | 41.777 |
| Seoul virus | 36.534 | 36.612 | 44.602 | 45.478 | 30.605 | 34.510 | 42.204 | 42.317 |
| Serang virus | 36.534 | 37.002 | — | — | — | — | — | — |
| Sin nombre virus | 27.570 | 30.404 | 31.607 | 37.009 | 20.828 | 28.611 | 30.415 | 34.393 |
| Sochi virus | 38.173 | 37.549 | 45.310 | 45.989 | 30.140 | 33.709 | 43.235 | 43.024 |
| Soochong virus | 36.534 | 35.597 | 45.141 | 43.383 | 30.326 | 33.976 | 42.535 | 40.719 |
| Tatenale virus | 11.833 | 22.240 | 12.680 | 24.409 | 9.995 | 21.887 | 12.354 | 23.623 |
| Thottapalayam virus | 52.594 | 45.341 | 56.043 | 51.221 | 38.128 | 37.883 | 55.036 | 50.048 |
| Tigray virus | — | — | — | — | 31.116 | 33.040 | — | — |
| Topografov virus | 4.640 | 17.729 | 8.963 | 21.976 | — | — | 7.673 | 20.384 |
| Tula virus | 17.799 | 24.299 | 18.190 | 27.680 | 13.767 | 24.684 | 18.054 | 26.581 |
| Wenling hagfish virus | — | — | — | — | 83.350 | 57.259 | — | — |
| Wenling red spikefish virus | — | — | — | — | 74.090 | 60.618 | — | — |
| Wenling yellow goosefish virus | — | — | — | — | 78.139 | 61.021 | — | — |
| Xuan Son virus | 44.731 | 40.796 | 52.004 | 47.418 | 34.407 | 35.446 | 49.665 | 45.486 |
| Yakeshi virus | 37.793 | 38.750 | 47.090 | 45.071 | — | — | 44.344 | 42.954 |
Discussion
Lasiopodomys brandtii belongs to the family Cricetidae and is primarily distributed across China, Mongolia, and Russia, with a concentration in the Hulunbuir region to the west of the Greater Hinggan Mountains. Notably, this species exhibits significant annual population fluctuations, with pronounced peaks occurring approximately every 12 years. L. brandtii is a major pest of the temperate grasslands of western Hulunbuir, where it lives in colonies within an extensive burrow system. During population outbreaks, these rodents cause substantial damage to grassland vegetation, contributing to land degradation, desertification, and exacerbating springtime dust storms.
Since 2020, the population density of L. brandtii has remained high, necessitating large-scale rodent control measures during the spring and summer of 2023. These efforts resulted in a relatively low number of rodents collected that year. However, L. brandtii continues to dominate the rodent population and is expected to rebound, posing an ongoing threat as a primary vector of local rodent-borne diseases. Other rodent species, such as Apodemus agrarius and Apodemus peninsulae, are also present in the Hulunbuir area and may carry pathogens, such as Yersinia pestis and Hantaan virus (HTNV). Current research on L. brandtii focuses on its role in disrupting the ecological balance of grasslands and monitoring severe infectious diseases such as plague and hemorrhagic fever with renal syndrome (HFRS). However, there is a lack of systematic studies on the unknown and potential pathogens that L. brandtii may harbour. Given the high infection rate of the Amugulang virus in this species, further research is needed to understand the risks of infection and transmission to predators, such as wolves, foxes, and weasels, and their impact on maintaining the local ecological balance.
The grasslands of Hulunbuir serve as traditional grazing areas for cattle and sheep, and L. brandtii is frequently found in grasslands, animal pens, and herder settlements. Notably, a significant abundance of L. brandtii has been observed in residential areas and near the sampling site in the Amugulang area. Their excrement and secretions can contaminate the environment and potentially human food sources. Although there is no definitive evidence linking L. brandtii to human infection, its genetic similarity to viruses, such as the Puumala virus (PUUV) and Muju virus, which can infect humans, raises concerns. Thus, the potential of L. brandtii to contribute to other febrile infectious diseases, particularly among vulnerable populations such as children and the elderly, warrants attention.
