Abstract
Background:
Colorectal cancer (CRC) is a major public health problem and one of leading cancer related death all over the world. One of the prognostic parameters that play a role in different types of cancer is HER2. However, the role of HER2 in CRC and its relation with clinicopathological features and survival is conflicting. We hypothesize that HER2 has different patterns of expression in CRC which may affect the prognosis of patients.
Material & Methods:
We studied sixty specimens of colorectal carcinoma for HER2 immunohistochemistry and gene amplification and correlate it with clinicopathological features and patients` survival.
Results:
Our data showed that negative HER2 expression was statistically associated with female gender (P = 0.010) and low & intermediate tumor budding (P = 0.030). There was a statistically significant relation between HER2 IHC and HER2 FISH amplification (P=0.000). Although neither HER2 immunoexpression and FISH amplification showed significant relation with overall survival nor disease free survival, HER2 amplified CRCs tended to have a worse survival compared with negative CRCs (40 months versus 50 months). The presence of male gender, lymphovascular invasion, nodal metastasis and distant metastasis (P = 0.013, 0.006, 0.006 and 0.000 respectively) were significantly statistically associated with poor overall survival. The presence of tumor grade III and high tumor budding (P = 0.035 and 0.007 respectively) were significantly statistically associated with shorter disease free survival.
Conclusions:
Our results showed that HER2 IHC 3+ staining is highly predictive of HER2 gene amplification in colorectal carcinomas. There is a tendency towards poorer prognosis in amplified HER2 CRC cases.
Introduction
Colorectal cancer (CRC) is a major public health problem and one of leading cancer related death all over the world [1]. It is the third most commonly diagnosed form of cancer globally, comprising 11% of all cancer diagnoses and the second most deadly cancer worldwide [1]. In spite of great advance in the diagnosis and developing therapeutic options, the prognosis of some patients is still grim. Many prognostic factors have been identified to date, with TNM (Tumor, Node, Metastasis) staging system one of the most important factors. However, patients with same stage may exhibit different outcome [2]. Therefor it is important to establish other prognostic & predictive parameters other than conventional ones. One of the prognostic parameters that play a role in different types of cancer is HER2 (human epidermal growth factor receptor) [3].
HER2 is a transmembrane glycoprotein located at the long arm of human chromosome 17 which belongs to the epidermal growth factor receptor (EGFR) epithelial tyrosine kinase protein family [4]. This family plays a central role in a variety of cellular responses including cell growth, survival, and differentiation via multiple signal transduction pathways and participate in cellular proliferation and differentiation [3].
It is well established that HER2 has a prognostic role in some types of cancer like breast and gastric carcinoma [5, 6]. It`s considered a therapeutic target, with targeted therapy against HER2 using the monoclonal antibody Trastuzumab (Herceptin) having become the standard for patients whose breast carcinomas exhibit HER2 gene amplification [5] and selected cases of gastric carcinoma [6]. However, HER2 overexpression and its relation with clinicopathological features and survival in CRC is conflicting [7]. In CRC, Diverse rates & patterns of HER2 overexpression have been reported [8], with cytoplasmic staining pattern showed an average around 30% [9] and only 2% - 11% showed membranous expression pattern [10].
To date, our knowledge about the concordance between HER2 expression by immunohistochemistry (IHC) and fluorescent in situ hybridization (FISH) in CRC is unclear. In addition, the relation between HER2 expression and patient prognosis is still limited. We hypothesize that HER2 has different patterns of expression in CRC and this may affect the prognosis of patients. To assess the credibility of our hypothesis, we investigated HER2 expression both by IHC & FISH techniques in CRC patients and correlate this expression with different clinicopathological parameters and patient’s survival.
Materials and Methods
Case selection
This is a retrospective study included sixty specimens of colorectal carcinoma. These were retrieved from biopsy specimens received at laboratory of pathology in south Egypt cancer institute at the period (2013-2016).
The available clinicopathological data of the cases were obtained from the hospital medical records. These data included: the age of patient at time of diagnosis, sex, tumor site, operation type, clinical follow up information as occurrence of distant metastasis or local recurrence and survival data including overall survival, disease free survival and progressive free survival. Follow up was performed for 2 years.
