Abstract
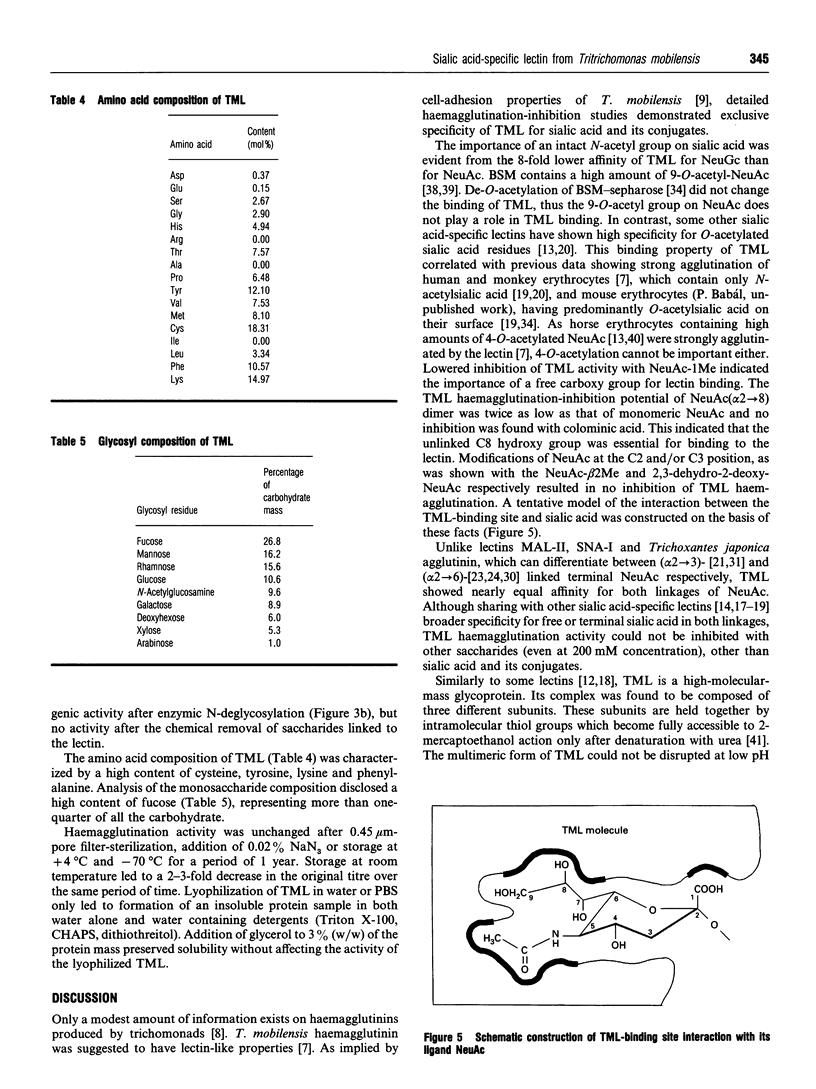
New sialic acid-specific lectin has been isolated from culture supernatant of the protozoan Tritrichomonas mobilensis. It was purified by adsorption by erythrocytes or bovine submaxillary gland mucin (BSM)-Sepharose affinity chromatography. The T. mobilensis lectin (TML) does not require bivalent cations for activity and agglutinates all human erythrocytes. The lectin forms multimeric complexes with molecular mass 556 and 491 kDa as determined by size-exclusion chromatography. SDS/PAGE under reducing conditions disclosed a large band of 343 kDa and three bands of 246, 265 and 286 kDa which, after denaturation with urea, were split into three subunits of 56, 61 and 66 kDa; under non-reducing conditions there were two bands, of 360 and 260 kDa. Western blots performed with anti-TML monoclonal antibodies revealed bands identical with those in the silver-stained gels, suggesting homogeneity of the BSM -Sepharose-purified lectin. TML is a highly glycosylated protein with approx. 8% of N-linked glycosides found by protein-N-glycanase F treatment; the total amount of saccharides revealed by chemical deglycosylation was 20%. Haemagglutination-inhibition studies documented exclusive specificity for sialic acid (NeuAc). Both (alpha 2-->6)- and (alpha 2-->3)-linked and free NeuAc were eight times more potent inhibitors than N-glycolylneuraminic acid. The lectin does not require O-acetyl groups on NeuAc for recognition. A spectrum of mono- and oligo-saccharides other than sialic acid had no inhibitory effect at 200 mM. Anti-TML monoclonal antibodies strongly inhibited the lectin activity. TML was stable at temperatures below 4 degrees C and lyophilized with 3% (w/w) glycerol.
