Abstract
Introduction:
Chronic kidney disease-mineral and bone disease (CKD-MBD) is a complication of chronic kidney disease (CKD) involving derangements in serum calcium and phosphate. This study aims to evaluate hypo- and hypercalcaemia and their associated outcomes among pre-dialysis CKD patients.
Methods:
A retrospective cohort study was performed and included all adult CKD stage 4–stage 5 patients who were on treatment for CKD-MBD between 2016 and 2017. Each patient was followed up for 3 years. Hypo- and hypercalcaemia were defined as serum corrected calcium (Ca2+) <2.10 and >2.46 mmol/L, respectively. Outcomes evaluated included all-cause mortality and cardiovascular events. Multivariate Cox regression analysis was done to evaluate the association of hypocalcaemia and/or hypercalcaemia with the clinical outcomes. Severity of hypocalcaemia episode was classified as ‘mild’ (Ca2+: between 1.90 and 2.10 mmol/L) and ‘severe’ (Ca2+: <1.90 mmol/L). Severity of hypercalcaemia was classified as ‘mild’ (Ca2+: between 2.47 and 3.00 mmol/L), moderate (Ca2+: between 3.01 and 3.50 mmol/L) and severe (Ca2+: >3.50 mmol/L).
Results:
Of the 400 patients, 169 (42.2%) and 94 (23.5%) patients experienced hypocalcaemia and hypercalcaemia, respectively. Severe hypocalcaemia was more prevalent in CKD stage 5 compared to CKD stage 4 (96 [40.5%] vs. 36 [25.9%], P = 0.004). Results from multivariate analyses after adjustment showed that hypocalcaemia and/or hypercalcaemia were not associated with all-cause mortality (P > 0.05) or the occurrence of cardiovascular events (P > 0.05).
Conclusion:
Hypocalcaemia and hypercalcaemia episodes were prevalent among pre-dialysis CKD patients. Studies with longer follow-up durations are required to assess the effects of calcium derangements on clinical outcomes.
Keywords: Chronic kidney disease, clinical outcomes, hypercalcaemia, hypocalcaemia
INTRODUCTION
Chronic kidney disease (CKD) is a major health problem that afflicts more than 10% of the global population and is associated with poor health-related outcomes.[1,2,3,4] Worldwide, the prevalence of CKD has been increasing, which is likely related to the rising incidence of diabetes mellitus and hypertension.[5,6] CKD-related mineral and bone disorder (CKD-MBD) constitutes one of the main complications experienced by CKD patients and refers to a complex systemic disorder that arises from dysregulation of calcium, phosphorus, intact parathyroid hormone (iPTH) and vitamin D levels.[7] These mineral derangements can manifest as vascular or soft tissue calcification and abnormalities in bone turnover or growth.[7]
Among patients with CKD-MBD, alterations in calcium levels, such as hypercalcaemia, are common.[8,9,10,11] Consequently, the Kidney Disease Improving Global Outcomes (KDIGO) and the National Kidney Foundation-Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) guidelines have recommended the routine monitoring of calcium levels.[12,13] The prevalence of hypercalcaemia among CKD patients on peritoneal dialysis and haemodialysis (HD) has been estimated to be 16.7%–36.6%.[10,11] Conversely, hypocalcaemia has been estimated to affect 23.8% of CKD patients.[8] A study which evaluated hypercalcaemia among CKD patients showed that previous history of hypercalcaemia, lower iPTH levels and immobility were independent risk factors associated with hypercalcaemia.[9] Another study by Vikrant et al.[8] found that hypocalcaemia tends to occur more frequently among pre-dialysis patients compared to dialysis patients.
Overall, positive calcium balance among CKD patients on dialysis has been shown to increase the risk of extraskeletal calcification and is associated with poor patient outcomes.[14] In a study by Obi et al.[15] involving 876 HD patients, hypercalcaemia was shown to increase the risk of mortality by 70%. Similar findings were also reported in two separate studies which enrolled end-stage renal disease patients.[16,17] At the other end of the spectrum, results pertaining to outcomes of hypocalcaemia in dialysis patients have been conflicting. A study by Miura et al.[18] which examined hypocalcaemia in CKD patients with heart failure showed that hypocalcaemia was an independent predictor of all-cause mortality. Another study which evaluated hypocalcaemia in incident dialysis patients similarly showed that hypocalcaemia was associated with increased risk of mortality and cardiovascular diseases.[19] However, a separate study by Fouque et al.[20] involving 8377 HD patients failed to find a significant association between hypocalcaemia and mortality. The reasons for these differing findings currently remain unclear.
Among the CKD pre-dialysis population, fewer studies have evaluated outcomes associated with hypercalcaemia and hypocalcaemia. A study conducted in the USA showed that hypercalcaemia and hypocalcaemia were associated with increased long-term and short-term mortality, respectively.[21] The study authors postulated that patients with higher calcium levels had a higher propensity of developing vascular calcification and cardiovascular events, while patients who had hypocalcaemia were more likely to develop cardiac arrhythmias, especially those not on calcium-based phosphate binders.
To date, there is paucity of data evaluating alterations in calcium levels and their associated outcomes, especially among Asian CKD pre-dialysis patients. Hence, the primary objective of the study was to determine the prevalence of hypocalcaemia and hypercalcaemia among CKD stage 4 and stage 5 pre-dialysis patients in our local patient population. Secondary objective of the study was to study the association of calcium dysregulation with clinical outcomes such as all-cause mortality and occurrence of cardiovascular events in this population of patients.
METHODS
A single-centre, retrospective cohort study was conducted in Singapore General Hospital, the largest tertiary medical centre in Singapore. The study included all adult CKD stage 4–5 pre-dialysis patients (i.e. estimated glomerular filtration rate [eGFR] <15–29 mL/min/1.73 m2) of age ≥21 years who were on treatment for CKD-MBD from 1 June 2016 to 31 May 2017. Patients were considered to be on CKD-MBD treatment if they had been prescribed phosphate binder(s) and/or vitamin D and its analogue(s). Types of phosphate binders included calcium carbonate, calcium acetate, sevelamer carbonate and lanthanum carbonate. Vitamin D and its analogues included calcitriol, alfacalcidol, cholecalciferol and ergocalciferol. Post-renal transplant or post-parathyroidectomy patients or those initiated on renal replacement therapy before the start of study or had no calcium levels performed during the study period were excluded from the study. Patient-related characteristics were charted between 1 June 2016 and 1 June 2019. Approval from the institutional review board was obtained before the initiation of study (CRIB 2020-2188).
