ABSTRACT
Thailand introduced a two-dose regimen of bivalent HPV vaccines for Grade 5 schoolgirls, approximately 11 years old, initially piloted in Ayutthaya province in 2014, and nationwide under the National Immunization Program (NIP) in 2017. This cross-sectional, case-control study evaluated the vaccine effectiveness in schoolgirls 7 years after a two-dose administration. Between May and June 2023, 211 grade 12 female students from Ayutthaya, who received the two-dose bivalent HPV vaccine CERVARIXⓇ (HPV types 16 and 18), and 376 grade 12 students from Nakhon Pathom who did not receive the HPV vaccine, were enrolled. HPV infection was detected by testing for HPV DNA in the first-void urine samples using real-time PCR (Cobas® 4800 and AnyplexTM HPV28). The study found that the HPV vaccine 100% effective against high-risk HPV (HR-HPV) types included in the vaccine (16, 18) and 32.8% effective against other HR-HPV types not included in the vaccine. Our findings indicated that the bivalent HPV vaccine does not provide cross-protection against non-vaccine HPV types. Prioritizing vaccines with the highest coverage of HR-HPV types, such as the nonavalent HPV vaccine, is crucial to effectively prevent a broader range of HR-HPV infections under the NIP.
KEYWORDS: HPV, vaccine, national immunization program, effectiveness, schoolgirls, adolescents
Introduction
Human papillomavirus (HPV) infection is a significant global concern as it is a leading cause of cervical and anal cancer. Most infections are transmitted through sexual intercourse and can also be spread through skin-to-skin contact and mucous membrane contact, such as oral sex.1 Cervical cancer is commonly found in Thailand, ranking second among women after breast cancer2 and is associated with HPV infection. According to a study by Chinchai et al., in patients with cervical cancer, high-risk HPV (HR-HPV) types are detected in up to 95% of patients.3 The HPV virus has various types, but those associated with cancer include 15 high-risk types4 and among these, Types 16 and 18 are the most common, accounting for approximately 70% of cervical cancer cases.5
Currently, HPV infection can be prevented with high efficacy through vaccination.6 There are several types of vaccines available, such as those that protect against 2 types (bivalent), 16 and 18, or 4 types (quadrivalent), which include 6, 11, 16, and 18 (6 and 11 are low-risk types that cause genital warts). There are also vaccines available that protect against 9 types (nonavalent) (types 6, 11, 16, 18, 31, 33, 45, 52, and 58), and have been widely adopted.7
In 2014, Thailand introduced a two-dose regimen of bivalent HPV vaccines for Grade 5 schoolgirls, approximately 11 years old, initially piloted in Ayutthaya province under a National Immunization Program (NIP). The vaccine has been administered to all female students aged 11–12 or in grade 5 since 2014. This initiative has been expanded to all Grade 5 schoolgirls nationwide since the year 2017. Due to the long interval between HPV infection and the onset of cervical cancer, clinical vaccine trials have used vaccine-type-specific infections or precancerous cervical lesions as substitute endpoints for assessing the efficacy of the HPV vaccine.8 Our earlier research indicated that HPV DNA detection from cervical swab samples or urine exhibited similar results, suggesting urine samples as a viable alternative for HPV DNA detection.9 However, the real-world effectiveness of Thai NIP against HPV infection has not yet been evaluated. Therefore, we conducted a study that aims to evaluate the effectiveness of the HPV vaccine against HPV infection in Thai female students 7 years after receiving the vaccine (Ayutthaya province). The long-term effectiveness data obtained from this study will be beneficial for future HPV vaccination programs under NPI.
Materials and methods
The study protocol was approved by the Research Ethics Committee of the Faculty of Medicine of Chulalongkorn University (IRB number 118/66). Written informed consent (or assent) was obtained from each guardian or participant before enrollment and this cross-sectional study was conducted in accordance with the Declaration of Helsinki.