Representative Hematoxylin and eosin-stained slides were examined for each specimen for detailed histopathological features including tumor histological type & grade (according to WHO (World Health Organization) classification of colon and rectal tumors, 5th Edition, 2019) [11], perineural and lymphovascular invasion (LVI) , depth of tumor invasion, lymph node metastasis, distant metastasis (according to the TNM classification of the American Joint Committee on Cancer (AJCC) 8th Edition, 2017) [12]. In addition, we evaluate tumor budding and presence of poorly differentiated clusters (according to The International Tumor Budding Consensus Conference (ITBCC) 2016 group) [13].
Immunohistochemical staining of HER2
Immunohistochemical staining was performed using avid-biotin immunoperioxidase method. Four-micrometer-thick formalin-fixed, paraffin embedded tissue sections were mounted over coated slides. Sections were dewaxed and rehydrated through graded alcohols to distilled water. The hydrogen peroxide block was applied and incubated for 15 minutes then for antigen retrieval; sections were treated in microwave of (600 watt) by immersion of the slides in citrate puffer solution (PH 7) for 20 minutes. Sections were incubated with primary antibody (c-erbB-2/HER-2/neu Ab-17) for 1 hour at room temperature in the humid chamber. Antibody used was Clone e 2-4001 + 3B5, Catalog #MS-730-P0 (0.1ml) supplied by Thermo Scientific Company of 1/400 concentration. Then, the slides were washed 2 to 3 times using phosphate buffer saline solution. After washing, immunostaining was performed using a universal staining kit (Thermo Scientific Company) according to the manufacturer`s instructions. Counter staining was done using Mayer’s hematoxylin.
Positive control
It was examined first to ascertain that all reagents are functioning properly. Sections from breast (known as HER2 score 3) was used as positive control for HER2
Negative control
Tumor tissue section was processed in the above-mentioned sequence but the primary antibody was not added and instead phosphate buffered saline (PBS) was used in this step.
Evaluation of HER2 protein expression
HER2 staining was known by brownish staining of the cytoplasm. The scoring method for HER2 expression in colorectal cancer according to HERACLES (HER2 Amplification for Colorectal Cancer Enhanced Stratification) criteria [14] is as following: IHC score 0: no staining, IHC score 1+: faint staining (segmental or granular); moderate staining < 50% of cells; intense staining < 10% cells, IHC score 2+: moderate staining in > 50% of cells and IHC score 3+: intense staining in > 50% of cells. Score 0 and 1 are considered as negative, score 2 is equivocal and score 3 is positive.
Fluorescent in situ hybridization (FISH) HER2 amplification probe
All cases of our study were evaluated by FISH probe. FISH was performed using the Cytocell HER-2 (ERBB2) Amplification probe. The HER2 amplification probe consists of a red probe spanning the HER2 (ERBB2) gene and neighboring regions and a green probe for the chromosome 17 centromere. The FISH procedure was performed according to the manufacturer` instructions. In brief, 4µm sections were dewaxed and rehydrated through xylene and ethanol and heated in pretreatment reagent at 98 - 100°C for 20 - 30 min. 5µl of probe mixture (as the probes were provided premixed in hybridization solution (Formamide; Dextran Sulphate; SSC)) was applied to the slide and overlaid with a coverslip then underwent denaturation at 80°C for 5 minutes and hybridization at 37°C for 12 – 16 hours. After hybridization, slides were rinsed in 0.4× SSC/0.3% NP-40 at 72°C for 2 minutes, and counterstained with 4,6-diamidino-2-phenylindole (DAPI).
Evaluation of HER2 amplification probe
HER2 FISH positive: an average of >6 HER2 gene copies/nucleus or HER2/CEP (centromere enumeration probe) 17 ratio of more than 2.2 in more than 10% of cells, where CEP17 is a centromeric probe for chromosome 17 on which the HER2 gene resides. The equivocal range for HER2 FISH assays: HER/CEP ratios from 1.8 to 2.2 or average gene copy number between 4.0 and 6.0. Negative HER2 FISH amplification: HER2/CEP17 ratio of less than 1.8 or an average of fewer than four copies of HER2 gene per nucleus [15].
