Abstract
Using density functional theory, we have studied the intrinsic properties of styrene. First, we determine the optimized structures, structural parameters and thermodynamic properties to make our simulations more realistic to experimental results and check the stability. Second, we investigate optoelectronic, electronic and global descriptors, transport properties of holes and electrons, natural bond orbital analysis, absorption and fluorescence properties. Finally, we study nonlinear optical (NLO) properties: first- and second-order hyperpolarizability, second and third-order optical susceptibilities, hyper-Rayleigh scattering hyperpolarizability, electro-optical Pockel effect, direct current Kerr effects and quadratic refractive index. The bandgap energy E g = 5.146 eV and dielectric constant show that styrene is a good insulator with an average electric field value of 4.43 × 108 Vm−1. Thermodynamic findings show that our molecule is thermodynamically and chemically stable. Electron and hole reorganization energies of 0.393 and 0.295 eV, respectively, show that styrene is more favourable to hole transport than electron transport. Styrene is transparent with linear refractive index n = 1.750 and quadratic n 2 = 1.748 × 10−20 m2 W−1. At the NLO, styrene has a non-zero value of which confirms the existence of first-order NLO activity. Globally the study shows that the styrene monomer is suitable for the architecture design of new polymer materials for NLO applications and optoelectronic by functionalization.
Keywords: styrene, DFT, functionalization, NLO, insulator, NBO
1. Introduction
Computation now plays a key role in materials innovation. It facilitates our understanding and design of materials, providing a comprehensive overview of their behaviour, from the Angstrom to the micrometre scale, and from the femtosecond to the millisecond [1,2]. For the understanding, prediction and design of materials, the density functional theory (DFT) method, which is an electronic structure method, can be used to predict with very good accuracy: (i) the fundamental properties of materials from their smallest constituents: atoms, unit cells and chemical bonds between atoms; and (ii) ground-state properties (ground-state energy and its derivatives, thermodynamics) as well as excited-state properties (optical absorption and emission) [1,3–5]. DFT is currently a mature and widely used method for material simulations [3,6–8]. With regards to the development of research into complex and non-complex materials, like nanocages [9], simulations are essential, as they enable materials to be assessed under pressure and temperature conditions that are difficult to access experimentally. They also make it possible to directly identify microscopic causes, as well as the origin of a macroscopic property [3]. Finally, one of the main advantages of simulations is that they can be reproduced, opened up and shared [3,10]. More recently, researchers have been experimenting with the use of machine learning to identify the descriptors that influence the performance of organic solar cells (OSCs) to design new small donor molecules suitable for improving OSCs performance [11,12].
The development and understanding of organic semiconductors have positioned this class of materials as the new electronics revolution of the early twenty-first century. Their applications span diverse fields, including flexible light sources, display devices, low-cost printed integrated circuits and plastic solar cells [13]. Organic semiconductors have led to applications in optoelectronic devices such as organic light-emitting diodes (OLEDs), organic field-effect transistors and organic photovoltaic solar cells [14–17]. In the field of nonlinear optical (NLO) properties, organic materials and organic semiconductors have several applications such as NLO devices, memory storage devices [18–20], optical communication, optical switching, laser technologies, dynamic image processing and optical computing [21–23]. In addition, organic materials offer numerous advantages over inorganic materials, such as high laser damage thresholds, high NLO susceptibilities, reduced dielectric constants, a wide transparency range, ultrafast response times and easier processing in the front line of NLO research [24,25]. In the same vein, some small organic compounds can also be used as models for the design of supramolecular systems, molecular logic gates, controllable switches and sensors, as well as in nanotechnology and signal processing [5,26–28].
In recent years, the design of new materials with high NLO response and good optoelectronic, transport and electronic properties has become the focus of most scientists and a major research topic worldwide. Many approaches have been developed including [29–32], the planar donor π-conjugated bridge acceptor (D-π-A) model, modification of the D-π-A structure [33,34], the use of halogen doping [32,35], alkali and super alkali metal doping [24], as well as organic dopants [36], organometallic design and complex formation [37], functionalization [38,39] and so on. One of the best approaches to designing this type of material is functionalization, an important process for modifying the physical and chemical properties of organic materials [40]. According to several researchers [2,4,5,38], functionalizing carbon chains is a suitable way of enhancing the electronic and optical properties of organic semiconductor compounds such as chromophores. According to Stadtmüller et al. [41] , chemical functionalization provides interesting possibilities for exploiting the tunability of structural and electronic properties of organic materials, which could lead to a new class of functional materials with applications in electronics and spintronics. In addition, functionalization serves as a powerful tool for designing new polymers and constructing macromolecular structures with predictable architectures from small organic molecules [42]. While several polymers are commonly recognized for their use in functionalizing organic materials for optical and optoelectronic applications, including polymethyl methacrylate (PMMA), polystyrene (PS) and polyacrylamide [38,42,43]. Our previous work focused on the study of methyl methacrylate (MMA) for optoelectronic and optical applications [4]. In this work, we are focusing on styrene monomer, which presents a credible alternative to MMA.
Styrene was first isolated in 1839 [44] and is one of the most widely used monomers with a variety of applications in the chemical industry to produce PS, acrylonitrile-butadiene-styrene rubber and many other polymers [45]. The advantages of styrene monomer and its derivatives that make them attractive compounds compared with other polymers are low cost and low density, durability and good resistance during processing, ease of processing and moulding, as well as other special PS characteristics, such as low moisture absorption and transparency. Indeed, the refractive index at 25°C of styrene monomer is n = 1.544, while PS offers high brightness with n = 1.592 and high transmission of all visible light wavelengths [46,47].
Nowadays, styrene monomer continues to be the focus of investigation for many research teams around the world owing to its numerous applications and uses [48,49]. PS and styrene monomers are widely used in various industries and are topical in many areas of research such as new effective commercial stabilizers [50], composite science and technology [51] for the manufacture and functionalization of transparent wood [52]. They are also used in the rubber industry [53], in electronics such as vacuum cleaners and telephone housings [47], in the food packaging industry [46,54,55], in ZnO/PS nanocomposites, in vinyl ester [52], in electronic devices [46,52] and in the design and synthesis of new organic semiconductors and optical materials [56].
In the midst of all these multiple and current applications of styrene monomer, we focus in this work, on the use of styrene monomer for the design of organic semiconductors, display devices, transparent and flexible electronics and NLO materials. In this respect, a prior understanding of the intrinsic behaviour of styrene monomers is essential to improve the quality of the design of new materials through styrene functionalization. Furthermore, the intrinsic behaviour of styrene monomer in terms of optics, electronics, thermodynamics and charge transport, before chemical reactions with other compounds, is not sufficiently documented in the literature, which in our view constitutes a lack of information that needs to be addressed. Indeed, to date, no theoretical studies were carried out on the determination of structural, electronic, optoelectronic, linear and NLO, thermodynamic, absorption and emission properties, as well as chemical reactivity descriptors, charge transfer and natural bond orbital (NBO) analysis of styrene monomer.
Therefore, this work aims to perform a DFT study of the electronic structure of styrene monomers. To this end, we will determine the above-mentioned electro-optical properties of styrene and highlight the advantages of styrene monomer over MMA monomer in the design of optoelectronic and optical devices through functionalization.
2. Methodology and computational details
Linear optical properties of styrene monomer are evaluated through physical parameters such as dipole moment , average polarizability ( ) and anisotropy (Δα) and first-order susceptibility ( ), which are given by the following equations:
| (2.1) |
| (2.2) |
| (2.3) |
| (2.4) |
For the characterization of the NLO behaviour, we calculate the first total hyperpolarizability, and the averaged second hyperpolarizability, of the monomer using the following equations:
| (2.5) |
| (2.6) |
where , and are given by
| (2.7) |
Regarding the electronic behaviour of styrene, the adiabatic ionization potential (IP) and adiabatic electron affinity (EA) are important parameters for describing charge transport processes and molecular chemical stability. They are obtained from the following relationships [57–62]:
| (2.8) |
| (2.9) |
where are the energies of the molecule, obtained after optimization of its cation and anion states respectively. is the energy of the neutral compound taken in the ground state at the end of the optimization. The bandgap energy was calculated as follows:
| (2.10) |
where is the energy of the highest occupied molecular orbital and the energy of the lowest unoccupied molecular orbital.
Optoelectronic properties of styrene monomer such as electric displacement (D), electric field in the material (E), relative permittivity of the material ( ), induced polarization (P) and refractive index (n) were calculated using equations available in the literature [5,63]:
| (2.11) |
| (2.12) |
In addition, the relative permittivity and the dielectric constant are given by and . The refractive index is obtained using equation .
Time-dependent DFT (TD-DFT) was used to study the excited states of styrene monomer including absorption and emission spectra, while DFT was used for the other properties. GaussView 6.0.16 software [64] was used for modelling and data visualization, while Gaussian 16 software [65] was used for all atomistic calculations, such as structural, thermodynamic, electronic, optoelectronic and NLO properties, as well as chemical reactivity descriptors, NBO and charge transport of styrene monomer. All calculations were performed in the gas phase at room temperature and standard pressure. Two functionals, B3LYP and ωB97XD were used. According to the literature, the B3LYP functional is suitable for studying the electronic, transport, NBO and thermodynamic properties of organic molecules [66–69]. Meanwhile, the ωB97XD functional is a corrected long-range hybrid functional that enables precise assessment of the optical and chemical quantum properties of organic molecules, as well as more realistic studies of excited states [10,35,66,70,71]. In this work, the ωB97XD functional will be used as a reference for the prediction of NLO properties, while the B3LYP will be the reference for the characterization of electronic, optoelectronic and transport properties. The basis set 6−311G(d,p) was used for all the calculations.
3. Results and discussion
3.1. Optimized structures
The optimized structures using ωB97XD and B3LYP functionals of our styrene monomer are shown in figure 1. No negative frequencies were observed after the optimizations, which leads to the existence of local minima on the potential energy surface at the end of the optimizations [72] and therefore, the optimized styrene monomer is geometrically stable.
Figure 1.

Optimized structures of the styrene monomer obtained using B97XD and B3LYP functionals.
It can be seen from figure 1 that the only and main difference between optimization with the ωB97XD and B3LYP methods is at the C3−C4 bond. Indeed, optimization using ωB97XD leads to a C=C double bond between C3 and C4, while a delocalized bond is obtained using the B3LYP method.
All bond lengths and some selected valence angles of styrene monomer are collected in table 1. The structural parameters of styrene monomer were determined experimentally at 87 K in 2001 by Yasuda et al. [73].
Table 1.
Bond lengths (in Å) and some selected angles (in °) of styrene monomer.
| bond lengths (Å) | angles (°) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| designation | B3LYP | ωB97XD | EXP a | ΔB3LYP | ΔωB97XD | designation | B3LYP | ωB97XD | EXP a |
| C1–C2 | 1.392 | 1.388 | 1.388 | 0.003 | 0.000 | C2–C1–C6 | 120.04 | 120.027 | 120.077 |
| C1–C6 | 1.392 | 1.389 | 1.390 | 0.001 | −0.001 | C2–C1–H7 | 120.151 | 120.01 | 120.375 |
| C1–H7 | 1.084 | 1.084 | 0.991 | 0.094 | 0.094 | C6–C1–H7 | 119.808 | 119.873 | 119.544 |
| C2–C3 | 1.396 | 1.391 | 1.392 | 0.003 | −0.001 | C1–C2–C3 | 119.453 | 119.511 | 119.432 |
| C2–H8 | 1.084 | 1.084 | 1.009 | 0.075 | 0.075 | C1–C2–H8 | 120.321 | 120.264 | 120.529 |
| C3=C4 | 1.389 | 1.386 | 1.388 | 0.001 | −0.001 | C3–C2–H8 | 120.226 | 120.224 | 120.025 |
| C3–H9 | 1.084 | 1.084 | 0.974 | 0.112 | 0.112 | C2–-C3–C4 | 120.423 | 120.355 | 120.483 |
| C4–C5 | 1.405 | 1.399 | 1.402 | 0.002 | −0.002 | C2–C3–H9 | 119.92 | 119.938 | 120.677 |
| C4–H10 | 1.084 | 1.084 | 0.983 | 0.102 | 0.102 | C4–C3–H9 | 119.657 | 119.705 | 118.840 |
| C5–C6 | 1.403 | 1.397 | 1.395 | 0.006 | 0.001 | C3–C4–C5 | 120.899 | 120.802 | 120.571 |
| C5–C12 | 1.472 | 1.474 | 1.474 | −0.001 | 0.000 | C3–C4–H10 | 119.222 | 119.348 | 119.051 |
| C6–H11 | 1.085 | 1.086 | 0.989 | 0.097 | 0.098 | C5–C12=C14 | 127.6628 | 126.817 | 126.790 |
| C12–H13 | 1.088 | 1.088 | 0.981 | 0.109 | 0.109 | H13–C12=C14 | 117.9266 | 118.3775 | 117.868 |
| C12=C14 | 1.335 | 1.330 | 1.325 | 0.008 | 0.004 | C12=C14 H15 | 122.8434 | 122.5279 | 123.381 |
| C14–H15 | 1.084 | 1.085 | 0.967 | 0.121 | 0.122 | C12=C14 H16 | 120.8199 | 120.819 | 119.975 |
| C14–H16 | 1.083 | 1.083 | 0.989 | 0.095 | 0.095 | H15–C14–H16 | 116.3366 | 116.6517 | 116.549 |
Experimental values from Yasuda et al. [73].