Baseline characteristics including patients’ demographics, clinical comorbidities, CKD-MBD–related laboratory test results and medication treatment were collected. Laboratory parameters related to CKD-MBD were monitored routinely in patients as per the recommendations by international guidelines, or more frequently as clinically indicated. For example, calcium levels were monitored at least every 3–6 months or 1–3 months for CKD stage 4 or 5 (non-dialysis) patients, respectively. During the study period, baseline and subsequent levels of serum calcium, phosphate and parathyroid hormone (PTH) were recorded at six-monthly intervals. Previous history of hypocalcaemia or hypercalcaemia episode was defined as the presence of at least one or more episodes of hypocalcaemia or hypercalcaemia experienced by each patient in the past 1 year from the study’s start date.
Corrected calcium was computed using the following formula:
corrected calcium (mmol/L) = serum calcium (mmol/L) + 0.02 [40 − albumin (g/L)].[22]
The normal reference range for serum calcium in the institution is between 2.10 and 2.46 mmol/L. Patients were deemed to have a hypocalcaemia episode if the serum corrected calcium was less than 2.10 mmol/L. Severity of hypocalcaemia episode was classified as ‘mild’ if the serum corrected calcium was between 1.9 and 2.1 mmol/L and ‘severe’ if the serum corrected calcium was less than 1.9 mmol/L.[23] Patients were deemed to be have a hypercalcaemia episode if the serum corrected calcium level was greater than 2.46 mmol/L. Severity of hypercalcaemia episode was classified as ‘mild’ if the corrected calcium was between 2.47 and 3.00 mmol/L, ‘moderate’ if the corrected calcium was between 3.01 and 3.50 mmol/L and ‘severe’ if the corrected calcium was greater than 3.50 mmol/L.[24] For patients who were commenced on renal replacement therapy or those who underwent renal transplant or parathyroidectomy during the study period, data were censored after the occurrence of these events.
Primary outcome measure included the proportion of hypocalcaemia and hypercalcaemia episodes. Secondary outcome measures included events of all-cause mortality, development of cardiovascular events, development of skeletal events and development of calciphylaxis. Development of cardiovascular events was defined as the presence of acute coronary syndrome or history of myocardial infarction or angina or coronary revascularisation or stroke or atherosclerotic transient ischaemic attack or peripheral arterial disease.[25] Development of skeletal events was defined as the presence of any skeletal fracture.
All statistical analyses were carried out using the Stata software, version 14.0 (Stata Corporation, College Station, TX, USA; 2016). Descriptive statistics was used to report the baseline characteristics of patients. Continuous variables were reported as mean ± standard deviation or median (interquartile range [IQR]), while categorical variables were reported as number (percentage). Shapiro–Wilk test was used to test for normality of data. Descriptive statistics was performed on patients’ demographic data and patients’ clinical characteristics. Chi-square test, Kruskal–Wallis and analysis of variance (ANOVA) test were used to test for differences, where appropriate. Univariate analysis was done to evaluate the association of hypocalcaemia and/or hypercalcaemia episodes with clinical outcomes. Multivariate Cox analysis was done to evaluate the association between hypocalcaemia and/or hypercalcaemia with all-cause mortality and the risk of cardiovascular events. In this study, as the incidence of skeletal events and calciphylaxis was low in the institution, these outcomes were excluded from multivariate Cox regression analysis. The analyses were adjusted for age and CKD staging. Survival probabilities were segregated by calcium status (presence of hypocalcaemia and/or hypercalcaemia episodes) and analysed using Kaplan–Meier curve. The results were compared using log-rank test. A two-tailed P value <0.05 was considered statistically significant.
RESULTS
Of the 688 patients eligible for recruitment into the study, 400 (58.1%) patients were included after excluding the patients who did not fulfil the inclusion criteria [Figure 1].
Figure 1.
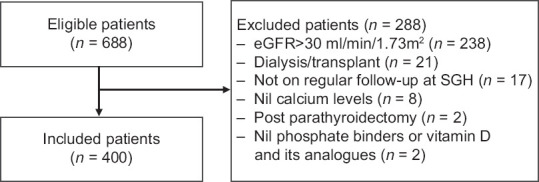
Flowchart of patients included in the study. eGFR: estimated glomerular filtration rate, SGH: Singapore General Hospital.
The demographics and clinical characteristics of the study patients are shown in Table 1. The median age of patients was 77 (IQR: 67–85) years. There were 187 (46.8%) and 213 (53.3%) patients who had CKD stage 4 and stage 5, respectively. Majority of the patients were Chinese (72.5%), female (53.5%), and the most common aetiology of renal dysfunction was diabetic nephropathy (n = 240, 60.0%). The median follow-up duration for all patients was 445.5 (IQR: 255.5–950.5) days.
Table 1.
Demographics and clinical characteristics of patients.