Statistical analysis
Results were statistically analyzed using statistical package for Social Sciences (SPSS version 22). Data were presented as frequency and percentage for qualitative variables, and as mean and standard deviation for quantitative variables. Chi-square test was used to compare qualitative variables. Kaplan Meyer (using Log-rank test) was done for overall survival, disease free survival and progression free survival. Multi-variate analysis was done using the Cox regression model. Significance was defined as P-value < 0.05.
Results
Clinicopathologic features of CRC Cases of the Study
The clinicopathologic characters of 60 patients with CRC involved in the present study were summarized in Table 1. Briefly, the age of patients ranges from 23 to 86 with the mean age was 50 years. 47 cases were adenocarcinoma, NOS and 13 cases were mucinous adenocarcinoma. 20 cases of CRC were in right colon and 40 cases were in left colon & rectum. 25 cases were grade I, 26 cases were grade II and 9 cases were grade III (Table 1).
Table 1.
Clinicopathological Features of the Studied Cases
| Clinicopathological features | No. (60) | % |
|---|---|---|
| Sex | ||
| Male | 30 | 50.00 |
| Female | 30 | 50.00 |
| Age: (years) | ||
| < 50 | 26 | 43.30 |
| ≥ 50 | 34 | 56.70 |
| Mean ± SD (Range) | 50.20 ± 13.70 (23.0-86.0) | |
| Tumor type | ||
| Adenocarcinoma | 47 | 78.30 |
| Mucinous adenocarcinoma | 13 | 21.70 |
| Site of tumor: | ||
| Right colon | 20 | 33.30 |
| Left colon & rectum | 40 | 66.70 |
| Grade | ||
| Grade I | 25 | 41.70 |
| Grade II | 26 | 43.30 |
| Grade III | 9 | 15.00 |
| Necrosis: | ||
| Positive | 16 | 26.70 |
| Negative | 36 | 60.00 |
| NA | 8 | 13.30 |
| LVI | ||
| Positive | 17 | 28.30 |
| Negative | 35 | 58.30 |
| NA | 8 | 13.30 |
| Tumor budding: | ||
| 1 | 16 | 26.70 |
| 2 | 14 | 23.30 |
| 3 | 18 | 30.00 |
| NA | 12 | 20.00 |
| Poorly differentiated clusters: | ||
| 1 | 23 | 38.30 |
| 2 | 11 | 18.30 |
| 3 | 14 | 23.30 |
| NA | 12 | 20.00 |
| Tumor invasion: | ||
| T2 | 2 | 3.30 |
| T3 | 49 | 81.70 |
| T4 | 1 | 1.70 |
| TX | 8 | 13.30 |
| Lymph node metastasis | ||
| N0 | 23 | 38.30 |
| N1 | 16 | 26.70 |
| N2 | 10 | 16.70 |
| NX | 11 | 18.30 |
| Distant metastasis: | ||
| M0 | 39 | 65.00 |
| M1 | 21 | 35.00 |
NA, not assessed
Immunohistochemical expression of HER2
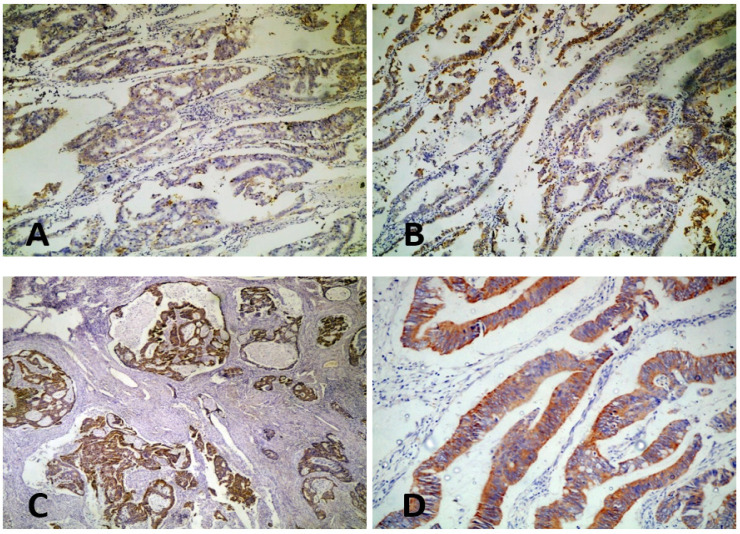
HER2/neu was expressed in colorectal carcinoma cells with mainly cytoplasmic staining pattern. Cytoplasmic staining was detected in 33/60 (55%) cases, while membranous staining was detected in only 1/60 case (1.7%). Positive HER2 staining (score 3) was noticed in 14 cases (23.3%), negative staining (score 0&1) in 28 cases (46.7%) and equivocal staining (score 2) in 18 cases (30%) (Figure 1)
Figure 1.