We found good agreement by comparing our DFT findings with experimental results. Indeed, as shown in table 1, the B3LYP and ωB97XD functionals give the same values for many bond lengths and angles. Our DFT methods are precise for the determination of carbon–carbon bond lengths and angles, while we have some small discrepancies on the evaluation of C−H bond length.
ωB97XD functional is slightly more precise than B3LYP for some bonds and angles. Moreover, the maximum difference between the present theoretical values and the experimental values does not exceed 0.13 whatever the functional used. Furthermore, we can conclude that the functionals and basis sets used for this study are well chosen, and that the results obtained may be more realistic at the end of the atomistic simulations.
It appears from the values in table 2 that the styrene monomer exhibits a planar structure relative to the B3LYP and ωB97XD optimizations. All atoms and bonds are predominantly present in the (x,y) plane.
Table 2.
Cartesians coordinates (in Å) of atoms of styrene monomer.
| designation | B3LYP | ωB97XD | ||||
|---|---|---|---|---|---|---|
| coordinates | x | y | z | x | y | z |
| C1 | −1.778 | −1.044 | 0.000 | −1.770 | −1.042 | 0.049 |
| C2 | −2.261 | 0.261 | 0.000 | −2.252 | 0.261 | 0.040 |
| C3 | −1.360 | 1.327 | 0.000 | −1.355 | 1.322 | −0.022 |
| C4 | 0.009 | 1.090 | 0.000 | 0.010 | 1.085 | −0.068 |
| C5 | 0.514 | −0.220 | 0.000 | 0.511 | −0.221 | −0.048 |
| C6 | −0.406 | −1.279 | 0.000 | −0.403 | −1.277 | 0.002 |
| H7 | −2.468 | −1.880 | 0.000 | −2.461 | −1.876 | 0.091 |
| H8 | −3.329 | 0.449 | 0.000 | −3.319 | 0.449 | 0.074 |
| H9 | −1.728 | 2.347 | 0.000 | −1.724 | 2.342 | −0.041 |
| H10 | 0.691 | 1.932 | 0.000 | 0.694 | 1.924 | −0.133 |
| H11 | −0.036 | −2.299 | 0.000 | −0.032 | −2.297 | 0.010 |
| C12 | 1.953 | −0.528 | 0.000 | 1.953 | −0.523 | −0.080 |
| H13 | 2.186 | −1.591 | 0.000 | 2.197 | −1.568 | −0.265 |
| C14 | 2.972 | 0.335 | 0.000 | 2.952 | 0.335 | 0.108 |
| H15 | 2.832 | 1.410 | 0.000 | 2.786 | 1.386 | 0.320 |
| H16 | 3.997 | −0.016 | 0.000 | 3.983 | 0.005 | 0.063 |
3.2. Optoelectronic properties
Determining and interpreting the optoelectronic properties of styrene monomer is important for understanding the optoelectronic properties of macromolecules based on the functionalization of certain chromophores by styrene. Optoelectronic properties provide information for characterizing light propagation in organic and inorganic media. They are also important to predict applications of studied materials in OSCs, ultrafast response and OLEDs, based on their ability to convert an optical signal into an electrical signal [4]. We have summarized in table 3, the obtained results of our DFT investigation through B3LYP functional.
Table 3.
Average electric field (E), electric polarization (P), average electric susceptibility (χ e), relative dielectric constant (εr), dielectric constant (ε), refractive index (n), electric displacement (D), electrical impermeability (η), coefficient of reflectivity (CR) of styrene monomer, obtained using B3LYP.
| parameters | B3LYP |
|---|---|
| E (×109 Vm−1) | 0.443 |
| P (×10−2 cm−2) | 0.809 |
| D (×10−2 cm−2) | 1.202 |
| ε (×10−11) | 2.711 |
| 3.062 | |
| 2.062 | |
| n | 1.750 |
| η | 0.327 |
| CR (%) | 7.400 |
The local electric field (E) results from the distribution of electric charges carried by the atoms within the molecule and applied to all charges in the molecular system. Depending on its intensity, it can influence the electrical and optical properties of the material. We obtained through the B3LYP; a value at least 13 times lower than that observed in MMA [4]. As a result, MMA monomer is a much better candidate than styrene monomer for the design of new high-field materials through functionalization. As regards, P, it is a measure of the distribution of electrical charges. It is created by the separation of positive and negative charges in the molecule, owing to the movement of electrons [25]. We obtained , a rather low value which highlights a weak charge distribution in the styrene monomer. Regarding, D, we obtained a value of , a value that reflects a low charge density in styrene. Comparing the P and D values of styrene monomer with those of MMA monomer under the same calculation conditions, we found that the electric polarization of MMA is 11 times greater than that of styrene. Similarly, the electric displacement of MMA is 12 times greater than that of styrene monomer. Styrene is therefore not a potential material for piezoelectric and pyroelectric applications.
As regards , it characterizes the material’s ability to store electrical energy and enables us to discuss potential applications of materials as insulators [4], styrene monomer exhibits a dielectric behaviour of . This is a significant value, making it a good insulator when compared with good insulators such as poly(ethylene terephthalate) ( ). In addition, styrene monomer has a slightly higher electrical energy storage capacity than MMA monomer in its cis ( ) and trans ( ) forms [4]. Regarding the refractive index, we obtained n = 1.750 at the B3LYP level. This value is 13% higher than the experimental value of 1.544 obtained at 25°C [42,47]. This value reflects a transparency comparable to that of glass with a refractive index of 1.5 [74,75]. By comparing with cis-MMA (n = 1.676) and trans-MMA (n = 1.730) at the B3LYP level, we can conclude that styrene monomer offers high transparency and the same ease of light propagation as MMA. Styrene monomer is therefore a good candidate for the design of transparent polymers through chromophore functionalization. Finally, when it comes to materials for optoelectronics and data transmission, such as optical fibers, highly transparent materials are the focus of researchers' attention, as they reduce reflections at the diopter level and enable better transmission of the light signal with little loss. We obtained a reflection coefficient of 7.40% in styrene monomer. Given that the reflection coefficients of common semiconductors are of the order of 30% [76], we conclude that there is about four times less loss in styrene than in common semiconductors during light signal transmission, making styrene a good potential functionalizer for the design of transparent semiconductors. Finally, the electrical impermeability of styrene is 0.327, a value very close to that of MMA monomer.
3.3. Charge mobility
Reorganization energy is a parameter that characterizes charge mobility in organic materials [77]. The reorganization energy of the electron (E e) as well as that of the hole (E h) can be calculated for an organic compound using the Marcus theory [78,79], through the following formulas:
| (3.1) |
| (3.2) |
where and are the energies of the neutral compound in the anionic and cationic states, respectively. is the energy of the neutral compound taken in the ground state at the end of optimization, whereas the energies and are obtained from the optimization of the cationic and anionic forms of the compound. and are the energies of the neutral form obtained from the optimized structures of the anion and cation, respectively. The integral charge carrier transfer coefficients of the electrons and holes are obtained from the following relations [60–62]:
| (3.3) |
| (3.4) |
The findings of the transport properties of the studied compound, calculated using the B3LYP and functionals are given in table 4. We obtained electron and hole reorganization energies of 0.393 and 0.295 eV, respectively. These values indicate that holes reorganize faster than electrons in styrene. Generally, the literature indicates that low values of electron and hole reorganization energies indicate better rates of charge transport and carrier mobility [59]. Consequently, styrene monomer is more favourable for hole transport than for electron transport. Comparing these values with those for MMA monomer, it can be seen that charge carriers reorganize faster and require lower energies in styrene than in MMA. In addition, the reorganization energy of electrons in MMA is two times that of styrene, while that of holes is four to nine times that of styrene. Styrene monomer is therefore more suitable than MMA for the functionalization of new charge-transporting molecules. In addition, the integral charge transfer coefficient assesses the charge transport properties of an organic molecule. Higher values of integral charge transfer coefficients reflect better charge carrier mobility [80]. In our study, we obtained an integral charge transfer coefficient of 0.430 eV for electrons and 0.375 eV for holes.
Table 4.
Reorganization energy (E h, E e) and integral charge transfer (t e, t h) of holes and electrons of styrene monomer calculated using the B3LYP and methods. (Values are given in eV.)
| parameters | B3LYP | |
|---|---|---|
| E e | 0.393 | 0.490 |
| E h | 0.295 | 0.380 |
| t e | 0.430 | 0.441 |
| t h | 0.375 | 0.402 |
Numerous current studies available in the literature on the problem of efficient charge transport in OSCs show that, in general, low values of reorganization energies promote or induce rapid movement of electrons and holes between the metal electrodes of OSCs [81–83]. To characterize the transport properties of styrene, we decided to compare its values with those of other materials recently published in the literature. Thus, in 2022, Janjua [84] designed and numerically studied new organometallic materials for hole and electron transport through halogen doping. The maximum electron and hole mobilities obtained were 0.198 and 0.201 eV, respectively. These values are 1.98 and 1.46 times smaller than those of the intrinsic styrene monomer. Interestingly, even in its neutral form, styrene despite being an insulator exhibits acceptable hole and electron transport properties. However, there are other molecules with transport parameters around E e = 0.022 eV and E h = 0.020 eV that enable the creation of OSCs with high fill factors and improved open-circuit voltage [81,85,86]. When comparing these values to those of styrene, it becomes evident that they are on average 12 to 15 times smaller than those of the styrene monomer. Styrene in its intrinsic form can thus serve as a basis for designing even more efficient molecules for rapid electron and hole transport, leading to enhanced OSC efficiencies.
3.4. Electronic properties and frontier molecular orbital analysis
Some electronic parameters of the styrene monomer were used to assess its electrical conduction properties. The ability of frontier orbitals to give up or receive electrons was also assessed. Parameters such as E LUMO, E HOMO, E g, IP, EA as well as the photon’s threshold wavelength (λ), Fermi energy (E Fermi) and fundamental gap (E f) were evaluated and reported in table 5. We obtained E g = 5.146 eV, a very high value (>4 eV), so the electron has difficulty crossing this energy barrier. As a result, there are very few free electrons in the conduction band and the material has low electric conduction, making it an electrical insulator.
Table 5.
E HOMO, E LUMO, E g, E Fermi, E f, IP and EA of styrene monomer, obtained at the B3LYP and levels of theory. (Energy values are given in eV and wavelength in nm.)
| parameters/method | B3LYP | |
|---|---|---|
| E LUMO | −1.154 | 0.772 |
| E HOMO | −6.300 | −8.279 |
| E g | 5.146 | 9.051 |
| λ | 240.954 | 137 |
| E Fermi | −3.727 | −3.753 |
| IPv | 8.252 | 8.401 |
| EAv | −0.727 | −0.878 |
| E f v | 8.979 | 9.279 |
| IPa | 8.105 | 8.205 |
| EAa | −0.532 | −0.623 |
| E f a | 8.637 | 8.828 |
The threshold wavelength of the photon that allows the electron to jump from the HOMO to the LUMO level is λ = 240.954 nm, while the Fermi energy level is E Fermi = −3.727 eV. Fermi energy represents the average energy of an electron in a material at thermodynamic equilibrium. In styrene monomer, the Fermi level is below the middle of the band gap.
With regards to , which is another measure of electronic stability and a parameter well suited to describing the reactivity of a molecule [87], we recorded values of 8.637 and 8.979 eV for the vertical and adiabatic determination. Both values indicate the existence of high electronic stability in styrene, which confirms the insulating nature of styrene, because for broadband organic semiconductors, the fundamental gap is generally between 4 and 7 eV [2]. We can also observe that the fundamental gap is highest when determined in the adiabatic regime.
As far as the IP is concerned, it provides an estimate of the energy barrier to be crossed for the extraction of charge carriers in an organic material [88,89]. We obtained a value of = 8.252 eV for the vertical potential, compared with a slightly lower value of = 8.105 eV for the adiabatic potential. The IP values show that the energy required to extract an electron from the HOMO level is high, giving the styrene molecule good stability and reactivity. Concerning EA, it is used to estimate the energy barrier to be crossed for the injection of charge carriers into an organic material [88,89]. For adiabatic and vertical EA, we obtained values of = −0.532 eV and = −0.727 eV, respectively, the larger value being that of the adiabatic model.
