Abstract
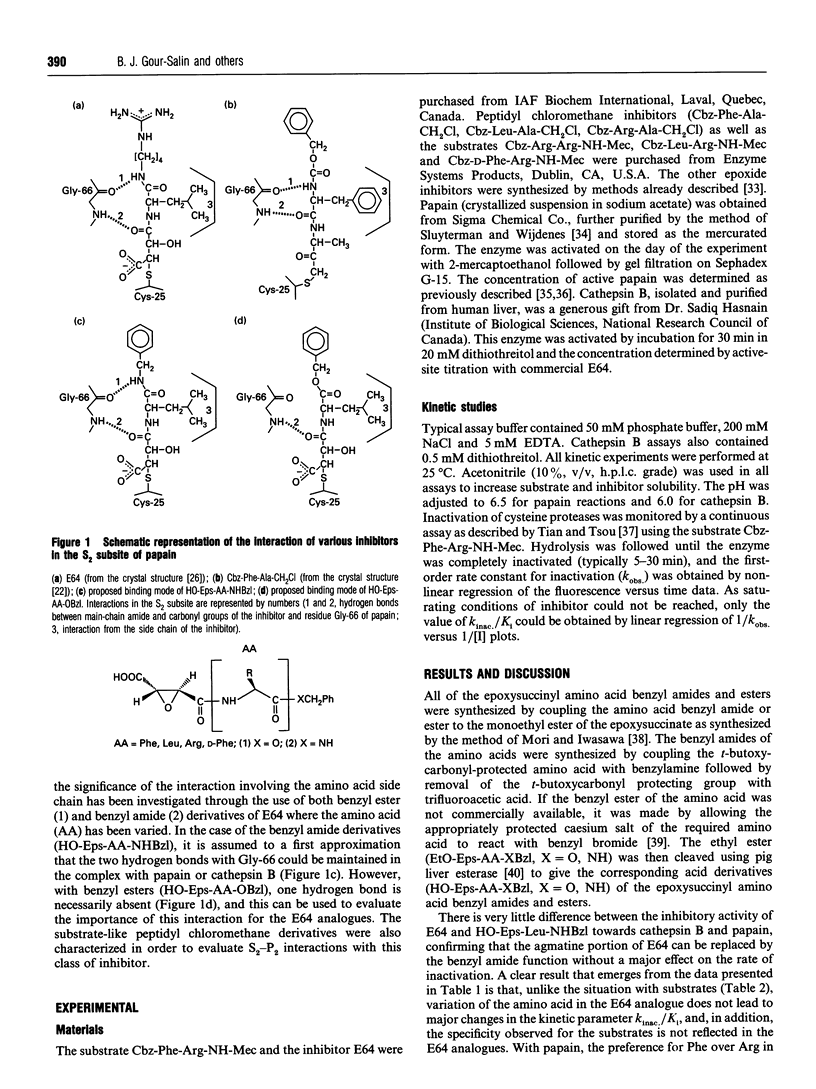
A number of epoxysuccinyl amino acid benzyl esters (HO-Eps-AA-OBzl) and benzyl amides (HO-Eps-AA-NHBzl) (where AA represents amino acid) were synthesized as analogues of E64, a naturally occurring inhibitor of cysteine proteinases. These inhibitors were designed to evaluate if selectivity for cathepsin B could be achieved by varying the amino acid on the basis of known substrate specificity. Contrary to the situation with substrates, it was found that variation of the amino acid in the E64 analogues does not lead to major changes in the kinetic parameter kinac./Ki and that the specificity of these analogues does not parallel that observed for substrates. This is particularly true in the case of the benzyl ester derivatives where the deviation from substrate-like behaviour is more important than with the benzyl amide derivatives. The results suggest that the amide proton of the benzyl amide group in HO-Eps-AA-NHBzl interacts in the S2 subsite in both cathepsin B and papain and contributes to increase the potency of these inhibitors. The kinetic data also suggest that differences in the orientation of the C alpha-C beta bond of the side chain in the S2 subsite of the enzyme might explain the differences between substrate and E64 analogue specificities. This hypothesis is supported by the fact that the order of inactivation rates with chloromethane inhibitors (which are believed to be good models of enzyme-substrate interactions) is indeed very similar to that observed with the corresponding amidomethylcoumarin substrates. In conclusion, the information available from S2-P2 interactions with substrates cannot be used to enhance the selectivity of the E64 analogues in a rational manner.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Berti P. J., Faerman C. H., Storer A. C. Cooperativity of papain-substrate interaction energies in the S2 to S2' subsites. Biochemistry. 1991 Feb 5;30(5):1394–1402. doi: 10.1021/bi00219a033. [DOI] [PubMed] [Google Scholar]
- Buck M. R., Karustis D. G., Day N. A., Honn K. V., Sloane B. F. Degradation of extracellular-matrix proteins by human cathepsin B from normal and tumour tissues. Biochem J. 1992 Feb 15;282(Pt 1):273–278. doi: 10.1042/bj2820273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaissé J. M., Eeckhout Y., Vaes G. In vivo and in vitro evidence for the involvement of cysteine proteinases in bone resorption. Biochem Biophys Res Commun. 1984 Dec 14;125(2):441–447. doi: 10.1016/0006-291x(84)90560-6. [DOI] [PubMed] [Google Scholar]
- Delaissé J. M., Ledent P., Vaes G. Collagenolytic cysteine proteinases of bone tissue. Cathepsin B, (pro)cathepsin L and a cathepsin L-like 70 kDa proteinase. Biochem J. 1991 Oct 1;279(Pt 1):167–174. doi: 10.1042/bj2790167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth H. U. Recent developments in inhibiting cysteine and serine proteases. J Enzyme Inhib. 1990;3(4):249–278. doi: 10.3109/14756369009030375. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T., Greenberg A. H., Egan S. E., Hamilton R. T., Wright J. A. Cysteine proteinase cathepsin L expression correlates closely with the metastatic potential of H-ras-transformed murine fibroblasts. Oncogene. 1987;2(1):55–59. [PubMed] [Google Scholar]