Population study
The study was conducted in 12th-grade students or third-year vocational school students who are approximately 18 years old in two provinces, which were Ayutthaya and Nakhon Pathom, between May and June 2023. Ayutthaya province was selected because it was the pilot province for administering the HPV vaccine under the NPI to all 5th-grade students in 2014. Seven years later, these 5th-grade students have grown up and are now in 12th-grade or third-year vocational school. Nakhon Pathom province was chosen as the control province due to its geographic similarities to Ayutthaya, but Nakhon Pathom did not administer the vaccine in 2014 and serves as the control province. Nakhon Pathom has similar characteristics in terms of population and occupation to Ayutthaya province (Figure 1), but the 5th-grade school girls in Nakhon Pathom received HPV vaccines according to the NIP in 2017.
Figure 1.
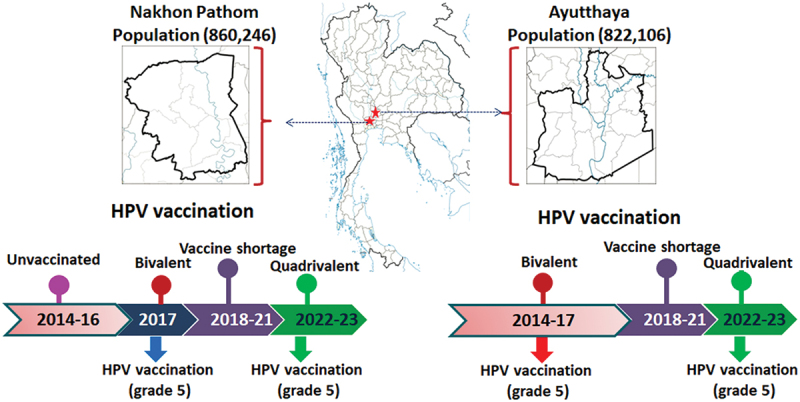
HPV national immunization program in Thailand 2014–2023.
The study design included a case-to-control ratio of 1:2. Therefore, it was planned to collect data from Nakhon Pathom in double the amount of Ayutthaya. The inclusion criteria included 1) students lived and studied in Ayutthaya or Nakhon Pathom province during primary school 2) students in Ayutthaya province reported in the self-administered questionnaire that they had received two doses of HPV vaccines six months apart. All schools are mixed-gender and selected based on convenience sampling. Similarly, students were also selected using convenience sampling. After receiving written consent, urine samples were collected.
Vaccine used in the study group
The vaccine used was bivalent Cervarix HPV vaccine (GlaxoSmithKline Biologicals, Rixensart, Belgium) administered in two doses 6 months apart.
Patient demographics and questionnaire
This self-administered questionnaire labeled with codes instead of names included questions on demographic data and sexual behavior. The first-void urine sample were collected.
Urine collection
Urine samples were collected using a Colli-Pee device (Novosanis, Wijnegem, Belgium) for HPV typing. Collected urine samples were stored at 2–8°C. All samples were processed at the Center of Excellence in Clinical Virology within 48 h after collection. The details of the processing have been reported elsewhere.9
Laboratory testing
HPV detection (cobas 4800)
The 20 mL urine sample was aliquoted 10 mL and centrifuged at 3000 rpm for 10 min. The 9 mL supernatant was discarded and the 1 mL pellet residue was resuspended and used for the Cobas®4800 assay (Roche Molecular Diagnostics, Pleasanton, CA). The Cobas® 4800 system uses automated processes for the extraction of nucleic acids from the sample. The extracted nucleic acids are then subjected to real-time Polymerase Chain Reaction (PCR) amplification and detection of HPV16, HPV18, and aggregated results for 12 other HR-HPV types (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68).9
HPV typing
Extracts from all Cobas-positive DNA were collected for HPV typing using AnyplexTM HPV28 (Seegene, Seoul, South Korea) to individually identify 28 HPV types (HR-HPV16, 18, 26, 31, 33, 35, 39, 45, 51,52, 53, 56, 58, 59, 66, 68, 69, 73, 82, and low-risk (LR) HPV6, 11, 40,42, 43, 44, 54, 61, 70), following the manufacturer’s protocol. The Anyplex™ HPV28 assay identifies multiple HPV types using multiplex real-time PCR technology. Details have been reported elsewhere.10
Statistical analysis
The sample size calculation was based on a study of 878 women aged 20–26 years, with a vaccination rate of 21.4%.11 From the calculation, a minimum of 210 target group samples and 420 control group samples are required. Additionally, approximately 5% more samples will be collected to account for ineligible samples. The odds ratio (OR) and the 95% confidence intervals (CI) were calculated using logistic regression models adjusted for age and sexual behavior to compare the vaccination odds between cases and controls. Vaccine effectiveness (VE) was estimated as (1 - OR) · 100, obtained from an adjusted model including the described covariates, and was expressed as a percentage.12 Statistical analysis was performed using IBM SPSS Statistics v21.0 (IBM Corp., Armonk, NY). Statistical significance was set as a p-value < .05.