The charge density distribution of the boundary molecular orbitals in the studied system is shown in figure 2. Regarding the HOMO and LUMO, they consist of negatively charged areas (indicated by the red colour) and positively charged zones (indicated by the green colour). The LUMO exhibits more positively and negatively charged regions than the HOMO. Both the HOMO and LUMO are delocalized throughout the styrene carbon skeleton. The styrene LUMO has many areas of potential interaction with other compounds, both on the phenyl group and on the ethylenic bond, as does the HOMO. The HOMO is located on orbital 28, while the LUMO is located on orbital 29. The energy barrier between these two orbitals is 5.146 eV and represents the band gap. The electronic transition between these two frontier orbitals is considered to be the most achievable and lowest-energy conversion that can enable a charge carrier to move from the HOMO to the LUMO of styrene [33,34] and it corresponds in this case on a π→π* transition. Overall, the styrene monomer has a total of 192 molecular orbitals, of which only 28 are occupied, leaving 164 free. This abundance of molecular orbitals opens up numerous possibilities for electronic transitions. The LUMO and HOMO energies are, respectively, E LUMO = −1.154 eV and E HOMO = −6.300 eV. Bearing in mind that the HOMO value generally required to be a charge transport polymer (CTP) [90] is between −5.5 and −6.0 eV, we found that the HOMO of styrene is −0.3 eV more than the maximum value required to be a CTP; a difference of 5% from the value that highlights the intrinsic suitability of styrene monomer for charge transport. Based on its HOMO value, functionalization or doping, to name but a few, could easily make styrene a good charge carrier. Relative to other materials available in the literature and recognized to date as potentially very suitable for the design and manufacture of OSC devices, the HOMO in these compounds is on average between −5.0 and −5.65 eV [11,81,84]. The maximum values are approximately 1.3 eV higher than those of styrene, which is an insulator. However, the styrene LUMO is on average 2 eV larger than that of good materials for hole and electron transport. To create effective charge carriers from styrene, it would be necessary to lower this LUMO. Overall, based on its HOMO and LUMO, the styrene monomer is a favourable material for charge transport.
Figure 2.

Frontiers molecular orbitals of styrene monomer, obtained using B3LYP.
3.5. Chemical descriptors of the reactivity
Chemical descriptors of reactivity allow us to discuss the reactivity and stability of the chemical behaviour of a molecule. These investigations can be achieved by the determination of global descriptor parameters such as the chemical potential (μ CP), chemical hardness (η), chemical softness (S), electrophilicity index (ω), nucleophilicity index (υ) and maximum charge transfer (ΔN max). These parameters for the styrene monomer are summarized in table 6.
Table 6.
Global reactivity descriptors of styrene monomer in vertical and adiabatic model, obtained using B3LYP.
| parameters/methods | vertical | adiabatic |
|---|---|---|
| μ CP (eV) | −3.763 | −3.787 |
| η (eV) | 4.490 | 4.319 |
| S (eV)−1 | 0.223 | 0.232 |
| ω (eV) | 1.577 | 1.660 |
| υ (eV)−1 | 0.634 | 0.602 |
| ∆N max | 0.838 | 0.877 |
μ CP represents the energy required for an electron to escape from the stable configuration of a molecule [4]. It can be interpreted as the ease with which electrons can abandon one stable molecular system for another [91]. We found that for styrene monomer, μ CP = −3.763 eV for vertical evaluation and −3.787 eV for adiabatic assessment. These values are intermediate between those of an insulator and a wide-bandgap semiconductor [2,25]. Based on the chemical potential, the reactivity of styrene monomer is greater than that of MMA monomer, for which μ CP = −4.414 eV. Indeed, an increase in the value of μ CP leads to an increase in reactivity and a decrease in stability.
Concerning , we obtained 4.490 and 4.319 eV for the vertical and adiabatic chemical hardness of styrene monomer, respectively. Consequently, despite its insulating nature, styrene monomer may yield electrons to the surrounding medium more readily than MMA monomer [4]. This suggests that styrene functionalization may be more favourable to intramolecular charge transfer, thereby enhancing or preserving the initial semiconducting character of the functionalized chromophore than MMA. Regarding , we obtained values of 1.577 and 1.660 eV for the vertical and adiabatic evaluation, respectively. These values show that the investigated system has a good capacity to accept electronic charges from others and remains stable. Styrene’s ability to emit and accept charges leads us to conclude that this molecule is suitable for intramolecular charge transfer processes. Indeed, the more semiconducting an organic material is, the higher the value of its . Similarly, low values of the index reflect insulating behaviour [2,4,25]. Finally, as regards to , we obtained 0.838 and 0.877 eV for vertical and adiabatic assessment, respectively. Compared with the value of 0.825 eV obtained in MMA, this confirms the better charge transport properties in styrene than in MMA.
3.6. Linear optical properties
Some selected linear optical parameters of styrene monomer such as μ, , Δα, χe, MR and χ(1)) in static mode were evaluated and collected in table 7.
Table 7.
Dipole moment (μ), average polarizability ( ), anisotropy of polarizability (Δα), molar refractivity (MR), first-order susceptibility tensor (χ (1)) and average electric susceptibility (χ e) in static mode of styrene monomer.
| parameters/method | B3LYP | ωB97XD |
|---|---|---|
| μ (D) | 0.192 | 0.149 |
| (×10−24 esu) | 12.958 | 12.638 |
| Δα (×10−24 esu) | 12.504 | 11.702 |
| MR (esu mol−1) | 32.691 | 31.885 |
| χ(1) xx | 3.155 | 3.211 |
| χ(1) xy | 0.122 | 0.134 |
| χ(1) yy | 2.153 | 2.269 |
| χ(1) xz | 0.000 | 0.009 |
| χ(1) yz | 0.000 | 0.027 |
| χ(1) zz | 0.876 | 0.948 |
| χe | 2.062 | 2.143 |
With regards to , a high value is very often synonymous with good optical properties, while a low value often indicates poor optical response [4]. Furthermore, a compound with zero dipole moment (polar molecule) cannot exhibit total static hyperpolarizability, internal electric field or electric polarization [25]. We obtained a value of 0.149 D for styrene monomer, a small value which proves that the behaviour of styrene monomer is close to that of polar compounds, but that it can exhibit NLO properties. Comparing with MMA monomer, with values of 1.843 and 1.698 D for cis- and trans-MMA, respectively, we can conclude that the dipole moment of styrene is 13 and 11 times lower than that of cis-MMA and trans-MMA, respectively.
As far as polarizability is concerned, it provides information on the distribution of electrons in the molecule, and plays an important role in determining the structure and orientation of a material [92]. Materials with low values are weakly polar. We recorded in the styrene monomer, a low value which indicates that styrene is a molecule that deforms very little under the action of an external electric field. Compared with MMA monomer with and 8.991×10−24 esu for cis- and trans-MMA [4], respectively, we find that styrene monomer offers greater polarizability than MMA. However, owing to their low values, both styrene and MMA can be used to improve materials such as optical fibres [93].
Regarding χ(1) tensor, we found that styrene monomer exhibits preferential directions of electron displacement. These are the (xx) and (yy) directions along which the material exhibits maximum susceptibility namely and . By contrast, the quasi-plane structure of styrene results in a zero response in the (xz) and (yz) directions and a weak response in the (zz) direction with . Styrene monomer has an average susceptibility χ e = 2.062, a value one unit lower than that of some organic broadband semiconductors, which have a first-order average susceptibility of between three and four [2]. This macroscopic response ability of styrene confirms the predictions of a good candidate material for the functionalization of chromophores for applications in nonlinear optics. Finally, directional analysis of linear optical behaviour in terms of polarizability and susceptibility shows that styrene monomer is anisotropic. We obtained a value Δα = 12.504 × 10−24 esu, a value at least two times higher than that of MMA monomer [4]. Finally, styrene monomer exhibits a molar refractivity of MR = 32.691 esu mol−1.
3.7. Nonlinear optical properties
3.7.1. Nonlinear optical properties in static mode
Some NLO properties of our styrene monomer were evaluated in static mode using ωB97XD functional. Thus, the values of βT, , and are presented in table 8.
Table 8.
First total hyperpolarizability (β T), averaged second hyperpolarizability ( ), second-order total susceptibility ( ) and third-order total susceptibility ( ) in static mode of styrene monomer.
| parameters/method | ωB97XD |
|---|---|
| β T (×10−30 esu) | 0.587 |
| (×10−36 esu) | 4.980 |
| (pm V−1) | 1.658 |
| (×10−22 m2 V−2) | 1.075 |
With regards to first-order hyperpolarizability in the static regime, we obtained a value of β T = 0.587 × 10−30 esu, a value that highlights the absence of centrosymmetry in styrene and the existence of a first-order microscopic response sufficient for the existence of NLO behaviour in styrene monomer. Comparing this value with that of MMA where β T = 0.153 × 10−30 esu, we see that styrene monomer is 3.83 times better than MMA. It could therefore be more suitable than MMA for the functional design of new NLO materials. With respect to the second-order hyperpolarizability, we obtained a value of , an insufficient value to consider styrene as a good material for NLO in the static regime. However, it is 1.66 times higher than that of the monomer MMA where .
Concerning the optical susceptibility, we obtained second- and third-order values in the static regime and , respectively. We note that at third order, styrene and MMA monomers offer almost the same behaviour, while at second order, the value for styrene is three times greater than that for MMA ( = 0.503 pmV−1).
3.7.2. Pockel’s electro-optic and direct current Kerr effects
The interaction between an organic material and a high-intensity laser source gives rise to several nonlinear optical effects that depend on the frequency and phase used by the laser. These include second harmonics generation (SHG) [24] and multiphoton absorption [94]. However, we can also distinguish two important effects which occur in the dynamic regime and which allow us to characterize the response of the material to a specific frequency [24,91,94]. These are the Pockel’s electro-optic effect( EOPE), which occurs for frequency (−ω;ω,0) and is related to , and the direct current Kerr (DC-KERR) effect, which occurs for frequency (−ω;ω,0,0) and is related to . The microscopic and macroscopic Pockel and KERR responses of styrene monomer were assessed and summarized in table 9.
Table 9.
EOPE first total hyperpolarizability ( ), DC-KERR averaged second hyperpolarizability ( ), EOPE second-order total susceptibility ( ) and DC-KERR third-order total susceptibility ( ) of styrene monomer using ωB97XD functional, obtained at wavelength λ = 1064 nm.
| parameters/molecules | styrene | MMA [4] |
|---|---|---|
| (×10−30 esu) | 0.699 | 0.153 |
| (×10−36 esu) | 5.509 | 3.000 |
| (pm V-1) | 1.974 | 0.510 |
| (×10−22 m2 V−2) | 2.609 | 0.856 |
The EOPE can be understood as the ability of a dielectric material to modify its polarization owing to a change in birefringence when a strong electric field is applied to it. A material with a high response can then find many applications in light modulation and switching devices, owing to its ability to modify the propagation of light within it, in the presence of an electric field. We determined the EOPE effect by evaluating and at the usual wavelength of 1064 nm. From the values in table 9, we obtain and , values which highlight the existence of the EOPE in styrene monomer. Compared with MMA, styrene monomer is therefore better suited to the functionalization of materials suitable for the electro-optical Pockel effect.
The DC-KERR effect, meanwhile, is related to and can be considered as the material’s ability to change its refractive index as a function of incident light intensity. A high value of allows us to postulate potential applications of the material in the manufacture of high-speed optical modulators, while a high value allows us to postulate potential applications in the generation of optical harmonics. For styrene monomer, we obtained and . Styrene monomer is therefore suitable for modulating its refractive index as a function of the intensity of the electrical excitation signal. However, its optical rigidity induces weak NLO responses, owing to its low intramolecular charge transfer.
3.7.3. Second harmonics generation and third harmonics generation
To assess the ability of styrene to generate specific frequency mixing processes such as SHG and third harmonics generation (THG), the first-order (β(−2 ω;ω,ω)) and second-order (γ(−2 ω;ω,ω,0)) as well as the resulting optical susceptibilities and at the wavelength of 1064 nm were determined and reported in table 10.
Table 10.
Some selected components of the frequency-dependent first-order hyperpolarizability (in 10−30 esu) and second-order hyperpolarizability (in 10−36 su), second- and third-order total optical susceptibilities and at λ = 1064 nm.
| parameters/method | ωB97XD |
|---|---|
| β xxx | 0.009 |
| β yyy | −0.121 |
| β zzz | −0.838 |
| β x | 0.020 |
| β y | −0.116 |
| β z | −0.838 |
| 0.847 | |
| γ xxxx | 0.161 |
| γ yyyy | 3.382 |
| γ zzzz | 26.323 |
| γ xxyy | 0.299 |
| γ yyzz | 0.505 |
| γ xxzz | 0.581 |
| 6.527 | |
| (pm V−1) | 2.392 |
| (×10−22 m2 V−2) | 1.410 |
Analysis of the β(−2 ω;ω,ω) and γ(−2 ω;ω,ω,0) tensors reveals strong anisotropy in the NLO response of styrene monomer. In the case of , intramolecular charge transfer predominates in the (zzz) direction, in contrast to linear optics, where the response is governed by the (xx) direction. The presence of an external electric field effectively modifies the polarization and topology of the electron pattern. Similarly, analysis of reveals a second-order nonlinear response strongly dominated by the γ zzzz component with a value of 26.323×10−36 esu. For styrene monomer, we obtained and . Comparing these values with experimental values for urea and [95], which is the reference molecule for NLO properties, we found that urea has a total first hyperpolarizability 2.71 times higher than styrene monomer. Similarly, styrene monomer has an average second hyperpolarizability at least 7.35 times lower than urea.
Consequently, the studied monomer is not a suitable candidate for devices requiring good NLO properties. On the other hand, compared with the NLO behaviour of MMA, styrene exhibits a 3.38 times greater than that of MMA, and a 1.86 times greater than that of MMA. As a result, styrene monomer is a much better material for harmonic generation than MMA monomer, and could be a better material than MMA for the design of new materials for NLO.
