Abstract
Objective:
This study aimed to discover the cytotoxic effect of YH239-EE and YH239 alone and their enantiomer potency in cytotoxic effect on the MCF7 cell line.
Methods:
We used the cytotoxic study on MDM2 cell lines by detecting the percentage of apoptosis and necrosis by annexin v methods.
Result:
This result shows that YH239-EE causes more apoptosis and necrosis 40% in comparison to YH239 without ethyl ester, about 4.92 %, and The (+) enantiomer of YH239-EE demonstrated a markedly higher induction of apoptosis and necrosis (84.48%) in MCF7 cells compared to the (-) enantiomer (48.71%).
Conclusion:
The ethyl ester group in YH239-EE might play a crucial role in enhancing the compound’s ability to induce cell death, and The high efficacy of the (+) enantiomer of YH239-EE in inducing cell death in MCF7 cells suggests it may be a more promising therapeutic candidate for breast cancer treatment, specifically for subtypes represented by MCF7 cells.
Key Words: MCF-7 Cells, Annexin A5, Apoptosis, Necrosis
Introduction
As early as 1,500 BC, the ancient Egyptians recorded breast cancer. Today, 3,500 years later, it has become one of the most commonly diagnosed cancers in modern women, with a high mortality rate [1,2]. Globally, breast cancer ranks among the primary causes of cancer-related illness and death. Breast cancer was the second most often diagnosed disease, accounting for more than 11.6% of all female cancer cases, according to the status report on the GLOBOCAN 2018 estimates of cancer incidence and death [3,4]. At number five on the list of common causes of cancer-related fatalities, About 500,000 people die from breast cancer every year in the world [5]. Among them, the number and mortality rate of women diagnosed with breast cancer rank second among all cancers [6,7].
The causes of breast cancer are divided into hereditary gene mutations and acquired environmental factors. Only about 5 to 15% of breast cancer cases in Cancer patients are caused by congenital inheritance, among which BRCA1 and BRCA2 gene mutations are the most famous [8]. Acquired breast cancer is usually thought to be caused by genetic mutations or expressions caused by the environment. Epigenetic changes lead to mutations in breast cells, including: 1) Cell cycle checkpoint disorders lead to cell failure Normal proliferation; 2) Loss of cell apoptosis ability, such as p53 protein mutation leading to the immortality of breast cancer cells (immortalization; 3) Changes in signaling pathways can also cause cells to become more cancerous. One of the more famous examples is the Abnormal signaling pathway that leads to increased growth and metastasis of breast cancer cells [9-11].
The current classification of breast cancer is mainly based on whether breast cancer cells show human epidermal receptor 2, estrogen receptor, progesterone receptor, and ki67 protein expression, divided into five subtypes types, including Luminal A, Luminal B, Her2-enriched, triple negative, and normal-like, among which Among them, triple-negative breast cancer cells proliferate, are highly metastatic, and have no hormone receptors to progress hormonal therapy and, therefore, the most malignant type of cancer [12,13].
The p53 protein is a transcription factor encoded by the tumor suppressor gene TP53 [14]. It can induce cell apoptosis, prevent abnormal division, and thereby inhibit the proliferation and transformation of cancer cells. Therefore, p53 is also called a “tumor suppressor protein” [15]. In normal cells, when there is a problem with gene replication, the p53 protein will terminate its replication, causing the cell to return to the G1 phase and promote the repair of gene damage. When gene repair fails to return cells to normal state, p53 will turn on downstream signaling pathways and induce and activate the transcription of its target genes, such as p21, BAX, PUMA, etc., thus promoting cell apoptosis [16,17].
MDM2 is a mouse double microbody gene encoding MDM2 protein. MDM2 was first discovered in the mouse BALB/c3T3 fibroblast cell line and was later confirmed in various human tissues. It is currently believed that MDM2 protein is a negative regulator of p53 protein, which can bind to p53 and inhibit its function to promote the occurrence and development of tumors [18,19].