Results
Adolescent female students in grade 12, totaling 587 individuals were enrolled. Overall, 211 participants were from Ayutthaya province, who received the HPV vaccine in grade 5, and 376 were from Nakhon Pathom province who did not receive the HPV vaccine. The mean age in years in the vaccinated group is 17.2 ± 0.4, while in the unvaccinted group it is 17.4 ± 0.7, with a statistically significant difference (p < .001). Similarly, sexual experience was reported by 37.4% in the vaccinated group which is significantly less than the unvaccinated group (46.8%; p = .028). Regarding condom usage, 2.5% of the vaccinated group never used condoms compared to 7.9% in the unvaccinated group. This difference in condom usage is not statistically significant (p = .09), as shown in Table 1.
Table 1.
Demographic data of hpv-vaccinated and unvaccinated schoolgirls and the result of HPV detection.
| Vaccinated HPV vaccine (Ayutthaya) N = 211 | Unvaccinated HPV vaccine (Nakhon Pathom) N = 376 | p-value | |
|---|---|---|---|
| Age in years, mean ± SD | 17.2 ± 0.4 | 17.4 ± 0.7 | <0.001a |
| Sexual experience, n (%) | 79 (37.4%) | 176 (46.8%) | 0.028b |
| Sexual debut age in years, mean ± SD | 15.8 ± 1.2 | 16.0 ± 1.1 | 0.232a |
| Condom usagec | 0.09b | ||
| Never used | 2 (2.5%) | 14 (7.9%) | |
| Ever used | 76 (96.2%) | 155 (88.1%) | |
| HPV detection | |||
| 16 | 0 | 3 (0.8%) | |
| 18 | 0 | 4 (1.1%) | |
| 16 + other HR | 0 | 1 (0.3%) | |
| 18 + other HR | 0 | 4 (1.1%) | |
| 16,18 + other HR | 0 | 1 (0.3%) | |
| other HR | 15 (7.1%) | 37 (9.8%) | |
| Total | 15 (7.1%) | 50 (13.3%) |
Abbreviation: HPV, human papillomavirus; HR, high-risk; SD, standard deviation.
aRepresents the mean difference in age between two groups using the independent samples t-test.
bRepresents the association in categorical variables between two groups using the Chi-square test.
cThe percentage of condom use was determined based on individuals with sexual experience.
The infection of HR-HPV types among adolescent female students in the Ayutthaya and Nakhon Pathom provinces is illustrated in Figure 2a. Among students from Ayutthaya province, HPV types 16 and 18 were not detected (Figure 2b), but other types of HR-HPV were found in 15 individuals, accounting for 7.1%. Conversely, among female students in Nakhon Pathom province, 13 individuals (3.5%) were found to have HR-HPV types 16 and 18 (Figure 2c), and 37 individuals (9.8%) were found to have other types of HR-HPV other than 16 and 18 using Anyplex HPV28 for detection. Details are presented in Table 1.
Figure 2.