Hantavirus evolution in molecular epidemiology involves three main mechanisms: genomic mutations, reassortment of segmented genomes between closely related hantaviruses, and genetic recombination. Various members of the Hantaviridae family, such as HTNV and Seoul virus (SEOV), are prevalent in northern China, including the northern and eastern parts of Inner Mongolia. These viruses can spread through the inhalation and ingestion of secretions, excreta, or bites from infected parasites. As animals migrate, the virus spreads. The Hulunbuir region’s extensive land border with Mongolia and Russia facilitates the seasonal migration of wild animals such as yellow sheep, wolves, and foxes. Active wild rodents on both sides of the border enable long-distance transmission of viruses, leading to interactions between Amugulang virus and HTNV both domestically and internationally. Recombination of hantaviruses, such as PUUV, increases the risk of human infection.
Rodents are generally considered the primary natural hosts of hantaviruses, adhering to the “one-mammal-one-hantavirus rule” or “single-host-single-virus systems” [22]. In this study, attempts to infect BALB/c and Kunming mice with Amugulang virus via oral, nasal, or intraperitoneal routes were unsuccessful, and the virus was not detected in samples from other species in the region. This suggests that the Amugulang virus is highly adapted and co-evolved with L. brandtii, following the “one-mammal-one-hantavirus rule.” However, this rule is not absolute, as recent discoveries of various hantaviruses have revealed a broader range of hosts, including shrews, bats, freshwater fish, geckos, and lower vertebrates [3]. Although hantaviruses typically select a specific host with which they co-adapt and evolve, exceptions exist, such as SEOV, which infects at least two rodent species. Humans are considered a “dead end” in hantavirus evolution, with epidemics not contributing to virus evolution [24].
The relatively strict “single-host-single-virus systems” of hantaviruses make isolation challenging. Even when successfully infecting cells, virus growth is slow and its titre remains low [25]. Previous attempts to isolate the Amugulang virus using conventional methods, such as inoculating Vero cells with organ homogenate from positive samples and conducting nucleic acid tests after three generations of blind passages, have been unsuccessful, as described in the literature. Vero cells, which are commonly used to isolate hantaviruses, have a low success rate. It is likely that the cells most suitable for isolating the Amugulang virus are primary cells of L. brandtii, which require long-term cultivation for successful isolation.
Hantaviridae are pleomorphic, with diameters ranging from 120–160 nm and variable shapes ranging from round to elongated. In this study, Amugulang virus particles exhibited a relatively regular shape, with diameters of 120–160 nm and spikes extending approximately 10 nm from the surface. The viral particles were observed at different cellular locations and displayed varying sizes and morphologies. It is generally believed that viral particles assemble in the Golgi apparatus, enter the Golgi pool through budding, and are released via exocytosis (Old World hantaviruses, distributed in Europe and Asia), transported to the cell surface, or directly released onto the plasma membrane (New World hantaviruses, distributed in North and South America) [16,23]. However, the details of this process remain unclear. In this study, virions located in Golgi vesicles appeared to be fully assembled, with free virus particles measuring 120–160 nm in diameter and visible capsules. Slender virions were not observed in this study. Additionally, no large clusters of viral particles were found within the cells, likely due to the high adaptation of the virus to its host cell. A slower proliferation rate may contribute to the persistent infections observed in host organisms.
Conclusion
A novel member of the Hantaviridae family, the Amugulang virus, was identified in L. brandtii from Hulunbuir, China, with an infection rate of 7.5%. Viral particles were localized in the cytoplasm of renal cells, measuring 120–160 nm, with a capsule membrane. The virus was unable to infect and replicate in BALB/c or Kunming mice, Vero cells, or BHK-21 cells, and did not achieve vertical placental transmission. Antigenic cross-reactivity with HFRS virus was observed. Phylogenetic analysis indicated that the Amugulang virus is most closely related to the Khabarovsk virus isolated from the Russian Far East.