YH239 and YH239 EE First discovered by Huang et al. in 2014, all Yh239 and its ethyl ester and enantiomers work by the same mechanism [20]; they block the formation of the p53-MDM2 complex by directly binding to MDM2, thereby inhibiting the degradation of p53 and enhancing the stability of p53 protein. [21,22]. The Yh239 showed high potency as an anticancer treatment of AML, a potent anticancer drug; however, there are no studies about its potency. However, several studies suggested the use of this medication shows more selectivity than traditional chemotherapeutic medication [23,24]. The Aim of this study was to discover the cytotoxic effect of YH239-EE and YH239 alone and their enantiomer potency in cytotoxic effect on the MCF7 cell line.
Materials and Methods
Cell Culture
The MCF7 cell line was obtained from the Iraqi Center for Genetics and Cancer Research at Al-Mustansiriyah University. First, it detached by being treated with Trypsin/EDTA Solution (Hangzhou Hyper Chemicals Limited Hangzhou China) and cultured in (TBS buffer pH 7.6 and RPMI complete media supplemented with Streptomycin obtained from Hangzhou Hyper Chemicals Limited at 37°C in a humidified atmosphere with 5% CO2 this form of methodology used in several articles[25].
Chemicals and Reagents
YH239-EE and YH239 were obtained from Hangzhou Hyper Chemicals, China, based on Annexin V-FITC Apoptosis Detection Kit. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was obtained from Hangzhou Hyper Chemicals Limited – China
Treatment Groups
Control group
Cells treated with vehicle only (TBS buffer and DMSO).
YH239-EE group
Cells treated with YH239-EE at varying concentrations.
YH239 group
Cells treated with YH239 at varying concentrations.
Enantiomer groups
Cells treated with (+) and (-) enantiomers of YH239-EE at varying concentrations.
Cytotoxicity Assay
MTT Assay
MCF7 cells were seeded in 96-well plates at a density of 10,000 cells per well and allowed to adhere overnight. Cells were treated with different concentrations of YH239-EE, YH239, and their enantiomers for specified time intervals. After treatment, MTT solution was added to each well and incubated for 4 hours at 37°C. DMSO was used to solubilize the formazan crystals, and the microplate reader was used to assess the absorbance at 570 nm. The proportion of the control group was used to compute the cell viability [26–29].
Annexin V-FITC/PI Assay
This method is used to determine the number of dead cells in a population and distinguish between apoptotic and necrotic cells. Annexin V is a 35-36 kDa calcium-dependent protein bound to a fluorescein isothiocyanate (FITC)-labeled antibody. It binds to phosphatidylserine (PS), which, after destabilization of the cell membrane during apoptosis, shifts from the inner membrane layer to the outer one and is thus made accessible to the dye Annexin V. Since the translocation of PS begins in the early stages of apoptosis, early apoptotic cells are also detected via staining with annexin V [31]. Six well plates containing MCF7 cells were seeded, and the proper amounts of YH239-EE, YH239, and their enantiomers were added, with about 20 micromolars for each one. Following treatment, the cells were taken out and resuspended in a binding buffer after being cleaned with cold PBS. As directed by the manufacturer, annexin V-FITC and propidium iodide (PI) were added to the cell solution. The percentage of necrotic (Annexin V-positive, PI-positive) and apoptotic (Annexin V-positive, PI-negative) cells in the samples was then determined by flow cytometry analysis [30-31].
Statistical analysis
The data were analyzed using GraphPad Prism software (GraphPad Software, La Jolla, CA, USA). The results were expressed as means ± standard error of the mean (SEM). The statistical significance was determined using the unpaired T-test by comparing the untreated result with the treated one. P values less than 0.05 were considered statistically significant [32,33].
Results
MCF7 cell line
The result of the current study (cytotoxicity study) on MCF7 breast cancer cells reported concentration-dependent and cell-specific effects.