| Characteristicsa | CKD stage 4 (n=187) | CKD stage 5 (n=213) | Overall (n=400) | P |
|---|---|---|---|---|
| Age (years) | 77 (67-85) | 76 (67-85) | 77 (67-85) | 0.50 |
|
| ||||
| Gender | ||||
| Male | 86 (46.0) | 100 (47.0) | 186 (46.5) | 0.85 |
|
| ||||
| eGFR (mL/min/1.73 m2) | 19.9 (17.4-23.4) | 10.6 (8.2-12.8) | 14.3 (10.2-19.7) | <0.001 |
|
| ||||
| Race | 0.09 | |||
| Chinese | 127 (67.9) | 163 (76.5) | 290 (72.5) | |
| Malay | 28 (15.0) | 31 (14.6) | 59 (14.8) | |
| Indian | 27 (14.4) | 13 (6.1) | 40 (10.0) | |
| Eurasian | 1 (0.5) | 2 (0.9) | 3 (0.8) | |
| Others | 4 (2.1) | 4 (1.9) | 8 (2.0) | |
|
| ||||
| Body mass index (kg/m2) | 24.8 (21.4-28.0) | 24.2 (21.2-27.9) | 24.4 (21.3-28.0) | 0.34 |
|
| ||||
| Smoking status | 0.96 | |||
| Non-smoker | 156 (83.4) | 179 (84.0) | 335 (83.8) | |
| Current smoker | 9 (4.8) | 9 (4.2) | 18 (4.5) | |
| Ex-smoker | 22 (11.8) | 25 (11.7) | 47 (11.8) | |
|
| ||||
| Aetiology of chronic kidney disease | 0.17 | |||
| Diabetic nephropathy | 121 (64.7) | 119 (55.9) | 240 (60.0) | |
| Chronic glomerulonephritis | 19 (10.2) | 38 (17.8) | 57 (14.3) | |
| Hypertensive nephrosclerosis | 23 (12.3) | 34 (16.0) | 57 (14.3) | |
| Polycystic kidney disease | 1 (0.5) | 3 (1.4) | 4 (1.0) | |
| Lupus nephritis | 1 (0.5) | 0 (0) | 1 (0.3) | |
| Obstructive uropathy | 2 (1.1) | 2 (0.9) | 4 (1.0) | |
| Others | 20 (10.7) | 17 (8.0) | 37 (9.3) | |
|
| ||||
| Comorbidities | ||||
| Hypertension | 169 (90.4) | 196 (92.0) | 365 (91.3) | 0.56 |
| Hyperlipidaemia | 143 (76.5) | 146 (68.5) | 289 (72.3) | 0.08 |
| Type 2 diabetes mellitus | 138 (73.8) | 143 (67.1) | 281 (70.3) | 0.15 |
| Cerebrovascular accident | 29 (15.5) | 42 (19.7) | 71 (17.8) | 0.27 |
| Ischaemic heart disease | 97 (51.9) | 75 (35.2) | 172 (43.0) | 0.001 |
| Liver disease | 13 (7.0) | 18 (8.5) | 31 (7.8) | 0.58 |
| Heart failure | 36 (19.3) | 27 (12.7) | 63 (15.8) | 0.07 |
| History of malignancy | 24 (12.8) | 24 (11.3) | 48 (12.0) | 0.63 |
| Osteoarthritis | 33 (17.7) | 27 (12.7) | 60 (15.0) | 0.17 |
| Hyperthyroidism | 6 (3.2) | 6 (2.8) | 12 (3.0) | 0.82 |
| Rheumatoid arthritis | 4 (2.1) | 1 (0.5) | 5 (1.3) | 0.13 |
|
| ||||
| Baseline lipid panel | ||||
| Total cholesterol, mmol/L | 4.2 (3.4-4.9) | 4.3 (3.6-5.1) | 4.3 (3.6-5.0) | 0.15 |
| HDL, mmol/L | 1.2 (1.0-1.4) | 1.1 (1.0-1.4) | 1.2 (1.0-1.4) | 0.79 |
| LDL, mmol/L | 2.2 (1.7-2.7) | 2.3 (1.8-3.0) | 2.3 (1.7-2.8) | 0.11 |
| TG, mmol/L | 1.5 (1.1-2.0) | 1.6 (1.2-2.2) | 1.6 (1.2-2.1) | 0.09 |
|
| ||||
| Baseline CKD-MBD parameters | ||||
| Serum corrected calcium-mmol/L | 2.3 (2.2-2.4) | 2.2 (2.1-2.3) | 2.3 (2.2-2.3) | 0.0001 |
| Serum phosphate-mmol/L | 1.4 (1.1-1.5) | 1.5 (1.3-1.8) | 1.4 (1.2-1.6) | <0.0001 |
| Serum iPTH-pmol/L | 13.1 (7.9-21.9) | 23.9 (10.9-33.9) | 18.2 (9.3-30.1) | <0.0001 |
| 25-Hydroxyl vitamin D-ng/mL | 18.9 (13.1-24.5) | 17.2 (11.7-24.5) | 17.6 (12.1-24.5) | 0.32 |
|
| ||||
| Baseline medication-related parametersb | ||||
| Vitamin D analogue use Phosphate binder use | 60 (32.1) | 56 (26.3) | 116 (29.0) | 0.20 |
| Calcium-based phosphate binder (calcium acetate and calcium carbonate) | 106 (56.7) | 139 (65.3) | 245 (61.3) | 0.08 |
| Non-calcium-based phosphate binder (lanthanum) | 1 (0.5) | 3 (1.4) | 4 (1.0) | 0.38 |
| Total elemental calcium per day (mg/day) | ||||
| Phosphate binder only | 683.4 (534.1) | 888.3 (738.2) | 799.7 (664.3) | 0.002 |
|
| ||||
| Phosphate binder + calcium supplement | ||||
| <1500 mg | 169 (90.4) | 179 (87.0) | 348 (87.0) | 0.06 |
| ≥1500 mg | 18 (9.6) | 34 (13.0) | 52 (13.0) | |
aVariables are expressed as number (percentage) or median (interquartile range) where appropriate. bThere were no patients who were on calcimimetics in the study. Bold values denote statistical significance at the P < 0.05 level. CKD: chronic kidney disease, CKD-MBD: chronic kidney disease-mineral and bone disorder, eGFR: estimated glomerular filtration rate, HDL: high-density lipoprotein, iPTH: intact parathyroid hormone level, LDL: low-density lipoprotein, TG: triglyceride.
The baseline characteristics of patients with CKD stage 4 and stage 5 were largely comparable, except for the presence of ischaemic heart disease (CKD stage 4: 97 [51.9%] vs. CKD stage 5: 75 [35.2%], P = 0.001). With regard to the baseline laboratory parameters, CKD stage 5 patients had lower baseline-corrected calcium level (CKD stage 5: 2.2 mmol/L [IQR: 2.1–2.3] vs. CKD stage 4: 2.3 mmol/L [IQR: 2.2–2.4], P = 0.0001), higher baseline phosphate levels (CKD stage 5: 1.5 mmol/L [IQR: 1.3–1.8] vs. CKD stage 4: 1.4 mmol/L [IQR: 1.1–1.5], P < 0.0001) and higher iPTH levels (CKD stage 5: 23.9 pmol/L [IQR: 10.9–33.9] vs. CKD stage 4: 13.1 pmol/L [IQR: 7.9–21.9], P < 0.0001) compared to patients who were CKD stage 4. The total elemental calcium per day from the phosphate binders was significantly lower for CKD stage 4 patients compared to CKD stage 5 patients (CKD stage 4: 683.4 mg/day [standard deviation {SD}: 534.1] vs. CKD stage 5: 888.3 mg/day [SD: 738.2], P = 0.002).