For second- and third-order susceptibilities, we obtained and . Our value of is 2.39 times greater than that of quartz ( ) [96], which is a reference material for SHG. It can, therefore, be considered a material suitable for SHG. On the other hand, the value of silica [97,98], which is a reference material for THG, is about 1.42 times higher than that of styrene monomer. Styrene monomer is therefore not sufficiently suitable for THG. However, the value of styrene is higher than that of MMA.
3.7.4. Hyper-Rayleigh scattering
Hyper-Rayleigh scattering (HRS) is one of the experimental techniques used to measure the intensity of the incoherently scattered frequency-doubled light generated after the interaction of a laser beam with a chromophore in an isotropic solution [99]. As optical communication networks evolve, the research and development of new compounds with NLO performance is of significant importance for improving this field [100,101]. Prediction of is essential for identifying, developing and improving raw materials suitable for use in optical communications [99], as well as for frequency doubling, ultrafast lasers and fast electro-optical modulation [102]. The value of HRS can be evaluated theoretically using the following equation [102]:
| (3.5) |
where and stand to the orientationally averaged tensor components. On the other hand, the depolarization ratio (DR), which is useful for measuring the dipolar to octupolar contribution of the studied compound, was evaluated as follows:
| (3.6) |
In addition, another important NLO parameter is the degenerate four-wave mixing response ( ) and the nonlinear quadratic refractive index (n 2), which allows us to discuss potential optoelectronic applications of the material as a wavelength converter or optical pulse modulator when the material exhibits large index values [103]. was assessed using the formula:
| (3.7) |
and n 2 using the following equation:
| (3.8) |
where n 0 is the linear refractive index and c the speed of light in vacuum. The values of these parameters are listed in table 11.
Table 11.
Frequency-dependent NLO response of styrene monomer, obtained at λ = 1064 nm: HRS response , , degenerate four-wave mixing of the third-order response and nonlinear refractive index .
| parameters/method | ωB97XD |
|---|---|
| (×10−30 esu) | 0.357 |
| 4.556 | |
| (×10−36 esu) | 6.024 |
| (×10−22 m2 V−2) | 1.891 |
| (×10-20 m2W−1) | 1.748 |
The values in table 11 show that , a non-zero value that confirms the existence of first-order NLO activity in styrene. This activity is half that of trans-MMA and slightly lower than that of cis-MMA [4]. The DR obtained is 4.556, a value higher than 4.5, giving styrene a dipolar contribution.
With regards to the response, we obtained , a value twice that of MMA. Styrene monomer has a greater capacity for second-order NLO response than first-order NLO response. Indeed, as a third-order susceptibility derived from , we obtained a value , smaller than that of silica [97,98], but still higher than that of MMA.
Finally, we obtained , a value 36% lower than that obtained in fused silica [104], and 32% lower than that obtained experimentally in the CS2 liquid [105], commonly used as reference material for . The value of styrene monomer is at least 40% higher than that of the two MMA isomers. Styrene should therefore be more suitable for the design of new degenerate four-wave mixing materials than MMA.
3.8. Thermodynamic properties
The thermodynamic stability of an organic system is a key criterion that it must meet to find applications in industry or to react with other compounds. We have evaluated some key parameters of the thermodynamic activity of styrene monomer and the results are given in table 12.
Table 12.
Thermodynamic properties of styrene monomer.
| parameters/methods | B3LYP | ωB97XD |
|---|---|---|
| EE (×103 kcal mol−1) | −194.357 | −194.282 |
| ZPVE (kcal mol−1) | 83.326 | 84.167 |
| H (×103 kcal mol−1) | −194.269 | −194.193 |
| G (×103 kcal mol−1) | −194.293 | −194.218 |
| E Th (kcal mol−1) | 87.596 | 88.378 |
| Cv (cal mol−1 K) | 26.134 | 25.859 |
| S (cal mol−1 K) | 82.898 | 81.721 |
The Gibbs free energy (G) is a fundamental criterion for the thermodynamic stability of a system. The lower the value for of compound, the more stable it is [2]. Another important aspect of G, is its ability to predict whether or not a system can react with another system. We obtained a value of −194.293×103 and −194.218×103 kcal mol−1 using B3LYP and ωB97XD, respectively. These negative values give styrene thermodynamic stability and the ability to react with other compounds. It can also be noted that styrene is less stable and slightly more reactive than MMA [4]. With regards to zero-point vibrational energy (ZPVE), we obtained a value of 83.326 kcal mol−1 through B3LYP and 84.167 kcal mol−1 through ωB97XD, values 8% higher than those for MMA. As far as thermal energy (E Th) is concerned, this is the kinetic energy of the microscopic agitation of a system, owing to the disordered agitation of its atoms and molecules [2]. For styrene monomer, we obtained E Th = 87.596 and 88.378 kcal mol−1 using B3LYP and ωB97XD, respectively, relatively low values owing to the small size of the styrene molecule, which favours its stability. Nevertheless, these values are higher than those for MMA by approximately 7%. For heat capacity, we obtained C v = 26.134 kcal mol−1 using B3LYP and Cv = 25.859 kcal mol−1 using ωB97XD, values 5% lower than those for MMA. These values show that styrene’s ability to withstand any increase in temperature is low. However, the values are sufficient to guarantee good thermal resistance. Regarding entropy (S), we obtained a value of 82.898 cal mol−1 K based on B3LYP and 81.721 cal mol−1 K based on ωB97XD, i.e. 5% more intramolecular disorder than in MMA. Finally, for enthalpy (H), another key parameter of thermodynamic stability, we obtained negative values H = −194.269 × 103 kcal mol−1 and H = −194.193 × 103 kcal mol−1 by B3LYP and ωB97XD, respectively, values which again suggest the thermodynamic stability of styrene.
Finally, the thermodynamic parameters of styrene show that it is stable and able to react with other compounds to form new ones. It can, therefore, be used to functionalize chromophores and other materials. In addition, it was found to offer virtually the same thermodynamic performance as MMA.
3.9. Ultraviolet-visible spectroscopy analysis
3.9.1. Ultraviolet-visible spectroscopy absorption spectra analysis
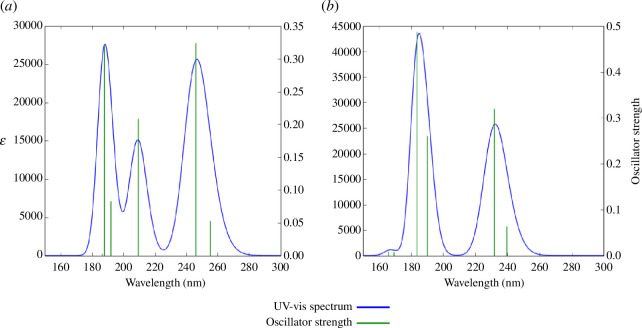
In this section, we evaluate the behaviour of styrene monomer in excited states after photon absorption, by determining the first allowed singlet-singlet excitation energies. The TD-DFT/B3LYP and TD-ωB97XD methods were used for this purpose. The absorption spectra of styrene are shown in figure 3, while the maximum absorption wavelength (λ), the first six electronic excitation energies (E), the oscillation strength (f) and their main electronic transitions are presented in table 13.
Figure 3.
TD-DFT absorption spectra of styrene monomer. (a) B3LYP and (b) 𝜔B97XD.
Table 13.
Singlet–singlet permitted excitation energies (E), maximum absorption wavelength (λ), oscillator strength (f), efficacity of absorption (η) and major electronic contribution to transitions of absorption spectra of styrene monomer obtained using TD-B3LYP and TD-ωB97XD.
| excited state/parameters | E (eV) | λ(nm) | f | η | major contribution |
|---|---|---|---|---|---|
| TD-B3LYP | |||||
| 1 | 4.860 | 255.10 | 0.053 | 0.115 | H−1 → L (42%), H → L (16%), H → L+1 (41%) |
| 2 | 5.039 | 246.04 | 0.324 | 0.526 | H−1 → L (15%), H → L (80%) |
| 3 | 5.927 | 209.19 | 0.209 | 0.381 | H−1 → L (39%), H → L+1 (51%) |
| 4 | 6.460 | 191.92 | 0.083 | 0.174 | H−2 → L (65%), H−1 → L+1 (17%), H → L+3 (16%) |
| 5 | 6.608 | 187.62 | 0.321 | 0.523 | H−1 → L+1(63%), H → L+3 (30%) |
| 6 | 6.650 | 186.43 | 0.003 | 0.006 | H → L+2 (98%) |
| TD-ωB97XD | |||||
| 1 | 5.174 | 239.62 | 0.063 | 0.136 | H−1 → L (37%), H → L (19%), H → L+1 (39%) |
| 2 | 5.353 | 231.62 | 0.320 | 0.521 | H−1 → L (14%), H → L (75%) |
| 3 | 6.523 | 190.08 | 0.261 | 0.452 | H−1 → L (43%), H → L+1 (51%) |
| 4 | 6.752 | 183.61 | 0.488 | 0.675 | H−1 → L+1 (78%) |
| 5 | 7.327 | 169.21 | 0.008 | 0.017 | H−3 → L (61%), H−2 → L (24%) |
| 6 | 7.474 | 165.89 | 0.009 | 0.021 | H−4 → L (16%), H −3→ L(26%), H−2 → L (36%), H → L+3 (13%) |
Figure 3 and table 13 reveal some differences depending on the functional used. Using the ωB97XD functional, two maximum absorption peaks were clearly identified. The low-intensity peak is located at wavelength λ = 231.62 nm with an oscillator strength of 0.320 and an absorption efficiency of 0.521. This transition corresponds to an electron jump from HOMO−1 to LUMO with 14% contribution and from HOMO to LUMO with 75% contribution.
The maximum absorption peak is located at wavelength λ = 183.61 nm with maximum oscillator strength and absorption efficiency of 0.488 and 0.675, respectively. This peak is attributed to an electron jump from HOMO−1 to LUMO+1 with 78% contribution. As far as the B3LYP functional is concerned, we have an average absorption band at λ = 209.19 nm and two intense absorption bands at λ = 246.04 and λ = 187.62 nm. Finally, whatever the functional used, like MMA [4], styrene absorbs in the ultraviolet. Both compounds thus appear as potential candidates for the manufacture of ultraviolet (UV) sensors.
3.9.2. Ultraviolet-visible spectroscopy emission spectra analysis
Emission properties are important parameters for suggesting applications of organic materials in display devices, as well as in light sources and in the manufacture of OLEDs. In this section, the emission spectrum of styrene and some important properties such as Stokes shift and radiative lifetime (τ) have been determined. The emission spectrum is shown in figure 4, while the emission properties are reported in table 14.
Figure 4.
Theoretical (a) TD-DFT/B3LYP and (b) TD-DFT/ωB3LYP emission spectra of styrene monomer.
Table 14.
Fluorescent emission wavelength (λ), fluorescence energy (E Flu), oscillator strength (f), Stokes shift (Δλ) and radiative lifetime ( ) of styrene monomer.
| parameters | TD-B3LYP | TD-ωB97XD |
|---|---|---|
| λ (nm) | 287.99 | 200.90 |
| E Flu(eV) | 4.315 | 6.201 |
| f | 0.330 | 0.371 |
| Δλ (nm) | 41.95 | 17.29 |
| (ns) | 3.863 | 1.662 |
The Stokes shift ( is the difference between a molecule’s maximum emission wavelength and its maximum absorption wavelength [106]. It indicates the relationship between the structure and properties of fluorescent molecules between their ground and excited states [107]. We have calculated the Stokes shift using the following equation [108]:
| (3.9) |
of styrene monomer given by the TD-ωB97XD method is 17.29 nm, a value seven times higher than that of the cis form of MMA, a result which allows us to conclude that styrene monomer is more chemically stable than cis-MMA. On the other hand, calculation by the TD-B3LYP method led to Δλ = 41.95 nm, a larger value than that calculated by TD-ωB97XD. Comparing this value obtained by the TD-B3LYP method with that of cis-MMA, it appears that the stability of styrene is three times greater than that of MMA, and that the value of the (Δλ) depends on the functional used. The low Stokes shift values of styrene monomer limit its use as a potential material for the manufacture of solar cell devices [93].
The emission spectra in figure 4 show two prominent emission bands, regardless of the method used. Using TD-ωB97XD, the fluorescent emission wavelength is 200.90 nm and is attributed to the HOMO−1 → LUMO transition with 44% contribution and to the HOMO → LUMO+1 transition with 50% contribution. In contrast, using TD-B3LYP, the fluorescent spectrum of styrene monomer shows a maximum emission wavelength of 287.99 nm, a difference of 87.09 nm compared with TD-ωB97XD. The transition involved in the maximum emission is essentially a HOMO → LUMO transition with 94% contribution and an oscillator strength of 0.330. According to the present results, styrene monomer emits only in the ultraviolet.
The time a fluorescent molecule remains in an excited state in the absence of a non-radiative transition is called its radiative lifetime [58]. It is defined (in arbitrary units) as follows [58,89,107]:
| (3.10) |
where E flu is the fluorescence energy, f the oscillator strength and c the speed of light. We obtained for styrene monomer τ = 3.863 ns using TD-B3LYP and τ = 1.662 ns using TD-ωB97XD.
