Abstract
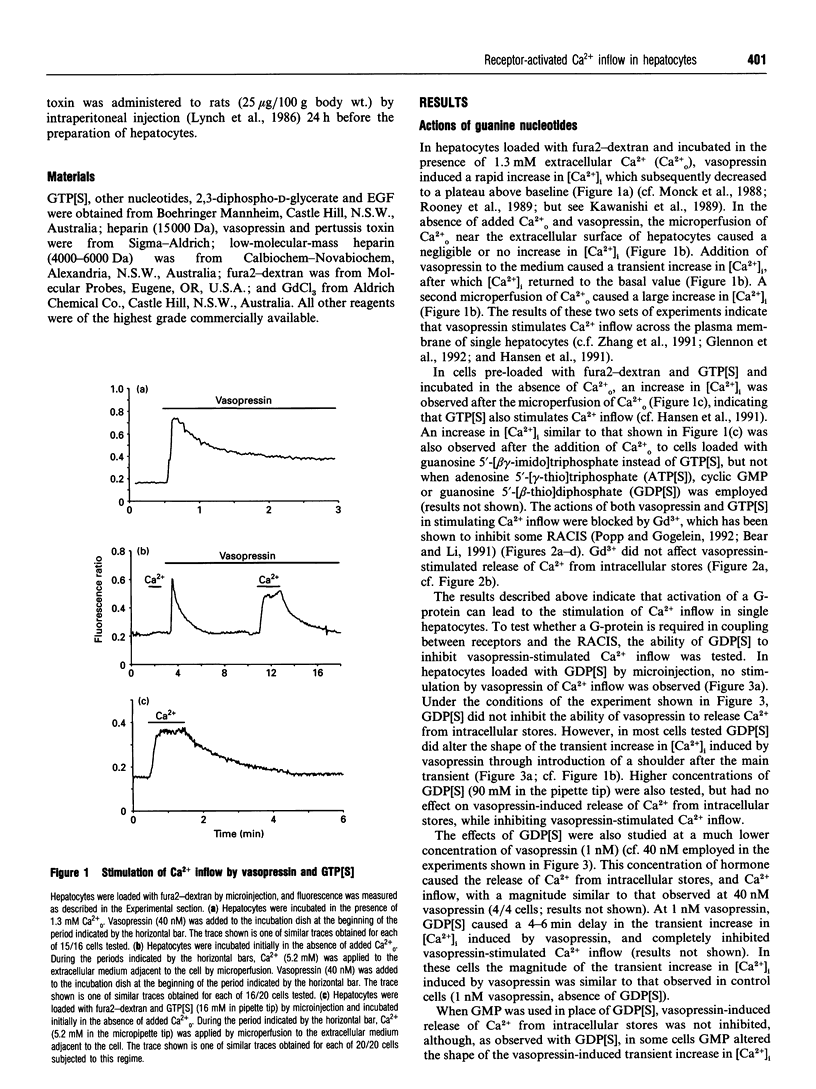
The roles of heterotrimeric GTP-binding regulatory proteins (G-proteins) and inositol polyphosphates in the mechanism by which vasopressin stimulates Ca2+ inflow in hepatocytes were investigated by using single cells loaded with fura2 by microinjection. Vasopressin-stimulated Ca2+ inflow was mimicked by microinjection of guanosine 5'-[gamma-thio]triphosphate (GTP[S]) or guanosine 5'-[beta gamma-imido]triphosphate to the cells, but not adenosine 5'-[gamma-thio]triphosphate (ATP[S]) or guanosine 5'-[beta-thio]diphosphate (GDP[S]). Extracellular Gd3+ (5 microM) inhibited both vasopressin- and GTP[S]-stimulated Ca2+ inflow. GDP[S], but not GMP, administered to hepatocytes by microinjection, completely inhibited vasopressin-stimulated Ca2+ inflow and partially inhibited vasopressin-induced release of Ca2+ from intracellular stores. The microinjection of pertussis toxin had no effect either on the release of Ca2+ from intracellular stores or on Ca2+ inflow induced by vasopressin, but completely inhibited changes in these processes induced by epidermal growth factor (EGF). Hepatocytes isolated from rats treated with pertussis toxin for 24 h exhibited no vasopressin- or GTP[S]-stimulated Ca2+ inflow, whereas the vasopressin-stimulated release of Ca2+ from intracellular stores was similar to that observed for control cells. Heparin or ATP[S] inhibited, or delayed the onset of, both vasopressin-induced release of Ca2+ from intracellular stores and vasopressin-stimulated Ca2+ inflow. Vasopressin-induced oscillations in intracellular [Ca2+] were observed in some heparin-treated cells. It is concluded that the stimulation by vasopressin of Ca2+ inflow to hepatocytes requires inositol 1,4,5-trisphosphate (InsP3) and, by implication, the pertussis-toxin-insensitive G-protein required for the activation of phospholipase C beta [Taylor, Chae, Rhee and Exton (1991) Nature (London) 350, 516-518], and another G-protein which is slowly ADP-ribosylated by pertussis toxin and acts between InsP3 and the putative plasma-membrane Ca2+ channel. EGF-stimulated Ca2+ inflow involves at least one G-protein which is rapidly ADP-ribosylated and is most likely required for InsP3 formation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barr F. A., Leyte A., Mollner S., Pfeuffer T., Tooze S. A., Huttner W. B. Trimeric G-proteins of the trans-Golgi network are involved in the formation of constitutive secretory vesicles and immature secretory granules. FEBS Lett. 1991 Dec 9;294(3):239–243. doi: 10.1016/0014-5793(91)81438-e. [DOI] [PubMed] [Google Scholar]
- Barritt G. J., Hughes B. P. The nature and mechanism of activation of the hepatocyte receptor-activated Ca2+ inflow system. Cell Signal. 1991;3(4):283–292. doi: 10.1016/0898-6568(91)90056-z. [DOI] [PubMed] [Google Scholar]
- Barritt G. J., Parker J. C., Wadsworth J. C. A kinetic analysis of the effects of adrenaline on calcium distribution in isolated rat liver parenchymal cells. J Physiol. 1981 Mar;312:29–55. doi: 10.1113/jphysiol.1981.sp013614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear C. E., Li C. H. Calcium-permeable channels in rat hepatoma cells are activated by extracellular nucleotides. Am J Physiol. 1991 Dec;261(6 Pt 1):C1018–C1024. doi: 10.1152/ajpcell.1991.261.6.C1018. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank J. L., Brattain K. A., Exton J. H. Activation of cytosolic phosphoinositide phospholipase C by G-protein beta gamma subunits. J Biol Chem. 1992 Nov 15;267(32):23069–23075. [PubMed] [Google Scholar]