Supplementary Material
Funding Statement
This study was supported by the Science and Technology Partnership Program of the Ministry of Science and Technology of China (KY201901014) and the National Key R&D Program of China (2022YFC2303700).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Ethical statement
The study was approved by Shenyang Agriculture University(Letter Number: 2021040701).
Authors’ contributions
Xiaohu Han and Zeliang Chen conceptualized the initial hypothesis, and conceived and designed the study. Feng Jiang, Xiaohu Han,Lianhong Zhang, Mei Han, Yongxiang Zhao,Ya Wen, Hua Deng, Feng Jiang, Mingxuan Zhang and Saijilahu performed the sample collection and molecular detection. Qin Dai, Jinguo Zhang and Lianhong Zhang collected sorted data. Qing Xin and Tianyu Yang, edited the tables. Xiaohu Han and Zeliang Chen conducted literature searches and participated in the writing process. Xiaohu Han, Lianhong Zhang performed the statistical analyses and wrote the first draft of the manuscript. Xiaohu Han and Zeliang Chen revised the manuscript. All authors contributed substantially to the data acquisition, interpretation, and revision andediting of the manuscript.
References
- 1.Abudurexiti A, Adkins S, Alioto D, et al. . Taxonomy of the order Bunyavirales: update 2019. Arch Virol. 2019;164(7):1949–1965. doi: 10.1007/s00705-019-04253-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laenen L, Vergote V, Calisher CH, et al. . Hantaviridae: current classification and future perspectives. Viruses. 2019;11(9):788. doi: 10.3390/v11090788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuhn JH, Schmaljohn CS.. A brief history of bunyaviral family Hantaviridae. Diseases. 2023;11(1):38. doi: 10.3390/diseases11010038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clement J, Maes P, Van Ranst M.. Hemorrhagic fever with renal syndrome in the new, and Hantavirus pulmonary syndrome in the old world: paradi(se)gm lost or regained? Virus Res. 2014;187:55–58. doi: 10.1016/j.virusres.2013.12.036 [DOI] [PubMed] [Google Scholar]
- 5.Avšič-Županc T, Saksida A, Korva M.. Hantavirus infections. Clin Microbiol Infect. 2019;21:e6–e16. doi: 10.1111/1469-0691.12291 [DOI] [PubMed] [Google Scholar]
- 6.Krüger DH, Ulrich R, Lundkvist Å. Hantavirus infections and their prevention. Microbes Infect. 2001 2001/11/01/;3(13):1129–1144. doi: 10.1016/S1286-4579(01)01474-5. [DOI] [PubMed] [Google Scholar]
- 7.Noh JY, Jung J, Song J-W.. Hemorrhagic fever with renal syndrome. Infect Chemother. 2019;51(4):405–413. doi: 10.3947/ic.2019.51.4.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brummer-Korvenkontio M, Vaheri A, Hovi T, et al. . Nephropathia Epidemica: detection of antigen in bank voles and serologic diagnosis of human infection. J Infect Dis. 1980;141(2):131–134. doi: 10.1093/infdis/141.2.131 [DOI] [PubMed] [Google Scholar]
- 9.Williams EP, Taylor MK, Demchyshyna I, et al. . Prevalence of hantaviruses harbored by murid rodents in northwestern Ukraine and discovery of a novel puumala virus strain. Viruses. 2021;13(8):1640. doi: 10.3390/v13081640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alemán A, Iguarán H, Puerta H, et al. . Hantaviruses: a global disease problem. Emerg Infect Dis. 1997;3(2):95–104. Revista De Salud Pública. 1997. doi: 10.3201/eid0302.970202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanada T, Seto T, Ozaki Y, et al. . Isolation of Hokkaido virus, genus Hantavirus, using a newly established cell line derived from the kidney of the grey red-backed vole (Myodes rufocanus bedfordiae). J Gen Virol. 2012;93(10):2237–2246. doi: 10.1099/vir.0.045377-0 [DOI] [PubMed] [Google Scholar]