3.10. Natural bond orbital analysis
3.10.1. Natural bond charge analysis
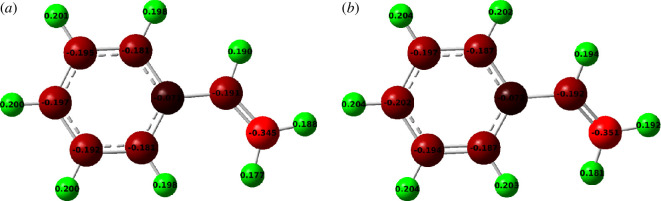
According to several authors [17,91,94,109], NBO analysis enables us to understand the direction of charge transfer taking place in the molecule, and to identify the atoms or groups of atoms that play the roles of donor and acceptor. NBO calculations were conducted using the two B3LYP and B97XD functionals, the results are depicted in figure 5.
Figure 5.
NBO charges of styrene monomer (a) B3LYP and (b) ωB97XD.
Using B3LYP, the NBO charges on the styrene monomer range from −0.345 e to +0.201 e. The maximum positive charge of +0.201 e is obtained on the H7-labelled hydrogen atom of the phenyl ring involved in the C1–H7 bond.
We found that all hydrogen atoms have an average positive charge of +0.194 e. It follows that the charges leave all the hydrogen atoms towards the carbon atoms, which are then all negatively charged. The system’s highest negative charge in absolute value −0.345 e is obtained on the C14-labelled carbon in the ethylene group [110]. The system’s highest negative charges are located on the carbon of the phenyl group. Thus, the probable direction of charge displacement is from the phenyl group, as charge donor, to ethylene, as receptor. Finally, all atoms are involved in intramolecular charge transfer, which helps us to understand the origin of the optical and transport properties observed in styrene monomer. The results obtained using ωB97XD are quite similar to those of B3LYP, even if slightly higher, and the overall behaviour observed on intramolecular charge transfer is the same.
3.10.2. Second order Fock matrix analysis
In NBO analysis, the second-order Fock matrix is a means of investigating the donor-acceptor interaction. The higher the degree of conjugation, the more stability of the molecule will be ensured [111]. The stabilization energy is a key parameter for this analysis. A higher value of E(2) indicates a strong interaction between the donor orbital, and the acceptor orbital, leading to a higher degree of electron delocalization [112]. Given a donor (i) and an acceptor (j), the diagonal members related to the orbital energies values of acceptor and donor and , respectively; the stabilization energy E(2) can be obtained through equation (3.11) [112–114]
| (3.11) |
where refers to the orbital occupancy and represents the elements of the Fock matrix. The results of styrene monomer bond interaction analysis are reported in table 15.
Table 15.
NBO second-order perturbation theory analysis of Fock matrix of styrene monomer, obtained using the B3LYP method.
| donor NBO (i) | type | acceptor NBO (j) | type | E(2) kcal mol−1 | E(i)−E(j) arbitrary units | F(i,j) arbitrary units |
|---|---|---|---|---|---|---|
| C1–C2 | σ(−1) | C1–C6 | σ*(1) | 3.17 | 1.28 | 0.057 |
| C1–C2 | σ(−2) | C3–C4 | σ*(2) | 19.9 | 0.29 | 0.068 |
| C1–C2 | σ(−2) | C5–C6 | σ*(2) | 20.07 | 0.29 | 0.068 |
| C1–C6 | σ(−1) | C1–C2 | σ*(1) | 3.15 | 1.28 | 0.057 |
| C1–C6 | σ(−1) | C5–C6 | σ*(1) | 3.52 | 1.26 | 0.06 |
| C1–C6 | σ(−1) | C5–C12 | σ*(1) | 3.32 | 1.18 | 0.056 |
| C1–H7 | σ(−1) | C2–C3 | σ*(1) | 3.79 | 1.09 | 0.057 |
| C1–H7 | σ(−1) | C5–C6 | σ*(1) | 4.16 | 1.08 | 0.06 |
| C2–C3 | σ(−1) | C3–C4 | σ*(1) | 3.12 | 1.28 | 0.057 |
| C2–H8 | σ(−1) | C1–C6 | σ*(1) | 3.85 | 1.1 | 0.058 |
| C2–H8 | σ(−1) | C3–C4 | σ*(1) | 3.81 | 1.1 | 0.058 |
| C3–C4 | σ(−1) | C2–C3 | σ*(1) | 3.11 | 1.27 | 0.056 |
| C3–C4 | σ(−1) | C4–C5 | σ*(1) | 3.71 | 1.27 | 0.061 |
| C3–C4 | σ(−1) | C5–C12 | σ*(1) | 3.59 | 1.18 | 0.058 |
| C3–C4 | σ(−2) | C1–C2 | σ*(2) | 19.98 | 0.28 | 0.068 |
| C3–C4 | σ(−2) | C5–C6 | σ*(2) | 19.88 | 0.29 | 0.068 |
| C3–H9 | σ(−1) | C1–C2 | σ*(1) | 3.74 | 1.1 | 0.057 |
| C3–H9 | σ(−1) | C4–C5 | σ*(1) | 4.25 | 1.08 | 0.061 |
| C4–C5 | σ(−1) | C3–C4 | σ*(1) | 3.46 | 1.27 | 0.059 |
| C4–C5 | σ(−1) | C5–C6 | σ*(1) | 4.07 | 1.25 | 0.064 |
| C4–C5 | σ(−1) | C5–C12 | σ*(1) | 3.17 | 1.17 | 0.054 |
| C4–H10 | σ(−1) | C2–C3 | σ*(1) | 3.91 | 1.09 | 0.058 |
| C4–H10 | σ(−1) | C5–C6 | σ*(1) | 4.31 | 1.08 | 0.061 |
| C5–C6 | σ(−1) | C1–C6 | σ*(1) | 3.27 | 1.27 | 0.058 |
| C5–C6 | σ(−1) | C4–C5 | σ*(1) | 4 | 1.25 | 0.063 |
| C5–C6 | σ(−2) | C1–C2 | σ*(2) | 20.93 | 0.28 | 0.069 |
| C5–C6 | σ(−2) | C3–C4 | σ*(2) | 19.31 | 0.28 | 0.067 |
| C5–C6 | σ(−2) | C12–C14 | σ*(2) | 14.8 | 0.29 | 0.063 |
| C5–C12 | σ(−1) | C4–C5 | σ*(1) | 3.04 | 1.22 | 0.054 |
| C5–C12 | σ(−1) | C12–C14 | σ*(1) | 3.4 | 1.31 | 0.06 |
| C6–H11 | σ(−1) | C1–C2 | σ*(1) | 3.79 | 1.09 | 0.058 |
| C6–H11 | σ(−1) | C4–C5 | σ*(1) | 4.46 | 1.08 | 0.062 |
| C12–H13 | σ(−1) | C4–C5 | σ*(1) | 4.67 | 1.07 | 0.063 |
| C12–H13 | σ(−1) | C14–H15 | σ*(1) | 4.84 | 0.94 | 0.06 |
| C12–C14 | π(−1) | C5–C12 | σ*(1) | 3.6 | 1.23 | 0.06 |
| C12–C14 | π(−2) | C5–C6 | σ*(2) | 11.32 | 0.31 | 0.057 |
| C14–C15 | σ(−1) | C12–H13 | σ*(1) | 4.86 | 0.96 | 0.061 |
| C14–H16 | σ(−1) | C5–C12 | σ*(1) | 6.88 | 1.01 | 0.074 |
| C4 | CR(−1) | C5 | RY*(2) | 2.32 | 11.57 | 0.146 |
| C14 | CR(−1) | C12 | RY*(2) | 2.67 | 11.23 | 0.155 |
| C1–C2 | σ*(−2) | C1 | RY*(3) | 2.65 | 0.58 | 0.086 |
| C1–C2 | σ*(−2) | C2 | RY*(3) | 2.77 | 0.57 | 0.087 |
| C5–C6 | σ*(−2) | C6 | RY*(3) | 3.4 | 0.6 | 0.094 |
We restricted ourselves mainly to interactions offering the highest stabilization energies (E(2) ≥ 3 kcal mol−1), and a few specific bonds with values of E(2) ≥ 2 kcal mol−1. We note the existence of several types of bonds in styrene including bonds: σ → σ*, π → σ*, π → RY*, CR → RY*, σ → RY*, σ*→ RY* but also σ → π *. The most predominant bond is σ → σ*. The main observation made is that the interactions between the C5−C6 and C1−C2 and also between the C1−C2 and C5−C6 bonds of the phenyl ring, lead to two σ→ σ* transitions, which, respectively display E(2) = 20.93 and E(2) = 20.07 kcal mol−1. These two values are the highest stabilization energy of the molecular system. It can be deduced that these interactions within the phenyl group are those which contribute most significantly to the overall stability and cohesion of the styrene monomer. However, these two bonds alone do not ensure the monomer’s stability and reactivity. In fact, there are several other high stabilization energies close to the above maximum values. These include 19.98, 19.90, 19.88 and 19.31 kcal mol−1 resulting, respectively, from interactions between C3−C4 and C1−C2 bonds and vice versa; and from interaction between C3−C4 and C5−C6 bonds and vice versa. All these bonds are of the σ → σ* type. The stability of the styrene monomer is essentially guaranteed by the interactions between the σ → σ* bonds within the phenyl group.
The system displays a number of π → σ* and σ → π* transitions, including those resulting from the interaction between the C12−C14 and C5−C6 bond, with energy E(2) = 11.32 kcal mol−1. It is the most important of the π → σ* transitions. Similarly, an energy E(2) = 14.8 kcal mol−1 is derived from the interaction between C5−C6 and C12−C14 for the most important σ → π* type transition. The two bonds, although second-neighbours, are quite strongly conjugated, with significant stabilization energies.
The C5−C6 bond interacts with the Ryberg state of the C6 carbon, giving rise to a stabilization energy of 3.4 kcal mol−1 and a σ*→ RY* type transition. A CR → RY* transition results from interaction between the Rydberg states of carbons C14 and C12, and an energy E(2) = 2.67 kcal mol−1 accompanies the transition.
Overall our intermolecular interactions, our molecular system has several σ → σ* transitions which are more favourable to a low delocalization of electrons that leads to weak NLO properties and an insulator electrical behaviour that has been previously observed.
3.11. Molecular electrostatic potential
The molecular electrostatic potential (MEP) surface is a hypothetical surface that maps over the molecular geometries, allowing us to visualize variably charged regions of a molecule [115]. MEP is also commonly used to study molecule polarity and identify reactive sites [116,117]. In fact, MEP highlights the nucleophilic and electrophilic sites of the molecule [118,119]. In figure 6, we present the MEP of the styrene monomer obtained using B3LYP and ωB97XD. On a MEP surface, the predominant colours are red and blue, indicating electron-withdrawing and electron-donating moieties, respectively. The general order of potential increase is: red < orange < yellow < green < blue [91]. The most electron-rich regions appear in red, while the red, orange and yellow regions correspond to a negative potential [120].
Figure 6.
MEP of styrene monomer: (a) B3LYP and (b) 𝜔𝐵97𝑋D.
Figure 6 shows that the most electron-rich areas of the styrene monomer are located in the phenyl ring and on the ethylene bond, whatever the functional used. Electrostatic potential values obtained using B3LYP range from −2.395 × 10⁻² to 2.395 × 10⁻² esu, while values obtained with ωB97XD range from −2.648 × 10⁻² to 2.648 × 10⁻² esu. The use of the long-range functional ωB97XD slightly influences the electrostatic potential values of the styrene monomer. Areas where electrons are weakest are shown in blue [120]. These regions have a positive potential and appear as preferred sites for nucleophilic attack [88]. In styrene monomer, we found that electrophilic sites are concentrated on hydrogen atoms, while nucleophilic sites are located along the numerous carbon-carbon bonds present in the monomer.
4. Conclusion
In this work, we studied the electronic structures of styrene monomers using DFT and TD-DFT methods. Based on the results of the geometric optimization, we found that the two functionals used, B3LYP and ωB97XD, accurately optimize the geometric structures with bond lengths and angles consistent with the experimental results available in the literature. At the optoelectronic level, styrene exhibits a relative dielectric constant value of 3.062 , making it a good insulator, while we have , which is 13% higher than the experimental index of 1.544 at 25°C. This refractive index value reflects a transparency comparable to that of glass, the reference material. The electronic findings show that the energy gap value is E g = 5.146 eV, giving styrene an insulating character. The high IP (8.252 eV) gives styrene monomer good stability and reactivity. Styrene is also thermodynamically stable thanks to its negative G value. Based on the findings of UV-visible spectroscopy analysis, styrene absorbs and emits mainly in the UV, at wavelengths of 183.61 and 200.90 nm, respectively. In addition, the Stokes shift of 41.95 nm is low and reduces potential applicability in devices such as solar cells. With regard to linear optical properties, we found that compared with MMA, styrene monomer has a very low of 0.192 D, but exhibits better linear optical properties such as and . From the transport properties, we obtained electron and hole reorganization energies of 0.393 and 0.295 eV, respectively, meaning that styrene monomer is more favourable to hole transport than electron transport. In terms of nonlinear optics, styrene has a non-zero value of β HRS, which confirms the existence of first-order NLO activity in styrene, while the DR gives styrene a dipolar contribution. In fact, showing that SHG response of styrene is 2.39 times greater than that of quartz, which is a reference material for SHG. Styrene is also more suitable for the potential generation of THG than MMA. For EOPE, styrene is ideally suited to the functional design of materials suitable for the electro-optical Pockel effect. Indeed, its EOPE susceptibility is almost times higher than that of MMA, making it a better functionalizer than MMA. Finally, compared with MMA, we can theoretically conclude that styrene monomer has good intrinsic potential for the functionalization-based design of new materials for optoelectronics, transparent organic semiconductors, NLO applications and charge transport.