The cell count was monitored every 24 hours using automated counting equipment. The results presented in (Figure 1) revealed that the cytotoxicity of MDM2 inhibitors was both concentration-dependent and cell line-specific. These findings highlighted the potential of YH239-EE and YH239 as effective agents for inducing cytotoxicity in MCF7 breast cancer cells.
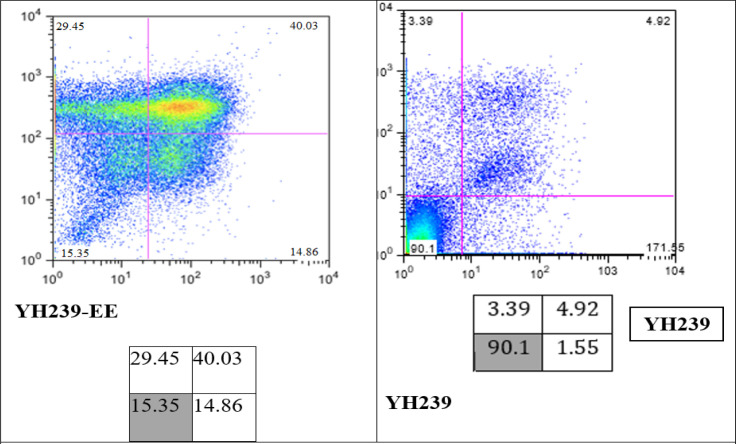
Figure 1.
Determination of Apoptotic and Necrotic Cells by Annexin V/PI Staining and FACS. Incubate the MCF7 cell lines with 20 μM YH239-EE and YH239 for 72 h. The values showed the sum of LUQ, RUQ, and RLQ in %.
Control Group (Placebo)
The control group, treated with a placebo, exhibited a modest inhibition rate of 17.28%± 0.17.
YH239-EE
YH239-EE, another compound under investigation, exhibited inhibition rates of (57.18%±0.22) and (61.68%± 5.57) at 10μM and 20μM concentrations, respectively. Presented in Table 1.
Table 1.
The Cytotoxic Effect of Medication on MCF7 Cell-Line
| Substance | Concentration (μM) | Inhibition rate (%) | SD | P-value |
|---|---|---|---|---|
| Placebo | 17.28 | 0.22 | ||
| YH239 | 1 | 17.043 | 2 | 0.00006 |
| YH239 | 5 | 25.86 | 1.33 | 0 |
| YH239 | 10 | 33.73 | 0.85 | 0 |
| YH239 | 20 | 41.4 | 1.14 | 0.00001 |
| YH239-EE | 1 | 40.56 | 0.57 | 0 |
| YH239-EE | 5 | 46.51 | 0.18 | 0 |
| YH239-EE | 10 | 57.18 | 0.22 | 0 |
| YH239-EE | 20 | 61.68 | 5.57 | 0.00035 |
STD is the standard deviation; P-value is the probability value (P<0.05), which is a significant difference, and lower than 0.01 (P<0.01) is considered statistically highly significant. The P value was calculated using the unpaired t-test (two-tail) method by comparing the result with the percentage of cytotoxicity in the placebo sample.
IC 50 result
IC50, the inhibitory concentration 50, reflects the chemicals’ ability to produce the cytotoxic effect. Increasing this value means the drug is less cytotoxic. If this value is low, it means these drugs or chemicals are more cytotoxic. As in Table 2, it is evident that the IC 50 of Yh239-EE is about 8.45 µM whereas IC 50 of Yh239 is 37.78 µM.
Table 2.
The Inhibitory Concentration 50 (IC50) Effect of Medication on the 3 Cell Lines
| IC50 | Mcf7 |
|---|---|
| Yh239 in µM | 37.78 |
| Yh239-EE in µM | 8.45 |
Determination of apoptotic and necrotic cells by Annexin V/PI staining
In our experiments, the efficacy of YH239-EE was, therefore, investigated in more detail. After treatment of Mcf7 cells with 20 μM YH239-EE or YH239 for 72 hours, the proportions of apoptotic and necrotic cells were determined by staining with annexin V/PI (Figure 1).