Full text
PDF



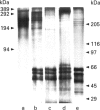
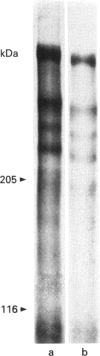
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed H., Chatterjee B. P., Kelm S., Schauer R. Purification of a sialic acid-specific lectin from the Indian scorpion Heterometrus granulomanus. Biol Chem Hoppe Seyler. 1986 Jun;367(6):501–506. doi: 10.1515/bchm3.1986.367.1.501. [DOI] [PubMed] [Google Scholar]
- Ahmed H., Gabius H. J. Purification and properties of a Ca2+-independent sialic acid-binding lectin from human placenta with preferential affinity to O-acetylsialic acids. J Biol Chem. 1989 Nov 5;264(31):18673–18678. [PubMed] [Google Scholar]
- Basu S., Sarkar M., Mandal C. A single step purification of a sialic acid binding lectin (AchatininH) from Achatina fulica snail. Mol Cell Biochem. 1986 Aug;71(2):149–157. doi: 10.1007/BF00214774. [DOI] [PubMed] [Google Scholar]
- Berg E. L., Robinson M. K., Mansson O., Butcher E. C., Magnani J. L. A carbohydrate domain common to both sialyl Le(a) and sialyl Le(X) is recognized by the endothelial cell leukocyte adhesion molecule ELAM-1. J Biol Chem. 1991 Aug 15;266(23):14869–14872. [PubMed] [Google Scholar]
- Brossmer R., Wagner M., Fischer E. Specificity of the sialic acid-binding lectin from the snail Cepaea hortensis. J Biol Chem. 1992 May 5;267(13):8752–8756. [PubMed] [Google Scholar]
- Buscher H. P., Casals-Stenzel J., Schaufer R. New sialic acids. Identification of N-glycoloyl-O-acetylneuraminic acids and N-acetyl-O-glycoloylneuraminic acids by improved methods for detection of N-acyl and O-acyl groups and by gas-liquid chromatography. Eur J Biochem. 1974 Dec 16;50(1):71–82. doi: 10.1111/j.1432-1033.1974.tb03873.x. [DOI] [PubMed] [Google Scholar]
- Danguy A., Kiss R., Pasteels J. L. Lectins in histochemistry. A survey. Biol Struct Morphog. 1988;1(3):93–106. [PubMed] [Google Scholar]
- Demes P., Pindak F. F., Wells D. J., Gardner W. A., Jr Adherence and surface properties of Tritrichomonas mobilensis, an intestinal parasite of the squirrel monkey. Parasitol Res. 1989;75(8):589–594. doi: 10.1007/BF00930953. [DOI] [PubMed] [Google Scholar]
- Erbe D. V., Watson S. R., Presta L. G., Wolitzky B. A., Foxall C., Brandley B. K., Lasky L. A. P- and E-selectin use common sites for carbohydrate ligand recognition and cell adhesion. J Cell Biol. 1993 Mar;120(5):1227–1235. doi: 10.1083/jcb.120.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farthing M. J., Pereira M. E., Keusch G. T. Description and characterization of a surface lectin from Giardia lamblia. Infect Immun. 1986 Feb;51(2):661–667. doi: 10.1128/iai.51.2.661-667.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa H. H., Paulson J. C. Sialylation of glycoprotein oligosaccharides with N-acetyl-, N-glycolyl-, and N-O-diacetylneuraminic acids. J Biol Chem. 1985 Jul 25;260(15):8838–8849. [PubMed] [Google Scholar]
- Knibbs R. N., Goldstein I. J., Ratcliffe R. M., Shibuya N. Characterization of the carbohydrate binding specificity of the leukoagglutinating lectin from Maackia amurensis. Comparison with other sialic acid-specific lectins. J Biol Chem. 1991 Jan 5;266(1):83–88. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marchialonis J. J., Edelman G. M. Isolation and characterization of a hemagglutinin from Limulus polyphemus. J Mol Biol. 1968 Mar 14;32(2):453–465. doi: 10.1016/0022-2836(68)90022-3. [DOI] [PubMed] [Google Scholar]