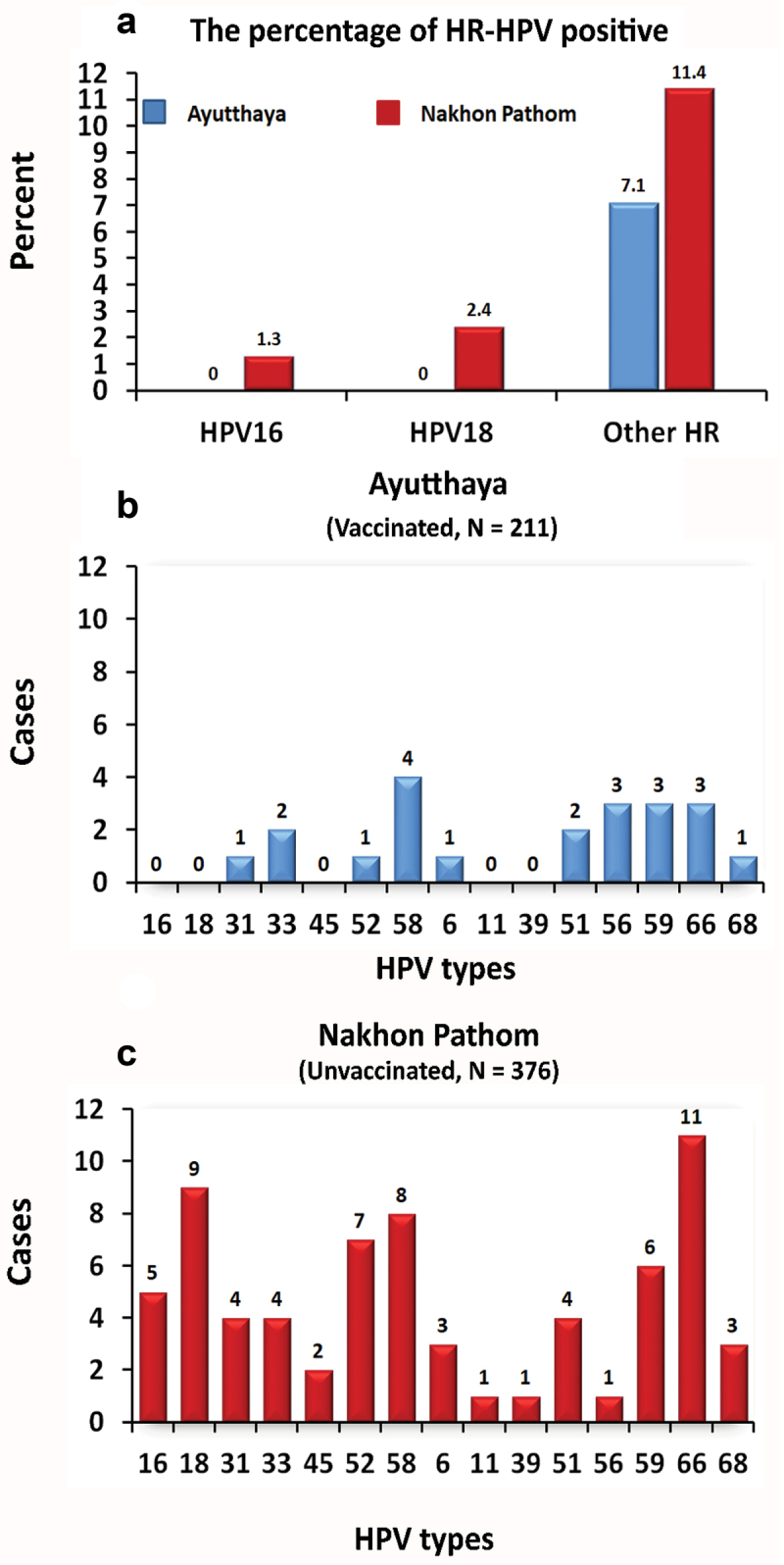
The percentage of HR-HPV-positive samples, in ayutthaya (vaccinated) and Nakhon Pathom (unvaccinated) provinces. (A) The number of hpv-positive samples in (B) vaccinated and (C) unvaccinated participants.
The long-term effectiveness of HPV infection prevention over 7 years in the NIP for vaccine types 16 and 18 was 100% (95% CI ND), while the effectiveness against non-16 or 18 HR-HPV types was 32.8% (95% CI [−26.1] to 64.2) as shown in Table 2. Our study showed that the bivalent HPV vaccine does not provide cross-protection against non-vaccine HPV types.
Table 2.