This result shows that YH239-EE shows 40% more apoptosis and necrosis in comparison to YH239 without ethyl ester, which was about 4.92 %
Analysis of (-) and (+) enantiomers of YH239-EE
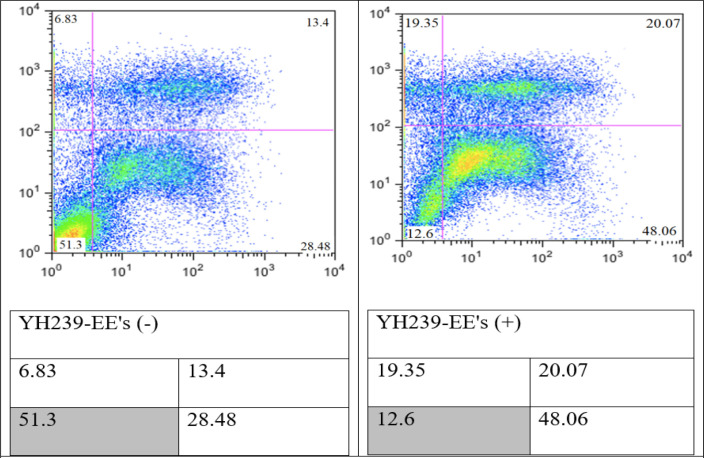
Chemically synthesized YH239-EE is present as an enantiomer mixture. Often, only one enantiomer has the desired biological properties. The racemic mixture was separated into the enantiomers YH239-EE (-) and YH239-EE (+) by preparative chiral SFC (supercritical fluid chromatography) from the provider (PubChem China). It tested the purified enantiomers for their biological activity. Both compounds were tested for their potency and ability to induce apoptosis and necrosis using an Annexin V/PI staining test. YH239-EE’s (+) enantiomer significantly increased the production of necrosis and apoptosis in MCF7 cells (84.48%) as opposed to its (-) enantiomer (48.71%) (Figure 2).
Figure 2.
Determination of Apoptotic and Necrotic Cells by Annexin V/PI Staining and FACS. Incubate the MCF7 cell lines with 20 μM enantiomers YH239-EE (-) and YH239-EE (+) or 72 h. The values show the sum of LUQ, RUQ, and RLQ in %.
Discussion
Several research studies have been conducted to make anticancer treatments more selective in treating cancer without side effects [34]. However, the MDM2 inhibitor drugs have a promising vision in this regard; in our research, the MTT assay shows that both YH239 and YH239-EE have cytotoxic effects with different IC 50 values (the IC 50 value of YH239 was about 37.78 and YH239-EE was about 8.45 µM). This result means YH239-EE showed more potency than YH239 in treating breast cancer cell lines. This result is the first time tested on the mcf7 cell line; however, this result is in line with the only article published in this regard conducted by Huang et al., they tested both drugs on Leukemia and showed that the YH239-EE has a superior effect compared with YH239 alone [20]. They thus hypothesize that the greater activity is caused by YH239-EE entering the cell more efficiently and being intracellularly activated by a cleavage process. Furthermore, they demonstrated that YH239-EE can efficiently and in concentration-dependent activate caspases 3 and 7, an early apoptotic marker.
The level of apoptosis and necrosis in YH239 and YH239-EE
The findings of the Annexin V/PI staining offer essential information on how well YH239-EE causes apoptosis and necrosis in MCF7 cells compared to its non-esterified equivalent, YH239. In MCF7 cells, YH239-EE revealed a very high level of necrosis and apoptosis (84.34%) after 72 hours. This robust outcome demonstrates the potent necrotic and apoptotic characteristics of YH239-EE in this breast cancer cell line.