Acknowledgements
We thank the University of Yaoundé I and Dr ABOMO ABEGA Francois Xavier.
Contributor Information
P. Noudem, Email: patrick.noudem@facsciences-uy1.cm; patnoudem@yahoo.com.
D. Fouejio, Email: fouejiodavid@gmail.com.
C. D. D. Mveme, Email: mvemed93@yahoo.com.
S. S. Zekeng, Email: sergezekeng@yahoo.fr.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
All input and Gaussian checkpoint file as well as output are available from the Dryad Digital Repository [110].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors’ contributions
P.N.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing—original draft, writing—review and editing; D.F.: data curation, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing—original draft, writing—review and editing; C.D.D.M.: data curation, formal analysis, methodology, software, validation, visualization, writing—original draft, writing— review and editing; S.S.Z.: methodology, project administration, resources, supervision, validation, visualization, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
No funding has been received for this article.
References
- 1. Shevlin S, Castro B, Li X. 2021. Computational materials design. Nat. Mater. 20 , 727. ( 10.1038/s41563-021-01038-8) [DOI] [PubMed] [Google Scholar]
- 2. Noudem P, Fouejio D, Mveme CDD, Zekeng SS, Tchangnwa Nya F, Ejuh GW. 2022. Hartree-Fock and DFT studies of the optoelectronic, thermodynamic structural, and nonlinear optical properties of photochromic polymers containing styrylquinoline fragments. Mater. Chem. Phys. 281 , 125883. ( 10.1016/j.matchemphys.2022.125883) [DOI] [Google Scholar]
- 3. Marzari N, Ferretti A, Wolverton C. 2021. Electronic-structure methods for materials design. Nat. Mater. 20 , 736–749. ( 10.1038/s41563-021-01013-3) [DOI] [PubMed] [Google Scholar]
- 4. Noudem P, Fouejio D, Mveme CDD, Nya FT, Zekeng SS. 2023. Electronic, nonlinear optical, UV–vis, and NBO analysis of methyl methacrylate for optoelectronic and optical applications: DFT study and impact of conformation. Spectrochim. Acta A 303 , 123267. ( 10.1016/j.saa.2023.123267) [DOI] [PubMed] [Google Scholar]
- 5. Noudem P, Fouejio D, Mveme CDD, Nya FT, Zekeng SS. 2023. Theoretical investigations of the electronic structure, Spectroscopic (IR, Raman, and UV–vis), optoelectronic, thermodynamic, and nonlinear optical properties of chromophores of 2 styrylquinoline and 2 (3 nitrostyryl)quinoline. Opt. Quantum Electron. 55 , 1240. ( 10.1007/s11082-023-05495-0) [DOI] [Google Scholar]
- 6. Hohenberg P, Kohn W. 1964. Inhomogeneous electron gas. Phys. Rev. 136 , B864–B871. ( 10.1103/PhysRev.136.B864) [DOI] [Google Scholar]
- 7. Van Noorden R, Maher B, Nuzzo R. 2014. The top 100 papers. Nat. New Biol. 514 , 550–553. ( 10.1038/514550a) [DOI] [PubMed] [Google Scholar]
- 8. Tsague LF, Ejuh GW, Ngoupo AT, Assatse YT, Kamsi RAY, Abe MTO, Ndjaka JMB. 2023. Ab-initio and density functional theory (DFT) computational study of the effect of fluorine on the electronic, optical, thermodynamic, hole, and electron transport properties of the circumanthracene molecule. Heliyon 9 , e19647. ( 10.1016/j.heliyon.2023.e19647) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Janjua MRS. 2021. Theoretical framework for encapsulation of inorganic B12N12 nanoclusters with alkaline earth metals for efficient hydrogen adsorption: a step forward toward hydrogen storage materials. Inorg. Chem. 60 , 2816–2828. ( 10.1021/acs.inorgchem.0c03730) [DOI] [PubMed] [Google Scholar]
- 10. Fouejio D, Noudem P, Mveme C, Zekeng S, Fankam Fankam JB. 2023. Impact of doping on the reactivity of photochromic polymers containing styrylquinoline fragments: Hartree-Fock and DFT study. Res. Highl. Sci. Technol. 8 , 162–197. ( 10.9734/bpi/rhst/v8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Janjua MRSA, Irfan A, Hussien M, Ali M, Saqib M, Sulaman M. 2022. Machine-learning analysis of small-molecule donors for fullerene-based organic solar cells. Energy Tech. 10 , 2200019. ( 10.1002/ente.202200019) [DOI] [Google Scholar]
- 12. Irfan A, Hussien M, Mehboob MY, Ahmad A, Janjua MRS. 2022. Learning from fullerenes and predicting for Y6: machine learning and high-throughput screening of small molecule donors for organic solar cells. Energy Tech. 10 , 2101096. ( 10.1002/ente.202101096) [DOI] [Google Scholar]
- 13. Brütting W. 2005. Introduction to the physics of organic semiconductors, pp. 1–14. Wiley-VCH, Weinheim, Germany. ( 10.1002/3527606637) [DOI] [Google Scholar]
- 14. Hofmann AI, Kroon R, Zokaei S, Järsvall E, Malacrida C, Ludwigs S, Biskup T, Müller C. 2020. Chemical doping of conjugated polymers with the strong oxidant magic blue. Adv. Electron. Mater. 6 , 2000249. ( 10.1002/aelm.202000249) [DOI] [Google Scholar]
- 15. Fahlman M, Fabiano S, Gueskine V, Simon D, Berggren M, Crispin X. 2019. Interfaces in organic electronics. Nat. Rev. Mater. 4 , 627–650. ( 10.1038/s41578-019-0127-y) [DOI] [Google Scholar]
- 16. Lund A, van der Velden NM, Persson NK, Hamedi MM, Müller C. 2018. Electrically conducting fibers for E-textiles: an open playground for conjugated polymers and carbon nanomaterials. Mater. Sci. Eng. R. Rep. 126 , 1–29. ( 10.1016/j.mser.2018.03.001) [DOI] [Google Scholar]
- 17. Noudem P, Fouejio D, Mveme CDD, Zekeng SS, Fankam Fankam JB. 2022. Impact of doping on the optoelectronic, electronic, and nonlinear optical properties and on the reactivity of photochromic polymers containing styrylquinoline fragments: Hartree-Fock and DFT study. Heliyon 8 , e11491. ( 10.1016/j.heliyon.2022.e11491) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murad AR, Iraqi A, Aziz SB, Abdullah SN, Brza MA. 2020. Conducting polymers for optoelectronic devices and organic solar cells. Polymers 12 , 2627. ( 10.3390/polym12112627) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ostroverkhova O. 2016. Organic optoelectronic materials: mechanisms and applications. Chem. Rev. 116 , 13279–13412. ( 10.1021/acs.chemrev.6b00127) [DOI] [PubMed] [Google Scholar]
- 20. Dou L, Liu Y, Hong Z, Li G, Yang Y. 2015. Low-bandgap near-IR conjugated polymers/molecules for organic electronics. Chem. Rev. 115 , 12633–12665. ( 10.1021/acs.chemrev.5b00165) [DOI] [PubMed] [Google Scholar]
- 21. Anthony JE. 2008. The larger acenes: versatile organic semiconductors. Angew. Chem. Int. Ed. 47 , 452–483. ( 10.1002/anie.200604045) [DOI] [PubMed] [Google Scholar]
- 22. Kosar N, Wajid S, Ayub K, Mahmood T. 2023. Excellent static and dynamic hyperpolarizabilities of TM@ C6O6Li6 C6O6Li6 (TM= SC, TI, V, Cr and mn) complexes to prove their NLO applications. Optik 276 , 170660. ( 10.1016/j.ijleo.2023.170660) [DOI] [Google Scholar]
- 23. Asif M, Sajid H, Qureshi S, Gilani MA, Mahmood T, Ayub K. 2023. Boron-rich triphenylene COF-based electrides having excellent nonlinear optical activity. Mater. Sci. Semicond. Process. 160 , 107468. ( 10.1016/j.mssp.2023.107468) [DOI] [Google Scholar]
- 24. Bano R, Arshad M, Mahmood T, Ayub K, Sharif A, Tabassum S, Gilani MA. 2022. Superalkali (Li2F, Li3F) doped Al12N12 electrides with enhanced static, dynamic nonlinear optical responses and refractive indices. Mater. Sci. Semicond. Process. 143 , 106518. ( 10.1016/j.mssp.2022.106518) [DOI] [Google Scholar]
- 25. Ribouem A Bessong CD, Ottou Abe MT,Ntieche Z,Noudem P, Olinga Mbala GF, Ndjaka JMB. 2023. Influence of lithium doping on optoelectronic, electronic, reactivity descriptors, thermodynamic, and nonlinear optical properties of dibenzo[B,Def]chrysene: insight by a DFT study. Opt. Quantum Electron. 55 . ( 10.1007/s11082-023-05294-7) [DOI] [Google Scholar]
- 26. Budyka MF. 2014. Design principles and action of molecular logic gates. Russ. Chem. Bull. 63 , 1656–1665. ( 10.1007/s11172-014-0651-2) [DOI] [Google Scholar]
- 27. Budyka MF, Potashova NI, Gavrishova TN, Lee VM. 2008. Molecular logic gates based on 2 styrylquinoline derivatives. Russ. Chem. Bull. 57 , 2586–2591. ( 10.1007/s11172-008-0372-5) [DOI] [Google Scholar]
- 28. Li VM, Gavrishova TN, Budyka MF. 2012. Microwave-assisted solvent-free synthesis of 2-styrylquinolines in the presence of zinc chloride. Russ. J. Org. Chem. 48 , 823–828. ( 10.1134/S1070428012060139) [DOI] [Google Scholar]
- 29. Janjua MRSA, Guan W, Yan L, Su Z-M, Karim A, Akbar J. 2010. Quantum chemical design for enhanced second-order NLO response of terpyridine-substituted hexamolybdates. Eur. J. Inorg. Chem. 2010 , 3466–3472. ( 10.1002/ejic.201000428) [DOI] [Google Scholar]
- 30. Janjua MRSA. 2017. Nonlinear optical response of a series of small molecules: quantum modification of Π-spacer and acceptor. J. Iran. Chem. Soc. 14 , 2041–2054. ( 10.1007/s13738-017-1141-x) [DOI] [Google Scholar]
- 31. Janjua MRSA, Guan W, Yan L, Su ZM, Ali M, Bukhari H. 2010. Prediction of robustly large molecular second-order nonlinear optical properties of terpyridine-substituted hexamolybdates: structural modelling towards a rational entry to NLO materials. J. Mol. Graph. Model. 28 , 735–745. ( 10.1016/j.jmgm.2010.01.011) [DOI] [PubMed] [Google Scholar]
- 32. Janjua MRSA, Su ZM, Guan W, Liu CG, Yan LK, Song P, Maheen G. 2010. Tuning second-order non-linear (NLO) optical response of organoimido-substituted hexamolybdates through halogens: quantum design of novel organic-inorganic hybrid NLO materials. Aust. J. Chem. 63 , 836. ( 10.1071/CH10094) [DOI] [Google Scholar]
- 33. Janjua MRSA. 2017. first theoretical framework of di-substituted donor moieties of triphenylamine and carbazole for NLO properties: quantum paradigms of interactive molecular computation. Mol. Simul. 43 , 1539–1545. ( 10.1080/08927022.2017.1332413) [DOI] [Google Scholar]
- 34. Mahmood R, Janjua MRSA, Jamil S. 2017. DFT molecular simulation for design and effect of core bridging acceptors (BA) on NLO response: first theoretical framework to enhance nonlinearity through BA. J. Clust. Sci. 28 , 3175–3183. ( 10.1007/s10876-017-1287-9) [DOI] [Google Scholar]
- 35. Mveme CDD, Nya FT, Ejuh GW, Kamsi RAY, Ndjaka JMB. 2020. Density functional theory study of optoelectronic, nonlinear optical, piezoelectric, and thermodynamic properties of Poly(3,4 ethylenedioxythiophene). Opt. Quantum Electron. 52 , 373. ( 10.1007/s11082-020-02492-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mveme CDD, Nya FT, Ejuh GW, Malloum A, Conradie J, Ndjaka JMB. 2021. DFT study of new organic materials based on PEDOT and 4-[2-(2-N, N-dihydroxy amino thiophene) vinyl] benzenamine. J. Mol. Model. 27 , 9. ( 10.1007/s00894-021-04827-9) [DOI] [PubMed] [Google Scholar]
- 37. Janjua MRSA, Jamil S, Mahmood A, Zafar A, Haroon M, Bhatti HN. 2015. Solvent-dependent non-linear optical properties of 5,59-disubstituted-2,29-bipyridine complexes of ruthenium(II): a quantum chemical perspective. Aust. J. Chem. 68 , 1502. ( 10.1071/CH14736) [DOI] [Google Scholar]