Table 2 shows the proportion of patients who experienced hypocalcaemia and hypercalcaemia episodes during the study period. A higher proportion of CKD stage 5 patients had the presence of at least one episode of hypocalcaemia in the past year compared to CKD stage 4 patients (CKD stage 5: 43 [20.2%] vs. CKD stage 4: 19 [10.2%], P = 0.006). Approximately half (169 [42.2%]) of the patients included in the study had at least one episode of hypocalcaemia experienced during the study period.
Table 2.
Proportion of patients who experienced hypocalcaemia and hypercalcaemia episodes and their corresponding risk factor.
| Characteristics | CKD stage 4 (n=187) | CKD stage 5 (n=213) | Overall (n=400) | P |
|---|---|---|---|---|
| Presence of at least one hypocalcaemia episode in the past 1 year | 19 (10.2) | 43 (20.2) | 62 (15.5) | 0.006 |
|
| ||||
| Presence of at least one hypercalcaemia episode in the past 1 year | 19 (10.2) | 26 (12.2) | 45 (11.3) | 0.52 |
|
| ||||
| Proportion of patients who experienced hypocalcaemia during the study period | 67 (35.8) | 102 (47.9) | 169 (42.2) | 0.40 |
| 1 episode | 36 (19.3) | 44 (20.7) | 80 (20.0) | |
| 2-3 episodes | 20 (10.7) | 38 (17.8) | 58 (14.5) | |
| >4 episodes | 11 (5.9) | 20 (9.4) | 31 (7.8) | |
|
| ||||
| Proportion of patients who experienced hypercalcaemia during the study period | 53 (28.3) | 41 (19.2) | 94 (23.5) | 0.08 |
| 1 episode | 39 (20.9) | 21 (9.9) | 60 (15.0) | |
| 2-3 episodes | 11 (5.9) | 15 (7.0) | 26 (6.5) | |
| >4 episodes | 3 (1.6) | 5 (2.3) | 8 (2.0) | |
Bold values denote statistical significance at the P < 0.05 level. CKD: chronic kidney disease
A total of 376 hypocalcaemia episodes and 162 hypercalcaemia episodes were documented among patients during the study period [Table 3]. CKD stage 5 patients had fewer episodes of mild hypocalcaemia (CKD stage 5: 141 [59.5%] vs. CKD stage 4: 103 [74.1%], P = 0.004), but more episodes of severe hypocalcaemia (CKD stage 5: 96 [40.5%] vs. CKD stage 4: 36 [25.9%], P = 0.004) compared to CKD stage 4 patients. The severity of hypercalcaemia did not differ between patients who were CKD stage 4 and stage 5. Majority of the hypercalcaemia episodes were of mild severity (n = 156, 96.3%).
Table 3.
Characteristics of patients’ hypocalcaemia and hypercalcaemia episodes.
| Characteristicsa | CKD stage 4 | CKD stage 5 | Overall | P |
|---|---|---|---|---|
| Total number of hypocalcaemia episodes during the study period | 139 | 237 | 376 | |
|
| ||||
| Total number of hypercalcaemia episodes during the study period | 80 | 82 | 162 | |
|
| ||||
| Severity of hypocalcaemia episodea | 0.004 | |||
| Mild | 103 (74.1) | 141 (59.5) | 244 (64.9) | |
| Severe | 36 (25.9) | 96 (40.5) | 132 (35.1) | |
|
| ||||
| Severity of hypercalcaemia episodeb | 0.68 | |||
| Mild | 78 (97.5) | 78 (95.1) | 156 (96.3) | |
| Moderate | 2 (2.5) | 4 (4.9) | 6 (3.7) | |
| Severe | 0 (0) | 0 (0) | 0 (0) | |
aSeverity of hypocalcaemia is defined as ‘mild’ if the corrected calcium is between 1.90 and 2.10 mmol/L and ‘severe’ if the corrected calcium is less than 1.90 mmol/L. bSeverity of hypercalcaemia is defined as ‘mild’ if the corrected calcium is between 2.47 and 3.00 mmol/L, ‘moderate’ if the corrected calcium is between 3.01 and 3.50 mmol/L and ‘severe’ if the corrected calcium is greater than 3.50 mmol/L. Bold values denote statistical significance at the P < 0.05 level. CKD: chronic kidney disease.
CKD patients who had experienced both hypocalcaemia and hypercalcaemia episodes during the study period had a higher incidence of cardiovascular events compared to the other three patient groups: patients who experienced neither hypocalcaemia episodes nor hypercalcaemia episodes, patients who experienced only hypocalcaemia episodes and patients who experienced only hypercalcaemia episodes (P = 0.001) [Table 4]. No difference was observed between the groups for the occurrence of events of all-cause mortality, presence of skeletal events and presence of calciphylaxis [Table 4].
Table 4.
Breakdown of outcomes associated with hypocalcaemia and/or hypercalcaemia episodes.
| Nil hypocalcaemia or hypercalcaemia episodes (n=169) | Only hypocalcaemia episodes experienced (n=137) | Only hypercalcaemia episodes experienced (n=62) | Both hypocalcaemia and hypercalcaemia episodes experienced (n=32) | P | |
|---|---|---|---|---|---|
| Number of patients who died | 73 (43.2) | 51 (37.2) | 24 (38.7) | 18 (56.3) | 0.23 |
|
| |||||
| Number of patients with occurrence of cardiovascular event | 33 (19.5) | 34 (24.8) | 19 (30.6) | 17 (53.1) | 0.001 a |
|
| |||||
| Number of patients with occurrence of skeletal event | 12 (7.1) | 13 (9.5) | 4 (6.5) | 3 (9.4) | 0.83 |
|
| |||||
| Number of patients with occurrence of calciphylaxis | 1 (0.01) | 0 | 0 | 0 | 1.00a |
aPairwise comparison showed that statistical difference was observed for patients who experienced both hypocalcaemia and hypercalcaemia episodes against the other three groups. Bold values denote statistical significance at the P < 0.05 level.
Figure 2 shows the Kaplan–Meier curve for the all-cause mortality stratified by the patients who experienced hypocalcaemia and/or hypercalcaemia episodes. There was no difference in 3-year mortality rates between the groups (P = 0.53). In the Cox regression model for incident cardiovascular event, the incidence of cardiovascular event was significantly higher in patients who experienced both hypocalcaemia and hypercalcaemia episodes compared to other patients (P = 0.04) [Figure 3].
Figure 2.