The ethyl ester group of YH239-EE could significantly boost the compound’s ability to kill cells. This may have more pronounced biological effects because of the increased cellular absorption, enhanced molecular target contact, or more excellent chemical stability. Our research concluded that the YH239 without ethyl ester can cause less percentage of necrosis and apoptosis (9.86%). This finding is consistent with another study, albeit it was not done on Leukemia (breast cancer cell lines) [20]. This implies that the compound’s ability to induce cell death effectively depends on the ethyl ester component [22]. Understanding how the ethyl ester modification enhances the efficacy of YH239 might be very helpful for developing cancer treatments.
The Annexin V/PI staining results highlight significant differences in the biological activities of the (-) and (+) enantiomers of YH239-EE, inducing apoptosis and necrosis in MCF7 cells. YH239-EE’s (+) enantiomer significantly increased the production of necrosis and apoptosis in MCF7 cells (84.48%) as opposed to its (-) enantiomer (48.71%). This significant discrepancy highlights how stereochemistry affects chiral substances’ biological function. The higher efficiency of the (+) enantiomer implies that it may be more effectively absorbed by the cells, resulting in a more enormous apoptotic and necrotic response or a greater affinity for molecular targets implicated in apoptotic pathways.
In conclusion, our study demonstrates that both YH239-EE and YH239 have a cytotoxic effect on the breast cancer cell line, and this is the first time this drug has been tested on this breast cancer cell line; moreover, we found that the ethyl ester of TH239 has a pharmacological effect more than the same drug without ethyl ester this is an open field of the importance of mild chemical modification in the field of cancer therapy, besides that the varying activity of enantiomers in their anticancer efficacy; this finding opens up new avenues for investigation into several drugs that are currently on the market that may have enantiomer types and demonstrates to researchers the critical role stereochemistry plays in drug design and pharmacology. The idea that enantiomers can have distinct pharmacokinetic, pharmacodynamic, and toxicological profiles is supported by this study, and this is essential information for developing and improving chiral medications for cancer treatment. The (+) enantiomer of YH239-EE is very effective in causing cell death in MCF7 cells, indicating that it might be a more attractive therapeutic option for the treatment of breast cancer, particularly for subtypes that are represented by MCF7 cells. Gaining knowledge of the processes underlying these enantiomers’ differing actions may help us better understand the molecular causes of necrosis and apoptosis in breast cancer cells.
More research is required to clarify the molecular processes behind YH239-EE’s increased effectiveness. Investigating its interactions with cellular targets, the features of absorption, distribution, metabolism, and excretion (ADME), and the function of the ethyl ester group in these processes might all be part of this.
Evaluating YH239-EE’s effects on in vivo models and other cancer cell lines will facilitate the assessment of its therapeutic potential and broader applicability.
Acknowledgements
General
We thank the Iraqi Medical Research Center for supporting us and the Department of Pharmacology College of Medicine, Baghdad, Iraq – for their tremendous support.
Scientific Approval
All research was conducted under ethical approval number 239\18\12\2020; no humans or animals were enrolled in this research.
Ethical Declaration
All research was conducted under ethical approval number 239\18\12\2020; no humans or animals were enrolled in this research.