- Blitzer R. D., Omri G., De Vivo M., Carty D. J., Premont R. T., Codina J., Birnbaumer L., Cotecchia S., Caron M. G., Lefkowitz R. J. Coupling of the expressed alpha 1B-adrenergic receptor to the phospholipase C pathway in Xenopus oocytes. The role of Go. J Biol Chem. 1993 Apr 5;268(10):7532–7537. [PubMed] [Google Scholar]
- Bourguignon L. Y., Jin H., Iida N., Brandt N. R., Zhang S. H. The involvement of ankyrin in the regulation of inositol 1,4,5-trisphosphate receptor-mediated internal Ca2+ release from Ca2+ storage vesicles in mouse T-lymphoma cells. J Biol Chem. 1993 Apr 5;268(10):7290–7297. [PubMed] [Google Scholar]
- Boyer J. L., Waldo G. L., Harden T. K. Beta gamma-subunit activation of G-protein-regulated phospholipase C. J Biol Chem. 1992 Dec 15;267(35):25451–25456. [PubMed] [Google Scholar]
- Brock T. A., Dennis P. A., Griendling K. K., Diehl T. S., Davies P. F. GTP gamma S loading of endothelial cells stimulates phospholipase C and uncouples ATP receptors. Am J Physiol. 1988 Nov;255(5 Pt 1):C667–C673. doi: 10.1152/ajpcell.1988.255.5.C667. [DOI] [PubMed] [Google Scholar]
- Burch R. M., Luini A., Axelrod J. Phospholipase A2 and phospholipase C are activated by distinct GTP-binding proteins in response to alpha 1-adrenergic stimulation in FRTL5 thyroid cells. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7201–7205. doi: 10.1073/pnas.83.19.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess G. M., Godfrey P. P., McKinney J. S., Berridge M. J., Irvine R. F., Putney J. W., Jr The second messenger linking receptor activation to internal Ca release in liver. Nature. 1984 May 3;309(5963):63–66. doi: 10.1038/309063a0. [DOI] [PubMed] [Google Scholar]
- Bushfield M., Griffiths S. L., Murphy G. J., Pyne N. J., Knowler J. T., Milligan G., Parker P. J., Mollner S., Houslay M. D. Diabetes-induced alterations in the expression, functioning and phosphorylation state of the inhibitory guanine nucleotide regulatory protein Gi-2 in hepatocytes. Biochem J. 1990 Oct 15;271(2):365–372. doi: 10.1042/bj2710365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butta N., Urcelay E., González-Manchón C., Parrilla R., Ayuso M. S. Pertussis toxin inhibition of alpha 1-adrenergic or vasopressin-induced Ca2+ fluxes in rat liver. Selective inhibition of the alpha 1-adrenergic receptor-coupled metabolic activation. J Biol Chem. 1993 Mar 25;268(9):6081–6089. [PubMed] [Google Scholar]
- Camps M., Hou C., Sidiropoulos D., Stock J. B., Jakobs K. H., Gierschik P. Stimulation of phospholipase C by guanine-nucleotide-binding protein beta gamma subunits. Eur J Biochem. 1992 Jun 15;206(3):821–831. doi: 10.1111/j.1432-1033.1992.tb16990.x. [DOI] [PubMed] [Google Scholar]
- Cantiello H. F., Patenaude C. R., Ausiello D. A. G protein subunit, alpha i-3, activates a pertussis toxin-sensitive Na+ channel from the epithelial cell line, A6. J Biol Chem. 1989 Dec 15;264(35):20867–20870. [PubMed] [Google Scholar]
- Catterall W. A. Excitation-contraction coupling in vertebrate skeletal muscle: a tale of two calcium channels. Cell. 1991 Mar 8;64(5):871–874. doi: 10.1016/0092-8674(91)90309-m. [DOI] [PubMed] [Google Scholar]