Expression of HER2 in Colorectal Carcinoma. A, Negative expression of HER2 in colorectal carcinoma (x10); B, Equivocal expression of HER2 in colorectal carcinoma (x10); C, Positive cytoplasmic expression of HER2 in colorectal carcinoma (x10); D, Positive cytoplasmic expression of HER2 in colorectal carcinoma (x40).
Relationship between HER2/neu protein expression and clinicopathological features
Statistically significant relation was detected between HER2 expression and sex of the patients as 67.9% of cases with negative HER2 expression were female gender (P = 0.010) . Also statistical significant relation was detected between HER2 expression and tumor budding as tumors with low & intermediate budding showed negative HER2 expression (P = 0.030) (Table 2).
Table 2.
Relationship between HER2 Expression and Clinicopathological Features
| Clinicopathological features | HER2 IHC | P-value | |||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Equivocal | |||||
| No. | % | No. | % | No. | % | ||
| Sex | |||||||
| Male | 7 | 50.00 | 9 | 32.10 | 14 | 77.80 | 0.010* |
| Female | 7 | 50.00 | 19 | 67.90 | 4 | 22.20 | |
| Age: (years) | |||||||
| < 50 | 5 | 35.70 | 10 | 35.70 | 11 | 61.10 | 0.191 |
| ≥ 50 | 9 | 64.30 | 18 | 64.30 | 7 | 38.90 | |
| Tumor type | |||||||
| Adenocarcinoma | 9 | 64.30 | 23 | 82.10 | 15 | 83.30 | 0.344 |
| Mucinous adenocarcinoma | 5 | 35.70 | 5 | 17.90 | 3 | 16.70 | |
| Site of tumor | |||||||
| Right colon | 6 | 42.90 | 8 | 28.60 | 6 | 33.30 | 0.651 |
| Left colon & rectum | 8 | 57.10 | 20 | 71.40 | 12 | 66.70 | |
| Grade | |||||||
| Grade I | 5 | 35.70 | 13 | 46.40 | 7 | 38.90 | |
| Grade II | 9 | 64.30 | 9 | 32.10 | 8 | 44.40 | 0.245 |
| Grade III | 0 | 0.00 | 6 | 21.40 | 3 | 16.70 | |
| Necrosis | |||||||
| Positive | 4 | 28.60 | 10 | 35.70 | 2 | 11.10 | 0.16 |
| Negative | 10 | 71.40 | 13 | 46.40 | 13 | 72.20 | |
| NA | 0 | 0.00 | 5 | 17.90 | 3 | 16.70 | |
| LVI | |||||||
| Positive | 2 | 14.30 | 9 | 32.10 | 6 | 33.30 | 0.194 |
| Negative | 12 | 85.70 | 14 | 50.00 | 9 | 50.00 | |