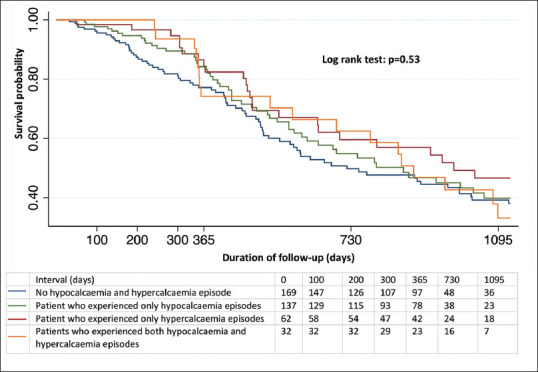
Kaplan–Meier curve for survival probability stratified by presence of hypocalcaemia and/or hypercalcaemia.
Figure 3.
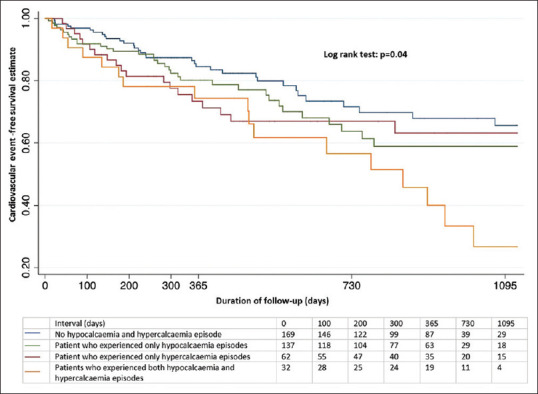
Cox regression model for incident cardiovascular event.
After adjustment for covariates which included patients’ age and CKD stage [see Appendix], hypocalcaemia and/or hypercalcaemia episodes were not associated with outcomes such as all-cause mortality (P > 0.05) and occurrence of cardiovascular event (P > 0.05) [Table 5].
Table 5.
Multivariable Cox regression analysis.
| All-cause mortality | Occurrence of cardiovascular event | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Unadjusted HR (95% CI) | P | Adjusted HRa (95% CI) | P | Unadjusted HR (95% CI) | P | Adjusted HRa (95% CI) | P | |
| Nil hypocalcaemia or hypercalcaemia episodes | Reference group | |||||||
|
| ||||||||
| Only hypocalcaemia episodes experienced | 0.86 (0.60, 1.24) | 0.42 | 1.11 (0.70, 1.78) | 0.64 | 1.34 (0.83, 2.16) | 0.24 | 1.33 (0.72, 2.44) | 0.37 |
|
| ||||||||
| Only hypercalcaemia episodes experienced | 0.71 (0.45, 1.13) | 0.15 | 0.74 (0.41, 1.34) | 0.33 | 1.40 (0.80, 2.46) | 0.24 | 1.13 (0.54, 2.40) | 0.74 |
|
| ||||||||
| Both hypocalcaemia and hypercalcaemia episodes experienced | 0.91 (0.54, 1.52) | 0.71 | 0.80 (0.38, 1.70) | 0.57 | 2.35 (1.31, 4.22) | 0.004 | 1.68 (0.72, 3.94) | 0.23 |
aHazard ratio was adjusted for covariates: age and CKD stage. Bold values denote statistical significance at the P < 0.05 level. CI: confidence interval, CKD: chronic kidney disease, HR: hazard ratio.
DISCUSSION
To the best of our knowledge, this is the first study done to examine the prevalence of hypocalcaemia and hypercalcaemia and their associated outcomes among CKD patients on MBD treatment in Singapore. Prevalence rates of hypocalcaemia (42.2%) and hypercalcaemia (23.5%) among pre-dialysis patients were high.
Pertaining to hypocalcaemia, the prevalence among our study population was significantly higher than that reported in other studies. The reported prevalence of hypocalcaemia among dialysis patients in the literature was 15%–25%, while a small study which evaluated CKD non-dialysis patients in India showed that hypocalcaemia affected 23.8% of patients.[8,11,17] Potential reasons for this differing finding may be due to higher rates of vitamin D insufficiency in our study population (median 25-hydroxyl vitamin D level: 17.6 ng/mL). A total of 60.0% (n = 90) of the patients in the study population had vitamin D levels less than 20 ng/mL. Vitamin D plays an important role in the regulation of CKD-MBD parameters, as vitamin D deficiency may worsen hypocalcaemia and lead to a compensatory increase in iPTH. Vitamin D deficiency is generally prevalent among patients with CKD, with the current literature citing an estimated prevalence of 60%–80% with a higher incidence observed to be present in patients with poorer kidney function.[26,27] A study by Massart et al.[28] done among dialysis patients proposed that correction of vitamin D deficiency via vitamin D supplementation can lead to improvements in CKD-MBD parameters, particularly increasing the proportion of patients attaining normalisation of serum calcium levels by up to twofold. Another potential reason for our findings could be attributed to the lower amounts of elemental calcium received from phosphate binders and supplementation in the study population. In a meta-analysis done by Palmer et al.,[29] which compared phosphate-binding agents in adults with CKD, the risk of hypercalcaemia was estimated to be 90% lower with the use of non–calcium-based phosphate binders (lanthanum and sevelamer) compared to calcium-based phosphate binders. Among the trials in the review, the mean calcium carbonate doses ranged from 2200 to 4800 mg/day (translating to 880–1920 mg elemental calcium per day), while the calcium acetate doses ranged from 2300 to 5300 mg/day (translating to 529–1219 mg elemental calcium per day).[29] As compared to the trials included in the meta-analysis, patients in the study population were observed to have a lower baseline serum phosphate level. Consequently, majority of our study population (87.0%) had a total elemental calcium of less than 1500 mg/day and the mean dose of calcium-based phosphate binders used was 799.7 mg of elemental calcium daily.