- Charest R., Prpić V., Exton J. H., Blackmore P. F. Stimulation of inositol trisphosphate formation in hepatocytes by vasopressin, adrenaline and angiotensin II and its relationship to changes in cytosolic free Ca2+. Biochem J. 1985 Apr 1;227(1):79–90. doi: 10.1042/bj2270079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra L. C., Twort C. H., Ward J. P., Cameron I. R. Effects of heparin on inositol 1,4,5-trisphosphate and guanosine 5'-O-(3-thio triphosphate) induced calcium release in cultured smooth muscle cells from rabbit trachea. Biochem Biophys Res Commun. 1989 Aug 30;163(1):262–268. doi: 10.1016/0006-291x(89)92130-x. [DOI] [PubMed] [Google Scholar]
- Crofts J. N., Barritt G. J. The liver cell plasma membrane Ca2+ inflow systems exhibit a broad specificity for divalent metal ions. Biochem J. 1990 Aug 1;269(3):579–587. doi: 10.1042/bj2690579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis T. N. What's new with calcium? Cell. 1992 Nov 13;71(4):557–564. doi: 10.1016/0092-8674(92)90590-9. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Cassel D., Levkovitz H., Lowe M., Selinger Z. Guanosine 5'-O-(2-thiodiphosphate). An inhibitor of adenylate cyclase stimulation by guanine nucleotides and fluoride ions. J Biol Chem. 1979 Oct 10;254(19):9829–9834. [PubMed] [Google Scholar]
- Furuichi T., Yoshikawa S., Miyawaki A., Wada K., Maeda N., Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989 Nov 2;342(6245):32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Glennon M. C., Bird G. S., Kwan C. Y., Putney J. W., Jr Actions of vasopressin and the Ca(2+)-ATPase inhibitor, thapsigargin, on Ca2+ signaling in hepatocytes. J Biol Chem. 1992 Apr 25;267(12):8230–8233. [PubMed] [Google Scholar]
- Guillemette G., Balla T., Baukal A. J., Catt K. J. Characterization of inositol 1,4,5-trisphosphate receptors and calcium mobilization in a hepatic plasma membrane fraction. J Biol Chem. 1988 Apr 5;263(10):4541–4548. [PubMed] [Google Scholar]
- Hansen C. A., Yang L. J., Williamson J. R. Mechanisms of receptor-mediated Ca2+ signaling in rat hepatocytes. J Biol Chem. 1991 Oct 5;266(28):18573–18579. [PubMed] [Google Scholar]
- Huang C. L., Takenawa T., Ives H. E. Platelet-derived growth factor-mediated Ca2+ entry is blocked by antibodies to phosphatidylinositol 4,5-bisphosphate but does not involve heparin-sensitive inositol 1,4,5-trisphosphate receptors. J Biol Chem. 1991 Mar 5;266(7):4045–4048. [PubMed] [Google Scholar]
- Hughes B. P., Barritt G. J. Evidence that guanosine 5'-[gamma-thio]triphosphate stimulates plasma membrane Ca2+ inflow when introduced into hepatocytes. Biochem J. 1989 Jan 15;257(2):591–598. doi: 10.1042/bj2570591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes B. P., Barritt G. J. The stimulation by sodium fluoride of plasma-membrane Ca2+ inflow in isolated hepatocytes. Evidence that a GTP-binding regulatory protein is involved in the hormonal stimulation of Ca2+ inflow. Biochem J. 1987 Jul 1;245(1):41–47. doi: 10.1042/bj2450041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes B. P., Crofts J. N., Auld A. M., Read L. C., Barritt G. J. Evidence that a pertussis-toxin-sensitive substrate is involved in the stimulation by epidermal growth factor and vasopressin of plasma-membrane Ca2+ inflow in hepatocytes. Biochem J. 1987 Dec 15;248(3):911–918. doi: 10.1042/bj2480911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F. 