Comparison of HPV detection among HPV-vaccinated and unvaccinated schoolgirls and estimated vaccine effectiveness.
| Vaccine status | No. of Participants (% HPV Positive)a | Crude OR (95%CI) | Adjusted ORb (95%CI) | VE (%) (95%CI) | p-value |
|---|---|---|---|---|---|
| Odds ratio and VE of bivalent HPV vaccine type against HPV 16 or 18 infection | |||||
| No Vaccination (n = 376) | 13 (3.46%) | 1 (reference) | 1 (reference) | ||
| Vaccination (n = 211) | 0 (0%) | 0 | 0 | 100% | .006 |
| Odds ratio and VE of quadrivalent HPV vaccine type against HPV other types infection | |||||
| No Vaccination (n = 376) | 43 (11.4%) | 1 (reference) | 1 (reference) | ||
| Vaccination (n = 211) | 15 (7.1%) | 0.593 (0.321 to 1.095) | 0.672 (0.358 to 1.261) | 32.8% (−26.1 to 64.2%) | .216 |
Abbreviation: HPV, human papillomavirus; OR, Odds ratio; VE, vaccine effectiveness.
aHPV positive defined as HPV 16, 18 or other types.
bOdds ratio and 95% Confidence Interval (95% CI) were estimated by logistic regression with age and sexual behaviors adjustment.
Discussion
It is well known that HPV, especially high-risk types, is closely associated with the development of cervical cancer in women.13 The types of HPV commonly found in patients with cervical cancer are types 16 and 18, which represent approximately 70% of cases followed by types 52 and 58 in Thailand,3,14 HPV infection is primarily transmitted through sexual contact or close contact; hence, it typically begins during adolescence or when individuals become sexually active.
In a study conducted among unvaccinated adolescent girls in Thailand, 16–18 years old, from Udon Thani and Buriram provinces, the prevalence of HR-HPV infection in Grade 10 and Grade 12 was 8.6% and 12.4%, respectively. The prevalent HPV strains included 16, 58, 51, 52, 66, 18, 56, 39, 59, and 68.10 Similar results were found in Nakhon Pathom, an unvaccinated control province.
In sexually mature women who underwent cervical cancer screening, 6.0% tested positive for HR-HPV types, indicating a high prevalence.15 Although some patients may naturally clear the infection, persistent infections can lead to cell changes and eventually cervical cancer.
The standard method for diagnosing HPV infection involves cervical swabs. However, acceptance of this method can be challenging. Therefore, alternative methods have been developed, such as self-swabbing from the cervix or using first void urine samples. The use of first-void urine samples collected with devices such as “Colli-Pee” has proven to be highly convenient and acceptable for adolescents.9,16 Studies have shown that this method yields convenient, specific, and accurate results, with a sensitivity and specificity of up to 89% and 78%, respectively.9 This study highlights the effectiveness of HPV detection by urine samples and emphasizes its acceptance, particularly among adolescents, over traditional methods such as cervical swabs or self-swabbing.
The HPV vaccine is highly effective in the long-term in preventing specific HPV types covered by the vaccine.17,18 Our study examined the effectiveness of administering a bivalent vaccine under the NIP to fifth-grade schoolgirls, aged approximately 11 years old, and followed-up when these students were in grade 12 or approximately 18 years old, which was 7 years after receiving the vaccine. We compared the vaccinated group with an unvaccinated group of students of the same age. Students in provinces where the vaccine was administered as part of the NPI were found to have 100% effectiveness in preventing HR-HPV types 16 and 18 infections compared to students who did not receive the vaccine. However, children in the province where the vaccine was administered still had a risk of contracting types outside the vaccine types, such as types 31, 33, 52, 58, 51, 56, 59, 66, 68, at an incidence of 7.1% compared to students in provinces that did not receive the vaccine, where 9.8% were found to be at risk. It can be concluded that the effectiveness in preventing HPV infections outside of the types of vaccine in the bivalent vaccine is 32.8%. Thus, protection remains specific to the types included in the HPV vaccine relevant to cervical cancer or high-risk groups, which can encompass up to 15 types controllable for disease prevention. It is necessary to use a vaccine composed of a large number of high-risk types, and currently, the nonavalent vaccine should be used instead of the bivalent vaccine.
A limitation of the study was the small sample size and very few cases of HR-HPV types 16, 18 reported in the unvaccinated group; however, the data obtained demonstrated the effectiveness of administering the vaccine in real world situations. In addition, using self-administered questionnaires labeled with codes instead of names to gather data on sexual behavior and condom use can present several challenges. Firstly, despite the anonymity provided by using codes, respondents might still experience discomfort or fear, leading to underreporting or dishonesty about sensitive behaviors. Secondly, participants might feel less compelled to answer every questions. This could lead to incomplete data.