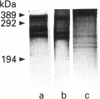
- McKenzie G. H., Sawyer W. H., Nichol L. W. The molecular weight and stability of concanavalin A. Biochim Biophys Acta. 1972 Apr 15;263(2):283–293. doi: 10.1016/0005-2795(72)90081-5. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Collawn J. F., Jr, Fish W. W. Purification and macromolecular properties of a sialic acid-specific lectin from the slug Limax flavus. J Biol Chem. 1982 Jul 10;257(13):7574–7580. [PubMed] [Google Scholar]
- Nagata Y., Burger M. M. Wheat germ agglutinin. Molecular characteristics and specificity for sugar binding. J Biol Chem. 1974 May 25;249(10):3116–3122. [PubMed] [Google Scholar]
- Nitta K., Takayanagi G., Kawauchi H., Hakomori S. Isolation and characterization of Rana catesbeiana lectin and demonstration of the lectin-binding glycoprotein of rodent and human tumor cell membranes. Cancer Res. 1987 Sep 15;47(18):4877–4883. [PubMed] [Google Scholar]
- Peumans W. J., Kellens J. T., Allen A. K., Van Damme E. J. Isolation and characterization of a seed lectin from elderberry (Sambucus nigra L.) and its relationship to the bark lectins. Carbohydr Res. 1991 Jun 25;213:7–17. doi: 10.1016/s0008-6215(00)90593-7. [DOI] [PubMed] [Google Scholar]
- Pindak F. F., Gardner W. A., Jr, Mora de Pindak M., Abee C. R. Detection of hemagglutinins in cultures of squirrel monkey intestinal trichomonads. J Clin Microbiol. 1987 Apr;25(4):609–614. doi: 10.1128/jcm.25.4.609-614.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pindak F. F., Mora de Pindak M., Gardner W. A., Jr, Abee C. R. Basic properties of Tritrichomonas mobilensis hemagglutinin. J Clin Microbiol. 1988 Aug;26(8):1460–1463. doi: 10.1128/jcm.26.8.1460-1463.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin J. I., Murphy C. F., Salata R. A., Guerrant R. L., Hewlett E. L. N-Acetyl-D-galactosamine-inhibitable adherence lectin of Entamoeba histolytica. I. Partial purification and relation to amoebic virulence in vitro. J Infect Dis. 1985 May;151(5):804–815. doi: 10.1093/infdis/151.5.804. [DOI] [PubMed] [Google Scholar]
- Ravindranath M. H., Higa H. H., Cooper E. L., Paulson J. C. Purification and characterization of an O-acetylsialic acid-specific lectin from a marine crab Cancer antennarius. J Biol Chem. 1985 Jul 25;260(15):8850–8856. [PubMed] [Google Scholar]
- Sakakibara F., Kawauchi H., Takayanagi G., Ise H. Egg lectin of Rana japonica and its receptor glycoprotein of Ehrlich tumor cells. Cancer Res. 1979 Apr;39(4):1347–1352. [PubMed] [Google Scholar]
- Sarris A. H., Palade G. E. The sialoglycoproteins of murine erythrocyte ghosts. A modified periodic acid-Schiff stain procedure staining nonsubstituted and O-acetylated sialyl residues on glycopeptides. J Biol Chem. 1979 Jul 25;254(14):6724–6731. [PubMed] [Google Scholar]
- Sata T., Lackie P. M., Taatjes D. J., Peumans W., Roth J. Detection of the Neu5 Ac (alpha 2,3) Gal (beta 1,4) GlcNAc sequence with the leukoagglutinin from Maackia amurensis: light and electron microscopic demonstration of differential tissue expression of terminal sialic acid in alpha 2,3- and alpha 2,6-linkage. J Histochem Cytochem. 1989 Nov;37(11):1577–1588. doi: 10.1177/37.11.2478613. [DOI] [PubMed] [Google Scholar]