'Quantal' Ca2+ release and the control of Ca2+ entry by inositol phosphates--a possible mechanism. FEBS Lett. 1990 Apr 9;263(1):5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Tracing the roots of ion channels. Cell. 1992 May 29;69(5):715–718. doi: 10.1016/0092-8674(92)90280-p. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Kass G. E., Llopis J., Chow S. C., Duddy S. K., Orrenius S. Receptor-operated calcium influx in rat hepatocytes. Identification and characterization using manganese. J Biol Chem. 1990 Oct 15;265(29):17486–17492. [PubMed] [Google Scholar]
- Katz A., Wu D., Simon M. I. Subunits beta gamma of heterotrimeric G protein activate beta 2 isoform of phospholipase C. Nature. 1992 Dec 17;360(6405):686–689. doi: 10.1038/360686a0. [DOI] [PubMed] [Google Scholar]
- Kawanishi T., Blank L. M., Harootunian A. T., Smith M. T., Tsien R. Y. Ca2+ oscillations induced by hormonal stimulation of individual fura-2-loaded hepatocytes. J Biol Chem. 1989 Aug 5;264(22):12859–12866. [PubMed] [Google Scholar]
- Khan A. A., Steiner J. P., Klein M. G., Schneider M. F., Snyder S. H. IP3 receptor: localization to plasma membrane of T cells and cocapping with the T cell receptor. Science. 1992 Aug 7;257(5071):815–818. doi: 10.1126/science.1323146. [DOI] [PubMed] [Google Scholar]
- Khan A. A., Steiner J. P., Snyder S. H. Plasma membrane inositol 1,4,5-trisphosphate receptor of lymphocytes: selective enrichment in sialic acid and unique binding specificity. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2849–2853. doi: 10.1073/pnas.89.7.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori S., Kawai M., Takewaki T., Ohashi H. GTP-binding protein involvement in membrane currents evoked by carbachol and histamine in guinea-pig ileal muscle. J Physiol. 1992 May;450:105–126. doi: 10.1113/jphysiol.1992.sp019118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M. N., Garrison J. C. The epidermal growth factor receptor is coupled to a pertussis toxin-sensitive guanine nucleotide regulatory protein in rat hepatocytes. J Biol Chem. 1991 Jul 15;266(20):13342–13349. [PubMed] [Google Scholar]
- Liang M., Garrison J. C. Epidermal growth factor activates phospholipase C in rat hepatocytes via a different mechanism from that in A431 or rat1hER cells. Mol Pharmacol. 1992 Nov;42(5):743–752. [PubMed] [Google Scholar]
- Lynch C. J., Prpic V., Blackmore P. F., Exton J. H. Effect of islet-activating pertussis toxin on the binding characteristics of Ca2+-mobilizing hormones and on agonist activation of phosphorylase in hepatocytes. Mol Pharmacol. 1986 Feb;29(2):196–203. [PubMed] [Google Scholar]
- Mauger J. P., Poggioli J., Guesdon F., Claret M. Noradrenaline, vasopressin and angiotensin increase Ca2+ influx by opening a common pool of Ca2+ channels in isolated rat liver cells. Biochem J. 1984 Jul 1;221(1):121–127. doi: 10.1042/bj2210121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy S. A., Bicknell R. Responses of pertussis toxin-treated microvascular endothelial cells to transforming growth factor beta 1. No evidence for pertussis-sensitive G-protein involvement in TGF-beta signal transduction. J Biol Chem. 1992 Oct 25;267(30):21617–21622. [PubMed] [Google Scholar]
- McDonald T. V., Premack B. A., Gardner P. Flash photolysis of caged inositol 1,4,5-trisphosphate activates plasma membrane calcium current in human T cells. J Biol Chem. 1993 Feb 25;268(6):3889–3896. [PubMed] [Google Scholar]