- 38. Samyn C, Verbiest T, Persoons A. 2000. Second-order non-linear optical polymers. Macromol. Rapid Commun. 21 , 1–15. () [DOI] [Google Scholar]
- 39. Guichaoua D, Kulyk B, Smokal V, Migalska-Zalas A, Kharchenko O, Krupka O, Kolendo O, Sahraoui B. 2019. UV irradiation induces NLO modulation in photochromic styrylquinoline-based polymers: computational and experimental studies. Org. Electron. 66 , 175–182. ( 10.1016/j.orgel.2018.12.022) [DOI] [Google Scholar]
- 40. Chavan V, Su Seo K, Wesdemiotis C, Quirk RP. 2019. Synthesis of poly(methyl methacrylate)-B-poly[(4-vinylphenyl)dimethylsilane] via atom transfer radical polymerization and its in-chain functionalization. Polym. Chem. 11 , 876–881. ( 10.1039/C9PY01512D) [DOI] [Google Scholar]
- 41. Stadtmüller B, et al. 2019. Strong modification of the transport level alignment in organic materials after optical excitation. Nat. Commun. 10 , 1470. ( 10.1038/s41467-019-09136-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Albu AM, Marculescu B, Vasilescu DS. 1999. Synthesis and characterization of some polymers with applications in non-linear optics II. Copolymerization of styrene with some monomer containing azo-dyes. Eur. Polym. J. 35 , 2203–2205. ( 10.1016/S0014-3057(99)00019-1) [DOI] [Google Scholar]
- 43. Soykan C, Erol I. 2003. Synthesis, spectral, and thermal properties of Homo- and copolymers of 2-[(5-Methylisoxazol-3-Yl)Amino]-2-Oxo-ethyl and determination of monomer reactivity ratios. Eur. Polym. J. 39 , 2261–2270. ( 10.1016/j.eurpolymj.2003.07.001) [DOI] [Google Scholar]
- 44. Schijndel JV, Molendijk D, Beurden KV, Canalle LA, Noël T, Meuldijk J. 2020. Preparation of bio-based styrene alternatives and their free radical polymerization. Eur. Polym. J. 125 , 109534. ( 10.1016/j.eurpolymj.2020.109534) [DOI] [Google Scholar]
- 45. Zhao L, Zhu W, Papadaki MI, Mannan MS, Akbulut M. 2019. Probing into styrene polymerization runaway hazards: effects of the monomer mass fraction. ACS Omega 4 , 8136–8145. ( 10.1021/acsomega.9b00004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pilevar AZ, Bahrami A, Beikzadeh S, Hosseini H, Jafari SM. 2019. Migration of styrene monomer from polystyrene packaging materials into foods: characterization and safety evaluation. Trends Food Sci. Technol. 91 , 248–261. ( 10.1016/j.tifs.2019.07.020) [DOI] [Google Scholar]
- 47. Satterthwaite MK. 2017. Plastics based on styrene. In Brydson’s plastics materials (ed. Gilbert M), pp. 311–328. Butterworth–Heinemann, Oxford, United Kingdom. ( 10.1016/B978-0-323-35824-8.00012-8) [DOI] [Google Scholar]
- 48. Zhao L, Shen Y, Sun Y, Mannan MS, Akbulut M. 2020. Thermal hazards analysis of styrene in contact with impurities. J. Loss Prev. Process Ind. 68 , 104315. ( 10.1016/j.jlp.2020.104315) [DOI] [Google Scholar]
- 49. Yildirim MB. 2012. Radiation induced copolymerization reactivity of different allyl monomers with styrene. Radiat. Phys. Chem. 81 , 840–845. ( 10.1016/j.radphyschem.2012.03.016) [DOI] [Google Scholar]
- 50. Kharchenko O, Smokal V, Krupka O, Virych P, Kutsevol N. 2020. Stabilization of polystyrene by styrylquinoline containing methacrylates. Mol. Cryst. Liq. Cryst. 699 , 51–56. ( 10.1080/15421406.2020.1732538) [DOI] [Google Scholar]
- 51. Eben M, Cithuraj K, Justus S, Bhagavathsingh AS. 2020. Synthesis and characterization of stretchable IPN polymers from biodegradable resins incorporated with styrene and methyl methacrylate monomers for enhanced mechanical strength. Eur. Polym. J. 138 , 109957. ( 10.1016/j.eurpolymj.2020.109957) [DOI] [Google Scholar]
- 52. Qiu Z, Wang S, Wang Y, Li J, Xiao Z, Wang H, Liang D, Xie Y. 2020. Transparent wood with thermo-reversible optical properties based on phase-change material. Compos. Sci. Technol. 200 , 108407. ( 10.1016/j.compscitech.2020.108407) [DOI] [Google Scholar]
- 53. Bronskaya G, Manuyko G, Balzamov D, Ignashina T, Bashkirov D, Kharitonova O, Kondrateva M, Khabibullina G. 2023. Copolymerization of butadiene and styrene under the influence of N-butyllithium. Arab. J. Chem. 16 , 105205. ( 10.1016/j.arabjc.2023.105205) [DOI] [Google Scholar]
- 54. Guazzotti V, Hendrich V, Gruner A, Fiedler D, Störmer A, Welle F. 2022. Migration of styrene in yogurt and dairy products packaged in polystyrene: results from market samples. Foods 11 , 2120. ( 10.3390/foods11142120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang J, Lee J, Kwon EE, Jeong S. 2023. Quantitative analysis of polystyrene microplastic and styrene monomer released from plastic food containers. Heliyon 9 , e15787. ( 10.1016/j.heliyon.2023.e15787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Erten H, Soykan C. 2014. Synthesis and characterization of novel poly(P-methyl styrene) containing azetidine moieties and their optical and semiconducting properties. Mater. Sci. Semicond. Process. 24 , 83–89. ( 10.1016/j.mssp.2014.03.012) [DOI] [Google Scholar]
- 57. Agda F, Nebbach D, Abram T, Bouachrine M, Taleb M. 2020. Photophysical properties of electroluminescent molecules based on thiophene and oxadiazole. Results Chem. 2 , 100068. ( 10.1016/j.rechem.2020.100068) [DOI] [Google Scholar]
- 58. Zaier R, Hajaji S, Kozaki M, Ayachi S. 2019. DFT and TD-DFT studies on the electronic and optical properties of linear Π-conjugated cyclopentadithiophene (CPDT) dimer for efficient blue OLED. Opt. Mater. (Amst.) 91 , 108–114. ( 10.1016/j.optmat.2019.03.013) [DOI] [Google Scholar]
- 59. Fuyu S, Jin R. 2017. DFT and TD-DFT study on the optical and electronic properties of derivatives of 1,4-bis(2-substituted-1,3,4-oxadiazole)benzene. Arab. J. Chem. 10 , 2988–2993. ( 10.1016/j.arabjc.2013.11.037) [DOI] [Google Scholar]
- 60. Jin R, Zhang X, Xiao W. 2020. Theoretical studies of photophysical properties of D−π−A−π−D-type diketopyrrolopyrrole-based molecules for organic light-emitting DIODES and organic solar cells. Molecules 25 , 667. ( 10.3390/molecules25030667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tedjeuguim CT, Tasheh SN, Alongamo CIL, Ghogomu JN. 2022. A DFT and TD-DFT study of charge transport and non-linear optical properties of N-(4- methoxybenzylidene)isonicotinohydrazone, 2,2’-bipyridine and their Fe2+, Ni2+, Cu2+, Pd2+ and Pt2+ complexes. J. Chem. Sci. 134 , 3. ( 10.1007/s12039-022-02060-2) [DOI] [Google Scholar]
- 62. Tendongmo H, Tasheh SN, Tamafo DAF, Bine FK, Numbonui Ghogomu J. 2022. Theoretical study of the structural, optoelectronic, and reactivity properties of N-5-(9-methyl-3-ISO-xazolyl)-3-(E)-1-(2-methylidene)amine and some of its Fe2+, Co2+, Ni2+, Cu2+, and Zn2+ complexes for OLED and OFET applications. J. Chem. 2022 , 1–18. ( 10.1155/2022/3528170) [DOI] [Google Scholar]
- 63. Bissi Nyandou PF, Noudem P, Mveme CDD, Fouejio D, Zekeng SS. 2024. DFT study of the intrinsic electronic, optical, NBO, transport, and thermodynamic properties of 12,12-dimethyl-7-phenyl-7,12-dihydrobenzo[A]acridine-3-carbonitrile (BACN). Chin. J. Phys. 89 , 1862–1882. ( 10.1016/j.cjph.2024.04.031) [DOI] [Google Scholar]
- 64. Dennington R, Millam JM. 2016. Gauss view 6.0. Shawnee Mission, KS: Semichem Inc. [Google Scholar]
- 65. Frisch MJ, et al. 2016. Gaussian 16 (revision C. 01). Wallingford, CT: Gaussian, Inc. [Google Scholar]
- 66. Abe FB, Ndjaka JMB, Nya FTM. 2017. Electronic structure, physico-chemical, linear and nonlinear optical properties analysis of coronene, 6B-, 6N-, 3B3N-substituted C24H12 using RHF, b3lyp, and wb97xd methods. Opt. Quant. Electron. 382. ( 10.1007/s11082-017-1221-2) [DOI] [Google Scholar]
- 67. Tchangnwa Nya F, Ejuh GW, Ndjaka JMB. 2017. Theoretical study of optoelectronic and thermodynamic properties of molecule 4-[2-(2-N,N-dihydroxy amino thiophene)vinyl]benzanamine: influence of hydroxyl position. Mater. Lett. 202 , 89–95. ( 10.1016/j.matlet.2017.05.064) [DOI] [Google Scholar]
- 68. Ejuh GW, Nouemo S, Nya FT, Ndjaka JM. 2016. Computational determination of the electronic and nonlinear optical properties of the molecules 2-(4-Aminophenyl) quinoline, 4-(4-Aminophenyl) quinoline, anthracene, anthraquinone, and phenanthrene. Mater. Lett. 178 , 221–226. ( 10.1016/j.matlet.2016.04.097) [DOI] [Google Scholar]
- 69. Tadjouteu Assatse Y, Ejuh GW, Yossa Kamsi RA, Tchoffo F, Ndjaka JMB. 2019. Theoretical studies of nanostructures modeled by the binding of uracil derivatives to functionalized (5,5) carbon nanotubes. Chem. Phys. Lett. 731 , 136602. ( 10.1016/j.cplett.2019.136602) [DOI] [Google Scholar]
- 70. Chai JD, Head-Gordon M. 2008. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 10 , 6615. ( 10.1039/b810189b) [DOI] [PubMed] [Google Scholar]
- 71. Fankam Fankam JB, Ejuh GW, Nya FT, Ndjaka JMB. 2020. Study of electronic structure, optoelectronics, linear and nonlinear optical properties, and chemical descriptors of dibromodinitrofluorescein isomers in gas phase and solvent media using AB Initio and DFT methods. Chin. J. Phys. 66 , 461–473. ( 10.1016/j.cjph.2020.05.015) [DOI] [Google Scholar]
- 72. Mubarik A, Rasool N, Hashmi MA, Mansha A, Zubair M, Shaik MR, Sharaf MAF, Awwad EM, Abdelgawad A. 2021. Computational study of structural, molecular orbitals, optical, and thermodynamic parameters of thiophene sulfonamide derivatives. Crystals 11 , 211. ( 10.3390/cryst11020211) [DOI] [Google Scholar]
- 73. Yasuda N, Uekusa H, Ohashi Y. 2001. Styrene at 83 K. Acta Crystallogr. Sect. E. Struct. Rep. 57 , 1189–1190. ( 10.1107/S1600536801019237) [DOI] [Google Scholar]
- 74. Hu Y, Lv J, Hao Q. 2021. Refractive index measurement of glass with arbitrary shape based on brewster’s law and a focusing probe beam. Sensors 21 , 2421. ( 10.3390/s21072421) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gunter P. 2000. Nonlinear optical effects and materials. Springer-Verlag, Berlin, Germany: Springer Nature. ( 10.1007/978-3-540-49713-4) [DOI] [Google Scholar]
- 76. Rebiai‐Benahmed S. 2020. Composants optoélectroniques. vol. 1. Algeria: Université des Frères Mentouri Constantine. [Google Scholar]
- 77. Aboulouard A, Mtougui S, Demir N, Moubarik A, idrissi ME, Can M. 2021. New non-fullerene electron acceptors based on quinoxaline derivatives for organic photovoltaic cells: DFT computational study. Synth. Met. 279 , 116846. ( 10.1016/j.synthmet.2021.116846) [DOI] [Google Scholar]
- 78. Mehboob MY, et al. 2020. Designing N-phenylaniline-triazol configured donor materials with promising optoelectronic properties for high-efficiency solar cells. Comput. Theor. Chem. 1186 , 112908. ( 10.1016/j.comptc.2020.112908) [DOI] [Google Scholar]
- 79. Mehboob MY, Hussain R, Khan MU, Adnan M, Ehsan MA, Rehman A, Janjua MRSA. 2021. Quantum chemical design of near‐infrared sensitive fused ring electron acceptors containing selenophene as π‐bridge for high‐performance organic solar cells. J. Phys. Org. Chem. 34 . ( 10.1002/poc.4204) [DOI] [Google Scholar]
- 80. Sun XQ, Qin GY, Lin PP. 2021. Theoretical derivatives with aryl substituents at the 2,6-position: thermally stable 'herringbone' stacking motifs. Phys. Chem. Chem. Phys. 23 , 12 679–12 691. ( 10.1039/d1cp00178g0) [DOI] [PubMed] [Google Scholar]