On the other hand, prevalence of hypercalcaemia among our study population was noted to be lower than that reported among Chinese dialysis patients, but similar to that of the CKD non-dialysis patients in the USA and in India. In the literature, prevalence of hypercalcaemia was noted to range from 30% to 40% among dialysis patients and from 5% to 27% among CKD non-dialysis patients.[8,11,21] Hypercalcaemia among CKD patients may be attributed to the use of vitamin D supplementation. Recent meta-analyses have supported the positive association between vitamin D usage and the risk of development of hypercalcaemia.[30,31] Additionally, results from the PRIMO and OPERA trials done among CKD non-dialysis patients showed that vitamin D usage predisposes patients to be at higher risk for hypercalcaemia without improvements in clinically relevant outcomes.[32,33] As such, the lower rates of hypercalcaemia seen in the present study compared to Chinese dialysis patients may have arisen from a more cautious prescribing of vitamin D. In this study, vitamin D usage among the study population was at 29.0% (n = 116), which was lower compared to another study done in China among dialysis patients, where vitamin D usage was reported to be at 67.4% of the study population.[11]
Concerning the association of hypercalcaemia with all-cause mortality, various epidemiological studies done among HD and pre-dialysis CKD patients have shown that hypercalcaemia is associated with increased risk of all-cause mortality.[16,17,21,34] A study by Zhu et al.[16] reported a threefold increase in mortality rates with serum calcium levels between 2.72 and 3.00 mmol/L. Studies looking at the association between hypocalcaemia and all-cause mortality have otherwise shown conflicting results.[16,17,34] In the present study, patients with hypocalcaemia and/or hypercalcaemia were not observed to be at increased risk of death. Potential reasons for the differing findings could be partly explained by the age of the patients recruited into the study and the characteristics of hypocalcaemia and hypercalcaemia episodes. Patients recruited in the present study were considerably older. Median age of patients recruited in the study was 77 years, compared to the patients from the other studies which had a mean age range of 60–68 years.[16,17,21,34] Clinically significant outcomes among CKD patients have been shown to differ between age groups, with older patients experiencing higher rates of death.[35] Additionally, majority of the hypocalcaemia (64.9%) and hypercalcaemia (96.3%) episodes documented were mild, which reduced patients’ exposure to extreme ranges of calcium values. A study by Kovesdy et al. showed that higher time-averaged calcium levels were associated with a 30.0% higher mortality rate, and it was hypothesised that higher time-averaged calcium levels lead to prolonged exposure of patients to hypercalcaemia, and thereby predispose them to be at risk of vascular calcification and mortality.[21] More studies are required in this area to better understand the association of different calcium levels across various time points with mortality outcomes.
In our study, initial analysis showed possible positive association between calcium dysregulation and occurrence of short-term risk of cardiovascular events. Subsequent multivariate Cox regression analysis after adjustment of covariates for age and CKD stage found no association of hypercalcaemia and hypocalcaemia events with these cardiovascular events. Numerous randomised control trials have reported the effects of calcium-based phosphate binders on arterial calcification among dialysis patients.[36,37,38] Collectively, these studies suggest that the use of calcium-based phosphate binders may predispose the patient to the risk of hypercalcaemia and may accelerate coronary arterial calcification, leading to an increased risk of cardiovascular events. As most studies which have evaluated long-term risk of cardiovascular disease with hypocalcaemia and hypercalcaemia were conducted in dialysis patients, more studies are required to evaluate if these risks present similarly among pre-dialysis CKD patients. A recent meta-analysis has suggested that there are other factors besides calcium, which may predispose CKD patients to be at risk for the occurrence of cardiovascular events.[39] These risk factors may include traditional general population cardiovascular risk factors, for example, age, gender, smoking, serum urate, haemoglobin and others. Additionally, other CKD-MBD studies have shown that derangements in other factors such as iPTH and phosphate levels may predispose patients to be at an increased risk of mortality and development of cardiovascular events.[16,34] Future studies could consider exploring the interplay of these factors in the occurrence of mortality and the development of cardiovascular events.
Limitations of the present study include the following. Firstly, the study only evaluated stage 4 and 5 CKD patients. While the prevalence of CKD-MBD is expected to be low in CKD stage 1–3 patients, more studies are required to evaluate the prevalence and outcomes associated with hypercalcaemia and hypocalcaemia in these patients. This would provide insight for physicians to better manage these patients in the early stages and mitigate their long-term risk. Secondly, as this is a single-centre study, results from the present study may not be generalisable to all other patients with CKD-MBD in Singapore. Lastly, patients in this study were followed up for 3 years. While our study showed no association of hypercalcaemia and hypocalcaemia with short-term mortality and cardiovascular events, more studies are needed to evaluate the long-term outcomes associated with dysregulation of calcium levels.
The prevalence of hypocalcaemia and hypercalcaemia was relatively high in our pre-dialysis CKD patients. While hypocalcaemia and hypercalcaemia were not associated with increased risk of short-term mortality and cardiovascular events among pre-dialysis CKD patients, more studies are required to analyse the impact of hypercalcaemia and hypocalcaemia on these patients’ long-term clinical outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Supplemental digital content
Appendix at http://links.lww.com/SGMJ/A127
APPENDIX
Supplementary Table 1.
Univariate analysis of baseline demographics between the groups of patients experiencing calcium dysregulation.