- Monck J. R., Reynolds E. E., Thomas A. P., Williamson J. R. Novel kinetics of single cell Ca2+ transients in stimulated hepatocytes and A10 cells measured using fura-2 and fluorescent videomicroscopy. J Biol Chem. 1988 Apr 5;263(10):4569–4575. [PubMed] [Google Scholar]
- Moriarty T. M., Sealfon S. C., Carty D. J., Roberts J. L., Iyengar R., Landau E. M. Coupling of exogenous receptors to phospholipase C in Xenopus oocytes through pertussis toxin-sensitive and -insensitive pathways. Cross-talk through heterotrimeric G-proteins. J Biol Chem. 1989 Aug 15;264(23):13524–13530. [PubMed] [Google Scholar]
- Neylon C. B., Nickashin A., Little P. J., Tkachuk V. A., Bobik A. Thrombin-induced Ca2+ mobilization in vascular smooth muscle utilizes a slowly ribosylating pertussis toxin-sensitive G protein. Evidence for the involvement of a G protein in inositol trisphosphate-dependent Ca2+ release. J Biol Chem. 1992 Apr 15;267(11):7295–7302. [PubMed] [Google Scholar]
- Nunn D. L., Taylor C. W. Liver inositol, 1,4,5-trisphosphate-binding sites are the Ca2(+)-mobilizing receptors. Biochem J. 1990 Aug 15;270(1):227–232. doi: 10.1042/bj2700227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandol S. J., Schoeffield-Payne M. S. Cyclic GMP mediates the agonist-stimulated increase in plasma membrane calcium entry in the pancreatic acinar cell. J Biol Chem. 1990 Aug 5;265(22):12846–12853. [PubMed] [Google Scholar]
- Park D., Jhon D. Y., Lee C. W., Lee K. H., Rhee S. G. Activation of phospholipase C isozymes by G protein beta gamma subunits. J Biol Chem. 1993 Mar 5;268(7):4573–4576. [PubMed] [Google Scholar]
- Parker I., Ivorra I. Characteristics of membrane currents evoked by photoreleased inositol trisphosphate in Xenopus oocytes. Am J Physiol. 1992 Jul;263(1 Pt 1):C154–C165. doi: 10.1152/ajpcell.1992.263.1.C154. [DOI] [PubMed] [Google Scholar]
- Peppelenbosch M. P., Tertoolen L. G., den Hertog J., de Laat S. W. Epidermal growth factor activates calcium channels by phospholipase A2/5-lipoxygenase-mediated leukotriene C4 production. Cell. 1992 Apr 17;69(2):295–303. doi: 10.1016/0092-8674(92)90410-e. [DOI] [PubMed] [Google Scholar]
- Pobiner B. F., Northup J. K., Bauer P. H., Fraser E. D., Garrison J. C. Inhibitory GTP-binding regulatory protein Gi3 can couple angiotensin II receptors to inhibition of adenylyl cyclase in hepatocytes. Mol Pharmacol. 1991 Aug;40(2):156–167. [PubMed] [Google Scholar]
- Polverino A. J., Hughes B. P., Barritt G. J. Inhibition of Ca2+ inflow causes an abrupt cessation of growth-factor-induced repetitive free Ca2+ transients in single NIH-3T3 cells. Biochem J. 1991 Sep 15;278(Pt 3):849–855. doi: 10.1042/bj2780849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp R., Gögelein H. A calcium and ATP sensitive nonselective cation channel in the antiluminal membrane of rat cerebral capillary endothelial cells. Biochim Biophys Acta. 1992 Jul 8;1108(1):59–66. doi: 10.1016/0005-2736(92)90114-2. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. The action of alpha-adrenergic agonists on plasma-membrane calcium fluxes in perfused rat liver. Biochem J. 1984 May 15;220(1):43–50. doi: 10.1042/bj2200043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. G., Choi K. D. Regulation of inositol phospholipid-specific phospholipase C isozymes. J Biol Chem. 1992 Jun 25;267(18):12393–12396. [PubMed] [Google Scholar]