- Drenth J., Kalk K. H., Swen H. M. Binding of chloromethyl ketone substrate analogues to crystalline papain. Biochemistry. 1976 Aug 24;15(17):3731–3738. doi: 10.1021/bi00662a014. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Erdel M., Trefz G., Spiess E., Habermaas S., Spring H., Lah T., Ebert W. Localization of cathepsin B in two human lung cancer cell lines. J Histochem Cytochem. 1990 Sep;38(9):1313–1321. doi: 10.1177/38.9.2201737. [DOI] [PubMed] [Google Scholar]
- Gour-Salin B. J., Lachance P., Plouffe C., Storer A. C., Ménard R. Epoxysuccinyl dipeptides as selective inhibitors of cathepsin B. J Med Chem. 1993 Mar 19;36(6):720–725. doi: 10.1021/jm00058a008. [DOI] [PubMed] [Google Scholar]
- Harris J. O., Olsen G. N., Castle J. R., Maloney A. S. Comparison of proteolytic enzyme activity in pulmonary alveolar macrophages and blood leukocytes in smokers and nonsmokers. Am Rev Respir Dis. 1975 May;111(5):579–586. doi: 10.1164/arrd.1975.111.5.579. [DOI] [PubMed] [Google Scholar]
- Hashida S., Towatari T., Kominami E., Katunuma N. Inhibitions by E-64 derivatives of rat liver cathepsin B and cathepsin L in vitro and in vivo. J Biochem. 1980 Dec;88(6):1805–1811. doi: 10.1093/oxfordjournals.jbchem.a133155. [DOI] [PubMed] [Google Scholar]
- Hasnain S., Hirama T., Huber C. P., Mason P., Mort J. S. Characterization of cathepsin B specificity by site-directed mutagenesis. Importance of Glu245 in the S2-P2 specificity for arginine and its role in transition state stabilization. J Biol Chem. 1993 Jan 5;268(1):235–240. [PubMed] [Google Scholar]
- Ii K., Hizawa K., Nonaka I., Sugita H., Kominami E., Katunuma N. Abnormal increases of lysosomal cysteinine proteinases in rimmed vacuoles in the skeletal muscle. Am J Pathol. 1986 Feb;122(2):193–198. [PMC free article] [PubMed] [Google Scholar]
- Johnson D., Travis J. Inactivation of human alpha 1-proteinase inhibitor by thiol proteinases. Biochem J. 1977 Jun 1;163(3):639–641. doi: 10.1042/bj1630639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katunuma N., Kominami E. Abnormal expression of lysosomal cysteine proteinases in muscle wasting diseases. Rev Physiol Biochem Pharmacol. 1987;108:1–20. doi: 10.1007/BFb0034070. [DOI] [PubMed] [Google Scholar]
- Khouri H. E., Plouffe C., Hasnain S., Hirama T., Storer A. C., Ménard R. A model to explain the pH-dependent specificity of cathepsin B-catalysed hydrolyses. Biochem J. 1991 May 1;275(Pt 3):751–757. doi: 10.1042/bj2750751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khouri H. E., Vernet T., Ménard R., Parlati F., Laflamme P., Tessier D. C., Gour-Salin B., Thomas D. Y., Storer A. C. Engineering of papain: selective alteration of substrate specificity by site-directed mutagenesis. Biochemistry. 1991 Sep 17;30(37):8929–8936. doi: 10.1021/bi00101a003. [DOI] [PubMed] [Google Scholar]
- Kominami E., Kunio I., Katunuma N. Activation of the intramyofibral autophagic-lysosomal system in muscular dystrophy. Am J Pathol. 1987 Jun;127(3):461–466. [PMC free article] [PubMed] [Google Scholar]