| Characteristics a | Nil hypocalcaemia or hypercalcaemia episodes (n = 169) | Only hypocalcaemia episodes experienced (n = 137) | Only hypercalcaemia episodes experienced (n = 62) | Both hypocalcaemia and hypercalcaemia episodes experienced (n = 32) | Overall (n = 400) | P value |
|---|---|---|---|---|---|---|
| Age (years) | 79 (71-86) | 74 (64-82) | 79 (65-86) | 76.5 (69-84) | 77(67-85) | 0.005 |
| Gender | ||||||
| Male | 75 (44.4) | 74 (54.0) | 24 (38.7) | 13 (40.6) | 186 (46.5) | 0.147 |
| CKD staging | 0.02 | |||||
| CKD stage 4b | 84 (49.7) | 50 (36.5) | 36 (58.1) | 17(53.1) | 187(46.8) | |
| CKD stage 5C | 85 (50.3) | 87 (63.5) | 26 (41.9) | 15 (46.9) | 213 (53.3) | |
| Race | 0.54 | |||||
| Chinese | 127 (75.2) | 97 (70.8) | 41 (66.1) | 25 (78.1) | 290 (72.5) | |
| Malay | 24 (14.2) | 23 (16.8) | 9(14.5) | 3 (9.4) | 59(14.8) | |
| Indian | 11 (6.5) | 15 (11.0) | 11 (17.7) | 3 (9.4) | 40(10.0) | |
| Eurasian | 2(1.2) | 1 (0.7) | 0 | 0 | 3 (0.8) | |
| Others | 5 (3.0) | 1 (0.7) | 1 (1-6) | 1 (3.1) | 8 (2.0) | |
| Body mass index (kg/m2) | 24.1 (21.1-27.1) | 25.3 (21.7-29.7) | 24.7 (20.8-29.6) | 23.3 (21.1-25.7) | 24.4 (21.3-28.0) | 0.08 |
| Smoking status | 0.43 | |||||
| Non-smoker | 144 (85.2) | 110(80.3) | 56 (90.3) | 25 (78.1) | 335 (83.8) | |
| Current smoker | 7(4.1) | 7(5.1) | 3 (4.8) | 1(3.1) | 18 (4.5) | |
| Ex-smoker | 18 (10.7) | 20 (14.6) | 3 (4.8) | 6(18.8) | 47(11.8) | |
| Aetiology of chronic kidney disease | 0.09 | |||||
| Diabetic nephropathy | 93 (55.0) | 84(61.3) | 44 (71.0) | 19 (59.4) | 240 (60.0) | |
| Chronic glomerulonephritis | 27(16.0) | 23 (16.8) | 4 (6.5) | 3 (9.4) | 57(14.3) | |
| Hypertensive nephrosclerosis | 27(16.0) | 20 (14.6) | 7(11.3) | 3 (9.4) | 57(14.3) | |
| Polycystic kidney disease | 1 (0.6) | 1 (0.7) | 1 (1-6) | 1 (3.1) | 4(1.0) | |
| Lupus nephritis | 1 (0.6) | 0 | 0 | 0 | 1 (0.3) | |
| Obstructive uropathy | 0 | 2(1.5) | 0 | 2(6.3) | 4(1.0) | |
| Others | 20(11.8) | 7(5.1) | 6 (9.7) | 4(12.5) | 37 (9.3) | |
| Comorbidities | ||||||
| Hypertension | 157 (92.9) | 124(90.5) | 56 (90.3) | 28 (87.5) | 365 (91.3) | 0.73 |
| Hyperlipidaemia | 120 (71.0) | 100 (73.0) | 46 (74.2) | 23 (71.9) | 289 (72.3) | 0.96 |
| Type 2 diabetes mellitus | 114(67.5) | 95 (69.3) | 50 (80.7) | 22 (68.8) | 281 (70.3) | 0.27 |
| Cerebrovascular accident | 29 (17.2) | 26(19.0) | 8(12.9) | 8 (25.0) | 71 (17.8) | 0.51 |
| Ischaemic heart disease | 73 (43.2) | 55 (40.2) | 26(41.9) | 18(56.3) | 172(43.0) | 0.43 |
| Liver disease | 12(7.1) | 11 (8.0) | 3 (4.8) | 5(15.6) | 31 (7.8) | 0.31 |
| Heart failure | 24 (14.2) | 17(12.4) | 12 (19.4) | 10(31.3) | 63 (15.8) | 0.05 |
| History of malignancy | 19(11.3) | 19(13.9) | 5(8.1) | 5(15.6) | 48 (12.0) | 0.60 |
| Osteoarthritis | 29 (17.2) | 20 (14.6) | 7(11.3) | 4(12.5) | 60(15.0) | 0.69 |
| Osteoporosis | 8 (4.7) | 3 (2.2) | 3 (4.8) | 1(3.1) | 15(3.8) | 0.66 |
| Hyperthyroidism | 4 (2.4) | 4 (2.9) | 2 (3.2) | 2 (6.3) | 12(3.0) | 0.70 |
| Rheumatoid arthritis | 1 (0.6) | 1 (0.7) | 2 (3.2) | 1 (3.1) | 5(1.3) | 0.29 |
aVariables are expressed as number (percentage) or median (interquartile range) where appropriate. bCKD stage 4: eGFR: 15-29 mL/min/1.73 nr. CCKD stage 5: eGFR: <15 mL/min/1.73m . Bold values denote statistical significance at the P < 0.05 level. CKD: chronic kidney disease, CKD-MBD: chronic kidney diseas ’-mineral and bone disorder, eGFR: estimated glomerular filtration rate.
REFERENCES
- 1.Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, et al. Global prevalence of chronic kidney disease-A systematic review and meta-analysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0158765. e0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–47. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 3.Wee H-L, Seng BJJ, Lee JJ, Chong KJ, Tyagi P, Vathsala A, et al. Association of anemia and mineral and bone disorder with health-related quality of life in Asian pre-dialysis patients. Health Qual Life Outcomes. 2016;14:94. doi: 10.1186/s12955-016-0477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang CW, Wee PH, Low LL, Koong YLA, Htay H, Fan Q, et al. Prevalence and risk factors for elevated anxiety symptoms and anxiety disorders in chronic kidney disease: A systematic review and meta-analysis. Gen Hosp Psychiatry. 2021;69:27–40. doi: 10.1016/j.genhosppsych.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Coresh J. Update on the Burden of CKD. J Am Soc Nephrol. 2017;28:1020–2. doi: 10.1681/ASN.2016121374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwek JL, Kee TYS. World Kidney Day 2020: Advances in preventive nephrology. Ann Acad Med Singap. 2020;49:175–9. [PubMed] [Google Scholar]
- 7.Chapter 1: Introduction and definition of CKD–MBD and the development of the guideline statements. Kidney Int. 2009;76:S3–8. doi: 10.1038/ki.2009.189. [DOI] [PubMed] [Google Scholar]
- 8.Vikrant S, Parashar A. Prevalence and severity of disordered mineral metabolism in patients with chronic kidney disease: A study from a tertiary care hospital in India. Indian J Endocrinol Metab. 2016;20:460–7. doi: 10.4103/2230-8210.183457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seng JJB, Tan YLC, Lim RW, Ng HTS, Lee PH, Wong J. Prevalence and risk factors for hypercalcemia among non-dialysis patients with chronic kidney disease-mineral and bone disorder. Int Urol Nephrol. 2018;50:1871–7. doi: 10.1007/s11255-018-1906-x. [DOI] [PubMed] [Google Scholar]
- 10.Soroka SD, Beard KM, Mendelssohn DC, Cournoyer SH, Da Roza GA, Geary DF. Mineral metabolism management in Canadian peritoneal dialysis patients. Clin Nephrol. 2011;75:410–5. doi: 10.5414/cnp75410. [DOI] [PubMed] [Google Scholar]
- 11.Kong X, Zhang L, Zhang L, Chen N, Gu Y, Yu X, et al. Mineral and bone disorder in Chinese dialysis patients: A multicenter study. BMC Nephrol. 2012;13:116. doi: 10.1186/1471-2369-13-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapter 4.1: Treatment of CKD-MBD targeted at lowering high serum phosphorus and maintaining serum calcium. Kidney Int. 2009;76113:S50–99. doi: 10.1038/ki.2009.192. [DOI] [PubMed] [Google Scholar]
- 13.K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42((4 Suppl 3)):S1–201. [PubMed] [Google Scholar]