- Schauer R. Chemistry, metabolism, and biological functions of sialic acids. Adv Carbohydr Chem Biochem. 1982;40:131–234. doi: 10.1016/s0065-2318(08)60109-2. [DOI] [PubMed] [Google Scholar]
- Shibuya N., Goldstein I. J., Broekaert W. F., Nsimba-Lubaki M., Peeters B., Peumans W. J. Fractionation of sialylated oligosaccharides, glycopeptides, and glycoproteins on immobilized elderberry (Sambucus nigra L.) bark lectin. Arch Biochem Biophys. 1987 Apr;254(1):1–8. doi: 10.1016/0003-9861(87)90074-9. [DOI] [PubMed] [Google Scholar]
- Shibuya N., Goldstein I. J., Broekaert W. F., Nsimba-Lubaki M., Peeters B., Peumans W. J. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(alpha 2-6)Gal/GalNAc sequence. J Biol Chem. 1987 Feb 5;262(4):1596–1601. [PubMed] [Google Scholar]
- Sojar H. T., Bahl O. P. Chemical deglycosylation of glycoproteins. Methods Enzymol. 1987;138:341–350. doi: 10.1016/0076-6879(87)38029-2. [DOI] [PubMed] [Google Scholar]
- Spicer S. S., Schulte B. A. Diversity of cell glycoconjugates shown histochemically: a perspective. J Histochem Cytochem. 1992 Jan;40(1):1–38. doi: 10.1177/40.1.1370305. [DOI] [PubMed] [Google Scholar]
- Taatjes D. J., Roth J., Peumans W., Goldstein I. J. Elderberry bark lectin--gold techniques for the detection of Neu5Ac (alpha 2,6) Gal/GalNAc sequences: applications and limitations. Histochem J. 1988 Sep;20(9):478–490. doi: 10.1007/BF01002646. [DOI] [PubMed] [Google Scholar]
- Tannich E., Ebert F., Horstmann R. D. Primary structure of the 170-kDa surface lectin of pathogenic Entamoeba histolytica. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1849–1853. doi: 10.1073/pnas.88.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomino Y., Inoue W., Watanabe S., Yagame M., Eguchi K., Nomoto Y., Sakai H. Detection of glomerular sialic acids in patients with diabetic nephropathy. Am J Nephrol. 1988;8(1):21–26. doi: 10.1159/000167548. [DOI] [PubMed] [Google Scholar]
- Varki A. Diversity in the sialic acids. Glycobiology. 1992 Feb;2(1):25–40. doi: 10.1093/glycob/2.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. A. The use of lectins in histopathology. Pathol Res Pract. 1989 Dec;185(6):826–835. doi: 10.1016/s0344-0338(89)80282-1. [DOI] [PubMed] [Google Scholar]
- Wang W. C., Cummings R. D. The immobilized leukoagglutinin from the seeds of Maackia amurensis binds with high affinity to complex-type Asn-linked oligosaccharides containing terminal sialic acid-linked alpha-2,3 to penultimate galactose residues. J Biol Chem. 1988 Apr 5;263(10):4576–4585. [PubMed] [Google Scholar]
- Ward H. D., Keusch G. T., Pereira M. E. Induction of a phosphomannosyl binding lectin activity in Giardia. Bioessays. 1990 May;12(5):211–215. doi: 10.1002/bies.950120504. [DOI] [PubMed] [Google Scholar]
- Ward H. D., Lev B. I., Kane A. V., Keusch G. T., Pereira M. E. Identification and characterization of taglin, a mannose 6-phosphate binding, trypsin-activated lectin from Giardia lamblia. Biochemistry. 1987 Dec 29;26(26):8669–8675. doi: 10.1021/bi00400a027. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Umetsu K., Suzuki T., Ohkura T. Purification and characterization of a Neu5Ac alpha 2-->6Gal beta 1-->4GlcNAc and HSO3(-)-->6Gal beta 1-->GlcNAc specific lectin in tuberous roots of Trichosanthes japonica. Biochemistry. 1992 Nov 24;31(46):11647–11650. doi: 10.1021/bi00161a052. [DOI] [PubMed] [Google Scholar]