- Rooney T. A., Sass E. J., Thomas A. P. Characterization of cytosolic calcium oscillations induced by phenylephrine and vasopressin in single fura-2-loaded hepatocytes. J Biol Chem. 1989 Oct 15;264(29):17131–17141. [PubMed] [Google Scholar]
- Rossier M. F., Bird G. S., Putney J. W., Jr Subcellular distribution of the calcium-storing inositol 1,4,5-trisphosphate-sensitive organelle in rat liver. Possible linkage to the plasma membrane through the actin microfilaments. Biochem J. 1991 Mar 15;274(Pt 3):643–650. doi: 10.1042/bj2740643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saida K., Twort C., van Breemen C. The specific GTP requirement for inositol 1,4,5-trisphosphate-induced Ca2+ release from skinned vascular smooth muscle. J Cardiovasc Pharmacol. 1988;12 (Suppl 5):S47–S50. [PubMed] [Google Scholar]
- Shears S. B., Kirk C. J. Determination of mitochondrial calcium content in hepatocytes by a rapid cellular fractionation technique. Vasopressin stimulates mitochondrial Ca2+ uptake. Biochem J. 1984 Jun 1;220(2):417–421. doi: 10.1042/bj2200417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striggow F., Bohnensack R. Verapamil and diltiazem inhibit receptor-operated calcium channels and intracellular calcium oscillations in rat hepatocytes. FEBS Lett. 1993 Mar 8;318(3):341–344. doi: 10.1016/0014-5793(93)80542-3. [DOI] [PubMed] [Google Scholar]
- Strong M., Gutman G. A. Missing link in ion channels. Nature. 1993 Mar 4;362(6415):26–26. doi: 10.1038/362026b0. [DOI] [PubMed] [Google Scholar]
- Taylor S. J., Chae H. Z., Rhee S. G., Exton J. H. Activation of the beta 1 isozyme of phospholipase C by alpha subunits of the Gq class of G proteins. Nature. 1991 Apr 11;350(6318):516–518. doi: 10.1038/350516a0. [DOI] [PubMed] [Google Scholar]
- Willcocks A. L., Nahorski S. R. ATP and the binding of [3H]inositol 1,4,5-trisphosphate to its receptor. Biochem J. 1988 Nov 1;255(3):1061–1061. doi: 10.1042/bj2551061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B. S., Palade G. E., Farquhar M. G. Endoplasmic reticulum-through-Golgi transport assay based on O-glycosylation of native glycophorin in permeabilized erythroleukemia cells: role for Gi3. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1681–1685. doi: 10.1073/pnas.90.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature. 1986 Feb 13;319(6054):600–602. doi: 10.1038/319600a0. [DOI] [PubMed] [Google Scholar]
- Yang L. J., Baffy G., Rhee S. G., Manning D., Hansen C. A., Williamson J. R. Pertussis toxin-sensitive Gi protein involvement in epidermal growth factor-induced activation of phospholipase C-gamma in rat hepatocytes. J Biol Chem. 1991 Nov 25;266(33):22451–22458. [PubMed] [Google Scholar]
- Yang L., Camoratto A. M., Baffy G., Raj S., Manning D. R., Williamson J. R. Epidermal growth factor-mediated signaling of G(i)-protein to activation of phospholipases in rat-cultured hepatocytes. J Biol Chem. 1993 Feb 15;268(5):3739–3746. [PubMed] [Google Scholar]
- Yau K. W., Baylor D. A. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Duszynski J., Hreniuk S., Waybill M. M., LaNoue K. F. Regulation of plasma membrane permeability to calcium in primary cultures of rat hepatocytes. Cell Calcium. 1991 Sep;12(8):559–575. doi: 10.1016/0143-4160(91)90075-p. [DOI] [PubMed] [Google Scholar]