- 14.Hill Gallant KM, Spiegel DM. Calcium balance in chronic kidney disease. Curr Osteoporos Rep. 2017;15:214–21. doi: 10.1007/s11914-017-0368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obi Y, Mehrotra R, Rivara MB, Streja E, Rhee CM, Lau WL, et al. Hidden hypercalcemia and mortality risk in incident hemodialysis patients. J Clin Endocrinol Metab. 2016;101:2440–9. doi: 10.1210/jc.2016-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu JG, Chen JB, Cheng BC, Lee CH, Long G, Chien YS. Association between extreme values of markers of chronic kidney disease: Mineral and bone disorder and 5-year mortality among prevalent hemodialysis patients. Blood Purif. 2018;45:1–7. doi: 10.1159/000478972. [DOI] [PubMed] [Google Scholar]
- 17.Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: The Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2008;52:519–30. doi: 10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 18.Miura S, Yoshihisa A, Takiguchi M, Shimizu T, Nakamura Y, Yamauchi H, et al. Association of hypocalcemia with mortality in hospitalized patients with heart failure and chronic kidney disease. J Card Fail. 2015;21:621–7. doi: 10.1016/j.cardfail.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi S, Hamano T, Doi Y, Oka T, Kajimoto S, Kubota K, et al. Hidden hypocalcemia as a risk factor for cardiovascular events and all-cause mortality among patients undergoing incident hemodialysis. Sci Rep. 2020;10:4418. doi: 10.1038/s41598-020-61459-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fouque D, Roth H, Pelletier S, London GM, Hannedouche T, Jean G, et al. Control of mineral metabolism and bone disease in haemodialysis patients: Which optimal targets? Nephrol Dial Transplantat. 2013;28:360–7. doi: 10.1093/ndt/gfs404. [DOI] [PubMed] [Google Scholar]
- 21.Kovesdy CP, Kuchmak O, Lu JL, Kalantar-Zadeh K. Outcomes associated with serum calcium level in men with non-dialysis-dependent chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:468–76. doi: 10.2215/CJN.06040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Payne RB, Little AJ, Williams RB, Milner JR. Interpretation of serum calcium in patients with abnormal serum proteins. Br Med J. 1973;4:643–6. doi: 10.1136/bmj.4.5893.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duval M, Bach K, Masson D, Guimard C, Le Conte P, Trewick D. Is severe hypocalcemia immediately life-threatening? Endocr Connect. 2018;7:1067–74. doi: 10.1530/EC-18-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician. 2003;67:1959–66. [PubMed] [Google Scholar]
- 25.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e563–95. doi: 10.1161/CIR.0000000000000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SM, Choi HJ, Lee JP, Kim DK, Oh YK, Kim YS, et al. Prevalence of vitamin D deficiency and effects of supplementation with cholecalciferol in patients with chronic kidney disease. J Ren Nutr. 2014;24:20–5. doi: 10.1053/j.jrn.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 27.LaClair RE, Hellman RN, Karp SL, Kraus M, Ofner S, Li Q, et al. Prevalence of calcidiol deficiency in CKD: A cross-sectional study across latitudes in the United States. Am J Kidney Dis. 2005;45:1026–33. doi: 10.1053/j.ajkd.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 28.Massart A, Debelle FD, Racape J, Gervy C, Husson C, Dhaene M, et al. Biochemical parameters after cholecalciferol repletion in hemodialysis: Results From the VitaDial randomized trial. Am J Kidney Dis. 2014;64:696–705. doi: 10.1053/j.ajkd.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 29.Palmer SC, Gardner S, Tonelli M, Mavridis D, Johnson DW, Craig JC, et al. Phosphate-binding agents in adults with CKD: A network meta-analysis of randomized trials. Am J Kidney Dis. 2016;68:691–702. doi: 10.1053/j.ajkd.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Li XH, Feng L, Yang ZH, Liao YH. Effect of active vitamin D on cardiovascular outcomes in predialysis chronic kidney diseases: A systematic review and meta-analysis. Nephrology (Carlton) 2015;20:706–14. doi: 10.1111/nep.12505. [DOI] [PubMed] [Google Scholar]
- 31.Xu L, Wan X, Huang Z, Zeng F, Wei G, Fang D, et al. Impact of vitamin D on chronic kidney diseases in non-dialysis patients: A meta-analysis of randomized controlled trials. PLoS One. 2013;8:e61387. doi: 10.1371/journal.pone.0061387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: The PRIMO randomized controlled trial. JAMA. 2012;307:674–84. doi: 10.1001/jama.2012.120. [DOI] [PubMed] [Google Scholar]
- 33.Wang AY, Fang F, Chan J, Wen YY, Qing S, Chan IH, et al. Effect of paricalcitol on left ventricular mass and function in CKD--The OPERA trial. J Am Soc Nephrol. 2014;25:175–86. doi: 10.1681/ASN.2013010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Floege J, Kim J, Ireland E, Chazot C, Drueke T, de Francisco A, et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant. 2011;26:1948–55. doi: 10.1093/ndt/gfq219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Hare AM, Choi AI, Bertenthal D, Bacchetti P, Garg AX, Kaufman JS, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007;18:2758–65. doi: 10.1681/ASN.2007040422. [DOI] [PubMed] [Google Scholar]
- 36.Chertow GM, Burke SK, Raggi P Treat to Goal Working Group. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245–52. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 37.Braun J, Asmus HG, Holzer H, Brunkhorst R, Krause R, Schulz W, et al. Long-term comparison of a calcium-free phosphate binder and calcium carbonate--phosphorus metabolism and cardiovascular calcification. Clin Nephrol. 2004;62:104–15. doi: 10.5414/cnp62104. [DOI] [PubMed] [Google Scholar]
- 38.Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, et al. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005;68:1815–24. doi: 10.1111/j.1523-1755.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 39.Major RW, Cheng MRI, Grant RA, Shantikumar S, Xu G, Oozeerally I, et al. Cardiovascular disease risk factors in chronic kidney disease: A systematic review and meta-analysis. PLoS One. 2018;13:e0192895. doi: 10.1371/journal.pone.0192895. [DOI] [PMC free article] [PubMed] [Google Scholar]