In summary, the HPV vaccine is highly effective in preventing HPV infections specific to the types included in the vaccine. Therefore, in practice, vaccines with the highest number of HPV high-risk types should be administered to enhance prevention effectiveness. It is also advisable to establish policies for the administration of the HPV vaccine to early adolescents under the NIP to benefit from the reduced incidence of cervical cancer.
Acknowledgments
The authors would like to thank the Thailand Ministry of Public Health study coordinators, Ayutthaya and Nakhon Pathom provincial health officers, staff of the health promotion center, and teachers at participating schools for their dedication, cooperation, and support. The authors acknowledge Yanathep Prasitsomsakul for creating the figure. In addition, the authors sincerely appreciate the staff of Chulalongkorn University laboratory for their support in this study and Education and Public Welfare Foundation CE Chulalongkorn University.
Biography
Yong Poovorawan heads The Center of Excellence in Clinical Virology at Chulalongkorn University, Bangkok. He earned his M.D. in 1974 and specialized in pediatrics in 1978 at Chulalongkorn University. In 1984, he was a Research Fellow in pediatric hepatology at King’s College Hospital, London. He joined Chulalongkorn’s Department of Pediatrics as a lecturer and became a full professor in 1991. Professor Poovorawan received many awards including the Outstanding Researcher Award (1997), Outstanding Scientist Award (1997), Mahidol University-B-Braun Award (2002), and Outstanding Achievement Doctor (2018). He is a leading expert in viral hepatitis, contributing significantly to reducing hepatitis A, B, and C in Thailand. His research also covers emerging viruses like avian influenza and COVID-19. He has led efforts in rapid COVID-19 detection, virus variant characterization, and vaccine immunity studies. He serves on several public health committees and has authored over 650 publications with an h-index of 74 on Google Scholar.
Funding Statement
This research was funded by the National Research Council of Thailand (NRCT), Health Systems Research Institute (HSRI), the Center of Excellence in Clinical Virology at Chulalongkorn University, King Chulalongkorn Memorial Hospital, the MK Restaurant Group and Aunt Thongkham Foundation, the Department of Disease Control (DDC) and the Education and Public Welfare Foundation. Additionally, Pornjarim Nilyanimit received support from the Second Century Fund (C2F) fellowship at Chulalongkorn University.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
All authors were involved in the conception and design of the study. RA, TT, SS, DP, NN collected specimens and data. PV, PN performed the laboratory tests. NS, PN analyzed the data. PN, NS, YP drafted manuscript. All authors critically revised the manuscript for intellectual content and approved the version to be published. All authors agree to be accountable for all aspects of the work.
Short biographical
Data availability statement
Due to the nature of the research and to ethical and legal restrictions, supporting data is not available.
References
- 1.Genital HPV Infection—Fact Sheet. Centers for Disease Control and Prevention . 2019. [accessed 2022 Apr 12] https://www.cdc.gov/std/hpv/stdfact-hpv.htm.