- 12.Hörling J, Chizhikov V, Lundkvist Å, et al. . Khabarovsk virus- a phylogenetically and serologically distinct Hantavirus isolated from Microtus fortis trapped in far-east Russia. J Gen Virol. 1996;77(4):8. doi: 10.1099/0022-1317-77-4-687 [DOI] [PubMed] [Google Scholar]
- 13.Ge X-Y, Yang W-H, Pan H, et al. . Fugong virus, a novel hantavirus harbored by the small oriental vole (Eothenomys eleusis) in China. Virol J. 2016;13(1):27. doi: 10.1186/s12985-016-0483-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chappell JG, Tsoleridis T, Onianwa O, et al. . Retrieval of the complete coding sequence of the UK-Endemic Tatenale Orthohantavirus reveals extensive strain variation and supports its classification as a novel species. Viruses. 2020;12(4):454. doi: 10.3390/v12040454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meheretu Y, Stanley WT, Craig EW, et al. . Tigray Orthohantavirus infects two related rodent species adapted to different elevations in Ethiopia. Vector-Borne Zoonotic Diseases. 2019;19(12):950–953. doi: 10.1089/vbz.2019.2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meier K, Thorkelsson SR, Quemin ERJ, et al. . Hantavirus replication cycle—An updated structural virology perspective. Viruses. 2021;13(8):1561. doi: 10.3390/v13081561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plyusnin A, Vapalahti O, Vaheri A.. Hantaviruses: genome structure, expression and evolution. J Gen Virol. 1996 Nov;77(Pt 11):2677–2687. PubMed PMID: 8922460; eng. doi: 10.1099/0022-1317-77-11-2677 [DOI] [PubMed] [Google Scholar]
- 18.Maes P, Klempa B, Clement J, et al. . A proposal for new criteria for the classification of hantaviruses, based on S and M segment protein sequences. Infect Genet Evol. 2009 2009/09/01/;9(5):813–820. doi: 10.1016/j.meegid.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Taki Y, Vincenot CE, Sato Y, et al. . Genetic diversity and population structure in the Ryukyu flying fox inferred from remote sampling in the Yaeyama archipelago. PLoS One. 2021;16(3):e0248672. doi: 10.1371/journal.pone.0248672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song KJ, Baek LJ, Moon S, et al. . Muju virus, a novel hantavirus harboured by the arvicolid rodent Myodes regulus in Korea. J Gen Virol. 2007 Nov;88(Pt 11):3121–3129. PubMed PMID: 17947538; PubMed Central PMCID: PMCPMC2253664. eng. doi: 10.1099/vir.0.83139-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parvate A, Williams EP, Taylor MK, et al. . Diverse morphology and structural features of old and new world hantaviruses. Viruses. 2019;11(9):862. doi: 10.3390/v11090862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mull N, Jackson R, Sironen T, et al. . Ecology of neglected rodent-borne American Orthohantaviruses. Pathogens. 2020;9(5):325. doi: 10.3390/pathogens9050325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Battisti AJ, Chu Y-K, Chipman PR, et al. . Structural studies of hantaan virus. J Virol. 2011;85(2):835–841. doi: 10.1128/jvi.01847-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plyusnin A, Morzunov SP.. Virus evolution and genetic diversity of hantaviruses and their rodent hosts. In: Schmaljohn CS, Nichol ST, editors. Hantaviruses. Berlin, Heidelberg: Springer Berlin Heidelberg; 2001. p. 47–75. [DOI] [PubMed] [Google Scholar]
- 25.Meyer BJ, Schmaljohn C.. Accumulation of terminally deleted RNAs may play a role in Seoul virus persistence. J Virol. 2000 Feb;74(3):1321–1331. PubMed PMID: 10627543; PubMed Central PMCID: PMCPMC111467. eng. doi: 10.1128/jvi.74.3.1321-1331.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.