- 81. Janjua M. 2022. All-small-molecule organic solar cells with high fill factor and enhanced open-circuit voltage with 18.25 % PCE: physical insights from quantum chemical calculations. Spectrochim. Acta A. 279 , 121487. ( 10.1016/j.saa.2022.121487) [DOI] [PubMed] [Google Scholar]
- 82. Mehboob MY, Hussain R, Adnan M, Farwa U, Irshad Z, Saeed Ashraf Janjua MR. 2022. Theoretical modeling of novel indandione-based donor molecules for organic solar cell applications. J. Phys. Chem. Solids 162 , 110508. ( 10.1016/j.jpcs.2021.110508) [DOI] [Google Scholar]
- 83. Janjua M. 2021. How does bridging core modification alter the photovoltaic characteristics of triphenylamine-based hole transport materials theoretical understanding and prediction. Chemistry 27 , 4197–4210. ( 10.1002/chem.202004299) [DOI] [PubMed] [Google Scholar]
- 84. Janjua M. 2022. Photovoltaic properties and enhancement in near-infrared light absorption capabilities of acceptor materials for organic solar cell applications: a quantum chemical perspective via DFT. J. Phys. Chem. Solids. 171 , 110996. ( 10.1016/j.jpcs.2022.110996) [DOI] [Google Scholar]
- 85. Haroon M, Janjua MRSA. 2021. High-throughput designing and investigation of D−A−π−A-type donor materials for potential application in greenhouse-integrated solar cells. Energy Fuels 35 , 12461–12472. ( 10.1021/acs.energyfuels.1c01726) [DOI] [Google Scholar]
- 86. Janjua M. 2021. Quantum chemical design of D–π–A‐type donor materials for highly efficient, photostable, and vacuum‐processed organic solar cells. Energy Tech. 9 , 2100489. ( 10.1002/ente.202100489) [DOI] [Google Scholar]
- 87. Fouejio D, Kamsi RAY, Assatse YT, Ejuh GW, Ndjaka JMB. 2021. DFT studies of the structural, chemical descriptors, and nonlinear optical properties of the drug dihydroartemisinin functionalized on C60 fullerene. Comput. Theor. Chem. 1202 , 113298. ( 10.1016/j.comptc.2021.113298) [DOI] [Google Scholar]
- 88. Irfan A. 2022. Effect of nitrogen doping and acene cores elongation on charge transport and electronic nature of organic semiconductor materials: a DFT study. Iran. J. Chem. Eng. 41 , 399–408. ( 10.30492/IJCCE.2020.132142.4267) [DOI] [Google Scholar]
- 89. Zou LY, Ren AM, Feng JK, Liu YL, Ran XQ, Sun CC. 2008. Theoretical study on photophysical properties of multifunctional electroluminescent molecules with different π-conjugated bridges. J. Phys. Chem. A 112 , 12172–12178. ( 10.1021/jp8032462) [DOI] [PubMed] [Google Scholar]
- 90. Ostroverkhova O. 2013. Handbook of organic materials for optical and (opto)electronic devices. In Properties and applications (ed. Ostroverkhova O). Oxford, UK: Woodhead Publishing Limited. ( 10.1533/9780857098764). See http://www.sciencedirect.com/science/book/9780857092656. [DOI] [Google Scholar]
- 91. Naeem J, Bano R, Ayub K, Mahmood T, Tabassum S, Arooj A, Gilani MA. 2022. Assessment of alkali and alkaline earth metals doped cubanes as high-performance nonlinear optical materials by first-principles study. J. Sci. Adv. Mater. Devices 7 , 100457. ( 10.1016/j.jsamd.2022.100457) [DOI] [Google Scholar]
- 92. Tedjeuguim CT, Tasheh SN, Julius Numbonui G. 2023. Theoretical investigation of the nonlinear optical and charge transport properties of N-(4-methoxybenzylidene) isonicotinohydrazone and some of its derivatives: a DFT and TD-DFT study. Adv. Mater. Sci. Eng. 2023 , 1–14. ( 10.1155/2023/6588603) [DOI] [Google Scholar]
- 93. Manzoni V, Gester R, Cunha Ad, Andrade-Filho T, Gester R. 2021. Solvent effects on Stokes shifts and NLO response of thieno[3,4-B]pyrazine: a comprehensive QM/MM investigation. J. Mol. Liq. 335 , 115996. ( 10.1016/j.molliq.2021.115996) [DOI] [Google Scholar]
- 94. Bano R, Hussain S, Arshad M, Rauf A, Mahmood T, Ayub K, Gilani MA. 2022. Lanthanum-doped corannulenes with enhanced static and dynamic nonlinear optical properties: a first-principle study. Physica B: Condensed Matter 641 , 414088. ( 10.1016/j.physb.2022.414088) [DOI] [Google Scholar]
- 95. Cassidy C, Halbout JM, Donaldson W, Tang CL. 1979. Nonlinear optical properties of urea. Opt. Commun. 29 , 243–246. ( 10.1016/0030-4018(79)90027-0) [DOI] [Google Scholar]
- 96. Jerphagnon J, Choy MM, Kurtz SK. 1979. Nonlinear dielectric susceptibilities (landolt-bornstein: numerical data and functional relationships in science and technology. Berlin, Germany: Springer. ( 10.1093/gji/74.2.644) [DOI] [Google Scholar]
- 97. Bosshard C, Gubler U. 2000. Optical third-harmonic generation of fused silica in gas atmosphere: absolute value of the third-order nonlinear optical susceptibility Χ(3). Phys. Rev. B. 61 , 10702–10710. ( 10.1103/PhysRevB.61.10702) [DOI] [Google Scholar]
- 98. Kajzar F, Okada-Shudo Y, Meritt C, Kafafi Z. 2001. Second- and third-order non-linear optical properties of multilayered structures and composites of C60 with electron donors. Synth. Met. 117 , 189–193. ( 10.1016/S0379-6779(00)00498-7) [DOI] [Google Scholar]
- 99. Santos FA, Cardoso CER, Rodrigues JJ, De Boni L, Abegão LMG. 2023. Nonlinear optical materials: predicting the first-order molecular hyperpolarizability of organic molecular structures. Photonics 10 , 545. ( 10.3390/photonics10050545) [DOI] [Google Scholar]
- 100. Gu B, Zhao C, Baev A, Yong KT, Wen S, Prasad PN. 2016. Molecular nonlinear optics: recent advances and applications. Adv. Opt. Photon. 8 , 328. ( 10.1364/AOP.8.000328) [DOI] [Google Scholar]
- 101. Reshef O, De Leon I, Alam MZ, Boyd RW. 2019. Nonlinear optical effects in epsilon-near-zero media. Nat. Rev. Mater. 4 , 535–551. ( 10.1038/s41578-019-0120-5) [DOI] [Google Scholar]
- 102. Plaquet A, Guillaume M, Champagne B, Castet F, Ducasse L, Pozzo JL, Rodriguez V. 2008. In silico optimization of merocyanine-spiropyran compounds as second-order nonlinear optical molecular switches. Phys. Chem. Chem. Phys. 10 , 6223–6232. ( 10.1039/b806561f) [DOI] [PubMed] [Google Scholar]
- 103. Tarazkar M, Romanov DA, Levis RJ. 2014. Higher-order nonlinearity of refractive index: the case of argon. J. Chem. Phys. 140 , 214316. ( 10.1063/1.4880716) [DOI] [PubMed] [Google Scholar]
- 104. Milam D. 1998. Review and assessment of measured values of the nonlinear refractive-index coefficient of fused silica. Appl. Opt. 37 , 546–550. ( 10.1364/ao.37.000546) [DOI] [PubMed] [Google Scholar]
- 105. Sheik-Bahae M, Said AA, Wei TH, Hagan DJ, Van Stryland EW. 1990. Sensitive measurement of optical nonlinearities using a single beam. IEEE J. Quantum Electron. 26 , 760–769. ( 10.1109/3.53394) [DOI] [Google Scholar]
- 106. Qiu X, Xu L, Ying S, Ye X, Xu P, Wang B, Hu D, Ma D, Ma Y. 2021. Novel 12,12-dimethyl-7,12-dihydrobenzo[A]-acridine as a deep-blue emitting chromophore for oleds with narrow band emission and suppressed efficiency roll-off. J. Mater. Chem. C 9 , 13697–13703. ( 10.1039/D1TC03898B) [DOI] [Google Scholar]
- 107. Van Bay M, Hien NK, Quy PT, Nam PC, Van DU, Quang DT. 2019. Using calculations of the electronically excited states for investigation of fluorescent sensors: a review. Vietnam J. Chem. 57 , 389–400. ( 10.1002/vjch.201900089) [DOI] [Google Scholar]
- 108. Ulla H, Garudachari B, Satyanarayan MN, Umesh G, Isloor AM. 2014. Blue organic light-emitting materials: synthesis and characterization of novel 1,8-naphthalimide derivatives. Opt. Mater. 36 , 704–711. ( 10.1016/j.optmat.2013.11.017) [DOI] [Google Scholar]
- 109. Reed AE, Weinhold F. 1983. Natural bond orbital analysis of near-Hartree–Fock water dimer. J. Chem. Phys. 78 , 4066–4073. ( 10.1063/1.445134) [DOI] [Google Scholar]
- 110. Noudem P, Fouejio D, Mveme CDD, Zekeng SS. 2024. Data from: Styrene monomer as a potential material for functionalization and design of chromophores for new optoelectronic and nonlinear optical polymers conception: a density functional theory study. Dryad Digital Repository ( 10.5061/dryad.5tb2rbpbg) [DOI] [Google Scholar]
- 111. Lakhera S, Rana M, Devlal K, Dhuliya V, Pandey N. 2023. Nonlinear optical behavior of gold and silver clusters adsorbed on 2,9-dimethylquinacridone. Optik 286 , 170983. ( 10.1016/j.ijleo.2023.170983) [DOI] [Google Scholar]
- 112. Zouitina S, El Azze S, Bensemlali M, Faqir M, Morad EB, El idrissi M, Tounsi A. 2022. First-principles study of nonlinear optical and electronic properties of A-P-D-P-A configured compounds with novel oligothiophenes. Mat. Sci. Energy Technol. 5 , 473–480. ( 10.1016/j.mset.2022.10.006) [DOI] [Google Scholar]
- 113. Olinga Mbala GF, Ottou Abe MT, Ntieche Z, Ejuh GW, Ndjaka JMB. 2021. Ab Initio investigation of nonlinear optical, electronic, and thermodynamic properties of BEDT-TTF molecule: doping with boron. Heliyon 7 , e07461. ( 10.1016/j.heliyon.2021.e07461) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Zaier R, Mahdhaoui F, Ayachi S, Boubaker T. 2019. Prediction of structural, vibrational, and nonlinear optical properties of small organic conjugated molecules derived from pyridine. J. Mol. Struct. 1182 , 131–140. ( 10.1016/j.molstruc.2019.01.043) [DOI] [Google Scholar]
- 115. Lakhera S, Devlal K, Rana M, Dhuliya V. 2024. Influence of the substitution of different functional groups on the gas sensing and light harvesting efficiency of zero-dimensional coronene quantum DOT: a first principle DFT study. Spectrochim. Acta A 308 , 123737. ( 10.1016/j.saa.2023.123737) [DOI] [PubMed] [Google Scholar]
- 116. Lakhera S, Rana M, Devlal K. 2024. Comprehensive quantum chemical study of the associative complex of para-aminobenzoic acid and 7-diethylamino 4-methyl coumarin by adsorption and aromatic bridges. J. Mol. Model. 30 , 37. ( 10.1007/s00894-023-05816-w) [DOI] [PubMed] [Google Scholar]
- 117. Lakhera S, Devlal K, Rana M. 2023. Nonlinear optical response of silver trimer adsorbed para-aminobenzoic acid: a DFT study. Phys. Scr. 98 , 115519. ( 10.1088/1402-4896/ad0084) [DOI] [Google Scholar]
- 118. Nosheen B, Perveen F, Ashraf Z, Bais A, Noor T. 2020. Charge transfer and opto-electronic properties of some newly designed polycatenar discotic liquid crystal derivatives: a DFT study. J. Mol. Model. 26 , 291. ( 10.1007/s00894-020-04550-x) [DOI] [PubMed] [Google Scholar]
- 119. Lakhera S, Devlal K, Rana M. 2023. Utilization of methyclothiazide adsorbed with malonamide for quantum chemical applications: a DFT and DFT-D2/D3 study. Optik 295 , 171485. ( 10.1016/j.ijleo.2023.171485) [DOI] [Google Scholar]
- 120. Azaid A, Dhifaoui S, Bourass M, Ben Haj Hassen L, Nasri H, Bouachrine M. 2021. DFT studies on molecular structure, absorption properties, NBO analysis, and Hirshfeld surface analysis of iron(III). Porphyrin Complex. Phys. Chem. Res. 9 , 701–713. ( 10.22036/pcr.2021.272919.1886) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All input and Gaussian checkpoint file as well as output are available from the Dryad Digital Repository [110].