| NA | 0 | 0.00 | 5 | 17.90 | 3 | 16.70 | |
| Tumor budding | |||||||
| 1 | 5 | 35.70 | 9 | 32.10 | 2 | 11.10 | |
| 2 | 5 | 35.70 | 6 | 21.40 | 3 | 16.70 | 0.030* |
| 3 | 2 | 14.30 | 5 | 17.90 | 11 | 61.10 | |
| NA | 2 | 14.30 | 8 | 28.60 | 2 | 11.10 | |
| Poorly differentiated clusters: | |||||||
| 1 | 8 | 57.10 | 7 | 25.00 | 8 | 44.40 | |
| 2 | 2 | 14.30 | 8 | 28.60 | 1 | 5.60 | 0.095 |
| 3 | 2 | 14.30 | 5 | 17.90 | 7 | 38.90 | |
| NA | 2 | 14.30 | 8 | 28.60 | 2 | 11.10 | |
| Tumor invasion: | |||||||
| T2 | 0 | 0.00 | 2 | 7.10 | 0 | 0.00 | |
| T3 | 14 | 100.00 | 20 | 71.40 | 15 | 83.30 | 0.338 |
| T4 | 0 | 0.00 | 1 | 3.60 | 0 | 0.00 | |
| TX | 0 | 0.00 | 5 | 17.90 | 3 | 16.70 | |
| Lymph node metastasis | |||||||
| N0 | 10 | 71.40 | 12 | 42.90 | 1 | 5.60 | |
| N1 | 3 | 21.40 | 7 | 25.00 | 6 | 33.30 | 0.11 |
| N2 | 0 | 0.00 | 4 | 14.30 | 6 | 33.30 | |
| NX | 1 | 7.10 | 5 | 17.90 | 5 | 27.80 | |
| Distant metastasis | |||||||
| M0 | 8 | 57.10 | 18 | 64.30 | 13 | 72.20 | 0.671 |
| M1 | 6 | 42.90 | 10 | 35.70 | 5 | 27.80 | |
No significant relationship was detected between HER2 expression and other parameters as; age, site of the tumor, histologic type and grade, presence of necrosis, lymphovascular emboli, poorly differentiated clusters, tumor invasion, lymph node metastasis and distant metastasis (P=0.191, P=0.651, P=0.060, P=0.334, P=0.651, P=0.245, P=0.160, P=0.194, P=0.095, P=0.338, P=0.110 and P=0.671 respectively) (Table 2).
Cytogenetic finding
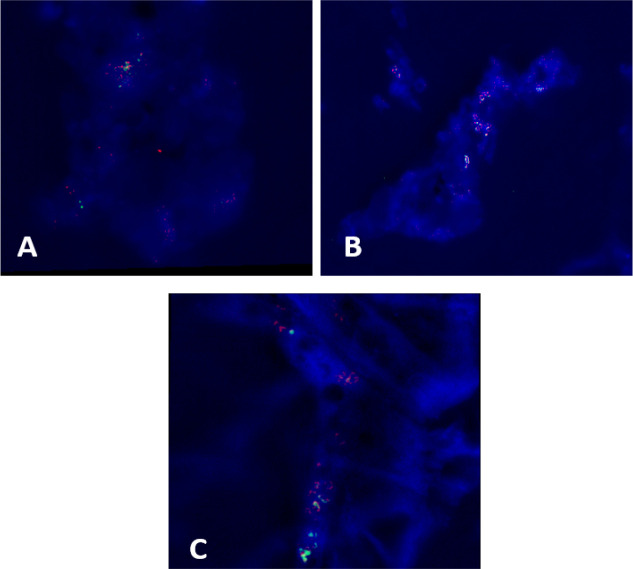
Amplification of HER2/neu FISH probe were noticed in 32 cases of CRC (53.3%) while 28 cases (46.7%) showed negative expression (Figure 2).
Figure 2.
A, B & C; HER2 Amplification in Colorectal Carcinoma
Relationship of HER2/neu FISH probe amplification and clinicopathological features
Statistically significant relation was detected between HER2/neu FISH probe amplification and male gender (P=0.010) and presence of grade 1 poorly differentiated clusters (P=0.045) (Table 3).
Table 3.