- Murata M., Miyashita S., Yokoo C., Tamai M., Hanada K., Hatayama K., Towatari T., Nikawa T., Katunuma N. Novel epoxysuccinyl peptides. Selective inhibitors of cathepsin B, in vitro. FEBS Lett. 1991 Mar 25;280(2):307–310. doi: 10.1016/0014-5793(91)80318-w. [DOI] [PubMed] [Google Scholar]
- Musil D., Zucic D., Turk D., Engh R. A., Mayr I., Huber R., Popovic T., Turk V., Towatari T., Katunuma N. The refined 2.15 A X-ray crystal structure of human liver cathepsin B: the structural basis for its specificity. EMBO J. 1991 Sep;10(9):2321–2330. doi: 10.1002/j.1460-2075.1991.tb07771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page A., Warburton M. J., Chambers T. J., Hayman A. R. Purification and characterisation of cysteine proteinases from human osteoclastomas. Biochem Soc Trans. 1991 Aug;19(3):286S–286S. doi: 10.1042/bst019286s. [DOI] [PubMed] [Google Scholar]
- Redwood S. M., Liu B. C., Weiss R. E., Hodge D. E., Droller M. J. Abrogation of the invasion of human bladder tumor cells by using protease inhibitor(s). Cancer. 1992 Mar 1;69(5):1212–1219. doi: 10.1002/cncr.2820690524. [DOI] [PubMed] [Google Scholar]
- Shaw E. Cysteinyl proteinases and their selective inactivation. Adv Enzymol Relat Areas Mol Biol. 1990;63:271–347. doi: 10.1002/9780470123096.ch5. [DOI] [PubMed] [Google Scholar]
- Sluyterman L. A., Wijdenes J. An agarose mercurial column for the separation of mercaptopapain and nonmercaptopapain. Biochim Biophys Acta. 1970 Mar 31;200(3):593–595. doi: 10.1016/0005-2795(70)90122-4. [DOI] [PubMed] [Google Scholar]
- Storer A. C., Lee H., Carey P. R. Relaxed and perturbed substrate conformations in enzyme active sites: evidence from multichannel resonance raman spectra. Biochemistry. 1983 Sep 27;22(20):4789–4796. doi: 10.1021/bi00289a027. [DOI] [PubMed] [Google Scholar]
- Tian W. X., Tsou C. L. Determination of the rate constant of enzyme modification by measuring the substrate reaction in the presence of the modifier. Biochemistry. 1982 Mar 2;21(5):1028–1032. doi: 10.1021/bi00534a031. [DOI] [PubMed] [Google Scholar]
- Varughese K. I., Ahmed F. R., Carey P. R., Hasnain S., Huber C. P., Storer A. C. Crystal structure of a papain-E-64 complex. Biochemistry. 1989 Feb 7;28(3):1330–1332. doi: 10.1021/bi00429a058. [DOI] [PubMed] [Google Scholar]
- Wang S-S, Gisin B. F., Winter D. P., Makofske R., Kulesha I. D., Tzougraki C., Meienhofer J. Facile synthesis of amino acid and peptide esters under mild conditions via cesium salts. J Org Chem. 1977 Apr 15;42(8):1286–1290. doi: 10.1021/jo00428a004. [DOI] [PubMed] [Google Scholar]
- Yamamoto D., Matsumoto K., Ohishi H., Ishida T., Inoue M., Kitamura K., Hanada K. The importance of Val-157 hydrophobic interaction for papain inhibitory activity of an epoxysuccinyl amino acid derivative. A structure-activity relationship based on the crystal structure of the papain-E-64-c complex. FEBS Lett. 1990 Apr 9;263(1):134–136. doi: 10.1016/0014-5793(90)80722-u. [DOI] [PubMed] [Google Scholar]
- van der Stappen J. W., Paraskeva C., Williams A. C., Hague A., Maciewicz R. A. Relationship between the secretion of cysteine proteinases and their inhibitors and malignant potential. Biochem Soc Trans. 1991 Nov;19(4):362S–362S. doi: 10.1042/bst019362s. [DOI] [PubMed] [Google Scholar]