Author Contribution Statement
H. A A: draft writing and conduct the research, methodology, M A J: Revision supervision
References
- 1.Takhellambam M, Singh A. Cancer disease and its’ understanding from the ancient knowledge to the modern concept. World J Adv Res Rev. 2022;15:169–76. [Google Scholar]
- 2.Al-Hussaniy HA. The effect of microrna-409-3p for treatment and response to tumor proliferation of lung cancer cell lines (in vitro) Asian Pac J Cancer Prev. 2022;23(9):3151–6. doi: 10.31557/APJCP.2022.23.9.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohammed AA, Elsayed FM, Algazar M, Rashed HE, Anter AH. Neoadjuvant chemotherapy in triple negative breast cancer: Correlation between androgen receptor expression and pathological response. Asian Pac J Cancer Prev. 2020;21(2):563–8. doi: 10.31557/APJCP.2020.21.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaaban SM, gaber Z, semary S, dewidar AM. Impact of vitamin b12 on outcome of early stage luminal a and b breast cancer, single center experience. Med Pharm J. 2023;2:17–27. [Google Scholar]
- 5.Rabiei R, Ayyoubzadeh SM, Sohrabei S, Esmaeili M, Atashi A. Prediction of breast cancer using machine learning approaches. J Biomed Phys Eng. 2022;12(3):297–308. doi: 10.31661/jbpe.v0i0.2109-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masoudi RF, Sahib AS, Mahmood HS. Exploring the impact of capecitabine treatment on hormonal and biochemical markers in women with breast cancer. Iraqi J Pharma Sci. 2023;32:99–103. [Google Scholar]
- 7.Saad AH, Salih H. Adherence and Beliefs to Adjuvant Hormonal Therapy in Patients with Breast Cancer: A Cross-Sectional Study (Conference Paper) Iraqi J Pharm Sci . 2021;30(Suppl.):31–9. [Google Scholar]
- 8.Chung SH, Woldenberg N, Roth AR, Masamed R, Conlon W, Cohen JG, et al. Brca and beyond: Comprehensive image-rich review of hereditary breast and gynecologic cancer syndromes. Radiographics. 2020;40(2):306–25. doi: 10.1148/rg.2020190084. [DOI] [PubMed] [Google Scholar]
- 9.Akeel H, Alburghaif A, Naji M. Tumor diagnosis by genetic markers protein p-53, p16, c-myc, n-myc, protein k-ras, and gene her-2 neu is this possible? Pak J Med Health Sci. 2021;15:2350–4. [Google Scholar]
- 10.Oleiwi M, Zalzala M. Synthesis, molecular docking study and cytotoxicity evaluation of some quinazolinone derivatives as nonclassical antifolates and potential cytotoxic agents. Iraqi J Pharm Sci. 2022;31:283–96. [Google Scholar]
- 11.Abd M, Abbas S. Association between serum insulin and interleukin-6 levels with breast cancer in post-menopausal iraqi women. J Fac Med Baghdad. 2024;65 [Google Scholar]
- 12.Geyer FC, Rodrigues DN, Weigelt B, Reis-Filho JS. Molecular classification of estrogen receptor-positive/luminal breast cancers. Adv Anat Pathol. 2012;19(1):39–53. doi: 10.1097/PAP.0b013e31823fafa0. [DOI] [PubMed] [Google Scholar]
- 13.Alabbody H, Nasiry B, Kadhim K. Applying food frequency questionnaire to evaluate the dietary pattern and life style on women with breast cancer. J Fac Med Baghdad. 2018;60:119–25. [Google Scholar]
- 14.Sammons MA, Nguyen TT, McDade SS, Fischer M. Tumor suppressor p53: From engaging DNA to target gene regulation. Nucleic Acids Res. 2020;48(16):8848–69. doi: 10.1093/nar/gkaa666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pundir S, Pundir CS. Detection of tumor suppressor protein p53 with special emphasis on biosensors: A review. Anal Biochem. 2020;588:113473. doi: 10.1016/j.ab.2019.113473. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh s, bhattacharjee m, jana nk. Gene regulation by p53 in human cancer system. Asian Pac J Canc Biol. 2022;7:97 –109. [Google Scholar]
- 17.Habib M, Ibrahim M, Daraji S. P53 in renal cell carcinoma: A biomarker for disease progression. J Fac Med Baghdad. 2011;53:54–6. [Google Scholar]