- 2.National Cancer Institute . Hospital-based cancer registry 2021 [internet]. Bangkok (BK): National Cancer Institute; c2022. [accessed 2023 June 1]. https://www.nci.go.th/e_book/hosbased_2564/index.html. [Google Scholar]
- 3.Chinchai T, Chansaenroj J, Swangvaree S, Junyangdikul P, Poovorawan Y.. Prevalence of human papillomavirus genotypes in cervical cancer. Int J Gynecol Cancer. 2012. Jul; 22(6):1063–6. doi: 10.1097/IGC.0b013e318259d904. [DOI] [PubMed] [Google Scholar]
- 4.Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, Snijders PJ, Meijer CJ, International Agency for Research on Cancer Multicenter Cervical Cancer Study Group . Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003 Feb 6; 348(6):518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 5.Clifford GM, Smith JS, Plummer M, Muñoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003 Jan 13; 88(1):63–73. doi: 10.1038/sj.bjc.6600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsagué X, Skinner SR, Apter D, Naud P, Salmerón J, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012. Jan. 13; (1):89–99. doi: 10.1016/S1470-2045(11)70286-8. [DOI] [PubMed] [Google Scholar]
- 7.Bergman H, Buckley BS, Villanueva G, Petkovic J, Garritty C, Lutje V, Riveros-Balta AX, Low N, Henschke N. Comparison of different human papillomavirus (HPV) vaccine types and dose schedules for prevention of hpv-related disease in females and males. Cochrane Database Syst Rev. 2019 Nov 22; 2019(11):CD013479. doi: 10.1002/14651858.CD013479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romanowski B, de Borba PC, Naud PS, Roteli-Martins CM, De Carvalho NS, Teixeira JC, Aoki F, Ramjattan B, Shier RM, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet. 2009 Dec 12; 374(9706):1975–85. doi: 10.1016/S0140-6736(09)61567-1. [DOI] [PubMed] [Google Scholar]
- 9.Nilyanimit P, Chaithongwongwatthana S, Oranratanaphan S, Poudyal N, Excler JL, Lynch J, Vongpunsawad S, Poovorawan Y. Comparable detection of HPV using real-time PCR in paired cervical samples and concentrated first-stream urine collected with colli-pee device. Diagn Microbiol Infect Dis. 2024. Mar. 108(3):116160. doi: 10.1016/j.diagmicrobio.2023.116160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vongpunsawad S, Rhee C, Nilyanimit P, Poudyal N, Jiamsiri S, Ahn HS, Lee J, Seo HW, Klinsupa W, Park S, et al. Prevalence of HPV infection among Thai schoolgirls in the north-eastern provinces in 2018: implications for HPV immunization policy. IJID Reg. 2023 Mar 4; 7:110–15. doi: 10.1016/j.ijregi.2023.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo F, Hirth JM, Berenson AB. Comparison of HPV prevalence between hpv-vaccinated and non-vaccinated young adult women (20-26 years). Hum Vaccin Immunother. 2015;11(10):2337–44. doi: 10.1080/21645515.2015.1066948. PMID: 26376014; PMCID: PMC4635939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Florentino PTV, Alves FJO, Cerqueira-Silva T, Oliveira VA, Júnior JBS, Jantsch AG, Penna GO, Boaventura V, Werneck GL, Rodrigues LC, et al. Vaccine effectiveness of CoronaVac against COVID-19 among children in Brazil during the omicron period. Nat Commun. 2022 Aug 13; 13(1):4756. doi: 10.1038/s41467-022-32524-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003. Jan. 16(1):1–17. doi: 10.1128/CMR.16.1.1-17.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chansaenroj J, Junyangdikul P, Chinchai T, Swangvaree S, Karalak A, Gemma N, Poovorawan Y. Large scale study of HPV genotypes in cervical cancer and different cytological cervical specimens in Thailand. J Med Virol. 2014. Apr. 86(4):601–7. doi: 10.1002/jmv.23769. [DOI] [PubMed] [Google Scholar]
- 15.Lindau ST, Drum ML, Gaumer E, Surawska H, Jordan JA. Prevalence of high-risk human papillomavirus among older women. Obstet Gynecol. 2008. Nov. 112(5):979–89. doi: 10.1097/AOG.0b013e31818b0df2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Pauw H, Donders G, Weyers S, De Sutter P, Doyen J, Tjalma WAA, Vanden Broeck D, Peeters E, Van Keer S, Vorsters A, et al. Cervical cancer screening using HPV tests on self-samples: attitudes and preferences of women participating in the VALHUDES study. Arch Public Health. [2021 Aug 30]. 79(1):155. doi: 10.1186/s13690-021-00667-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skinner SR, Apter D, De Carvalho N, Harper DM, Konno R, Paavonen J, Romanowski B, Roteli-Martins C, Burlet N, Mihalyi A, et al. Human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine for the prevention of cervical cancer and hpv-related diseases. Expert Rev Vaccines. 2016;15(3):367–87. doi: 10.1586/14760584.2016.1124763. [DOI] [PubMed] [Google Scholar]
- 18.Wheeler CM, Castellsagué X, Garland SM, Szarewski A, Paavonen J, Naud P, Salmerón J, Chow SN, Apter D, Kitchener H, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012. Jan. 13; (1):100–10. doi: 10.1016/S1470-2045(11)70287-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to the nature of the research and to ethical and legal restrictions, supporting data is not available.