- 18.Kelsall IR. Non-lysine ubiquitylation: Doing things differently. Front Mol Biosci. 2022;9:1008175. doi: 10.3389/fmolb.2022.1008175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S, Chen FE. Small-molecule mdm2 inhibitors in clinical trials for cancer therapy. Eur J Med Chem. 2022;236:114334. doi: 10.1016/j.ejmech.2022.114334. [DOI] [PubMed] [Google Scholar]
- 20.Huang Y, Wolf S, Beck B, Köhler LM, Khoury K, Popowicz GM, et al. Discovery of highly potent p53-mdm2 antagonists and structural basis for anti-acute myeloid leukemia activities. ACS Chem Biol. 2014;9(3):802–11. doi: 10.1021/cb400728e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mustafa ES, Al-Jameel WH, Al-Mahmood SS. Immunohistochemical detection of p53 and mdm2 and its correlation with histological grading system in ovine pulmonary adenocarcinoma. Iraqi J Vet Sci. 2021;35(4):687–92. [Google Scholar]
- 22.Fang Y, Liao G, Yu B. Small-molecule mdm2/x inhibitors and protac degraders for cancer therapy: Advances and perspectives. Acta Pharm Sin B. 2020;10(7):1253–78. doi: 10.1016/j.apsb.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hadi HM, shahada AR, hussein NM, hussein E. Doxorubicin side effects and its uses a new update: A narrative review. Arabian J Drug Res. 2021;1:1–6. [Google Scholar]
- 24.Akeel H, Mohammed Z, Alburghaif A, Naji M. Panax ginseng as antioxidant and anti-inflammatory to reduce the cardiotoxicity of doxorubicin on rat module. Res J Pharm Technol. 2022:4594–600. [Google Scholar]
- 25.Fathi SM AI. Cytotoxic effect of the alcoholic extract of conocarpus erectus leaves on mda-mb 231 and mcf7 breast cancer cell lines. Iraqi J Sci. 2023;64(1):84–90. [Google Scholar]
- 26.Bhandary L, Bailey PC, Chang KT, Underwood KF, Lee CJ, Whipple RA, et al. Lipid tethering of breast tumor cells reduces cell aggregation during mammosphere formation. Sci Rep. 2021;11(1):3214 . doi: 10.1038/s41598-021-81919-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SJ, Jeong JH, Lee J, Park HY, Jung JH, Kang J, et al. Microrna-496 inhibits triple negative breast cancer cell proliferation by targeting del-1. Medicine (Baltimore) 2021;100(14):e25270. doi: 10.1097/MD.0000000000025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahangdale P, Wankhade AM. Ganoderma lucidum ethanolic extract for the treatment of androgenic alopecia in rats with testosterone-induced baldness. Med Pharm J. 2033;2:107–20. [Google Scholar]
- 29.Altalebi RR, Al-Hussaniy HA, Al-Tameemi ZS, Al-Zobaidy MA, Albu-Rghaif AH, Alkuraishy HM, et al. Non-alcoholic fatty liver disease: Relation to juvenile obesity, lipid profile, and hepatic enzymes. J Med Life. 2023;16(1):42–7. doi: 10.25122/jml-2022-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ha AH, Sameer AH, Oraibi HN. The relationship between statin therapy and adipocytokine/inflammatory mediators in dyslipidemic nondiabetic patients: A comparative study. Pharmacia. 2023;70:581–5. [Google Scholar]
- 31.Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin v for flow cytometric detection of phosphatidylserine expression on b cells undergoing apoptosis. Blood. 1994;84(5):1415–20. [PubMed] [Google Scholar]
- 32.Milliana A, Listiyana A, Mutiah R, Annisa R, Firadusi AF, Faradila VA, et al. The potential of eleutherine bulbosa in inducing apoptosis and inhibiting cell cycle in breast cancer: A network pharmacology approach and in vitro experiments. Asian Pac J Cancer Prev. 2023;24(11):3783–94. doi: 10.31557/APJCP.2023.24.11.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arsić A, nikić-spiegel I. The tail domain of neurofilament light chain accumulates in neuronal nuclei during oxidative injury. bioRxiv. 2022 [Google Scholar]
- 34.Al-Hussaniy HA, Alburghaif AH, Alkhafaje Z, Al-Zobaidy MAJ, Alkuraishy HM, Mostafa-Hedeab G, et al. Chemotherapy-induced cardiotoxicity: A new perspective on the role of digoxin, atg7 activators, resveratrol, and herbal drugs. J Med Life. 2023;16(4):491–500. doi: 10.25122/jml-2022-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]