Relation between Her2/neu FISH Probe Amplification and Clinicopathological Features
| Clinicopathological features | HER2 FISH probe | P-value | |||
|---|---|---|---|---|---|
| Amplified | Negative | ||||
| No. | % | No. | % | ||
| Sex | |||||
| Male | 21 | 65.60 | 9 | 32.10 | 0.010* |
| Female | 11 | 34.40 | 19 | 67.90 | |
| Age: (years) | |||||
| < 50 | 16 | 50.00 | 10 | 35.70 | 0.265 |
| ≥ 50 | 16 | 50.00 | 18 | 64.30 | |
| Tumor type | |||||
| Adenocarcinoma | 24 | 75.00 | 23 | 82.10 | 0.503 |
| Mucinous adenocarcinoma | 8 | 25.00 | 5 | 17.90 | |
| Site of tumor | |||||
| Right colon | 12 | 37.50 | 8 | 28.60 | 0.464 |
| Left colon & rectum | 20 | 62.50 | 20 | 71.40 | |
| Grade | |||||
| Grade I | 12 | 37.50 | 13 | 46.40 | |
| Grade II | 17 | 53.10 | 9 | 32.10 | 0.197 |
| Grade III | 3 | 9.40 | 6 | 21.40 | |
| Necrosis | |||||
| Positive | 6 | 18.80 | 10 | 35.70 | |
| Negative | 23 | 71.90 | 13 | 46.40 | 0.133 |
| NA | 3 | 9.40 | 5 | 17.90 | |
| LVI | |||||
| Positive | 8 | 25.00 | 9 | 32.10 | |
| Negative | 21 | 65.60 | 14 | 50.00 | 0.427 |
| NA | 3 | 9.40 | 5 | 17.90 | |
| Tumor budding | |||||
| 1 | 7 | 21.90 | 9 | 32.10 | |
| 2 | 8 | 25.00 | 6 | 21.40 | 0.159 |
| 3 | 13 | 40.60 | 5 | 17.90 | |
| NA | 4 | 12.50 | 8 | 28.60 | |
| Poorly differentiated clusters: | |||||
| 1 | 16 | 50.00 | 7 | 25.00 | |
| 2 | 3 | 9.40 | 8 | 28.60 | 0.045* |
| 3 | 9 | 28.10 | 5 | 17.90 | |
| NA | 4 | 12.50 | 8 | 28.60 | |
| Tumor invasion: | |||||
| T2 | 0 | 0.00 | 2 | 7.10 | |
| T3 | 29 | 90.60 | 20 | 71.40 | 0.179 |
| T4 | 0 | 0.00 | 1 | 3.60 | |
| TX | 3 | 9.40 | 5 | 17.90 | |
| Lymph node metastasis: | |||||
| N0 | 11 | 34.40 | 12 | 42.90 | |
| N1 | 9 | 28.10 | 7 | 25.00 | 0.914 |
| N2 | 6 | 18.80 | 4 | 14.30 | |
| NX | 6 | 18.80 | 5 | 17.90 | |
| Distant metastasis | |||||
| M0 | 21 | 65.60 | 18 | 64.30 | 0.914 |
| M1 | 11 | 34.40 | 10 | 35.70 | |
*Statistical significant difference (P < 0.05); NA, not assessed
No significant relation was detected between HER2/neu FISH probe amplification and other parameters as; age, site of the tumor, histologic type and grade, presence of necrosis, lymphovascular emboli, tumor budding, tumor invasion, lymph node metastasis and distant metastasis (P=0.265, P=0.464, P=0.396, P=0.197 P=0.133, P=0.427, P=0.194, P=0.159, P=0.179, P=0.914 and P=0.592 respectively) (Table 3).
Relation between HER2/neu immunoexpression and HER2/neu FISH probe amplification
According to our study, all 28 cases with negative HER2 expression showed negative Fish probe. All positive (14) and equivocal (18) cases showed amplified Fish probe. There was a statistically significant correlation between HER2/neu immunoexpression and HER2/neu FISH amplification (P=0.000) (Table 4).
Table 4.
Relation between HER2 Immunoexpression and HER2 FISH Probe Amplification
| HER2 IHC | HER2 FISH probe | P-value | |||
|---|---|---|---|---|---|
| Amplified | Negative | ||||
| No. | % | No. | % | ||
| Positive | 14 | 43.80 | 0 | 0.00 | 0.000* |
| Negative | 0 | 0.00 | 28 | 100.00 | |
| Equifocal | 18 | 56.30 | 0 | 0.00 | |
*Statistical significant difference (P < 0.05)
Survival Analysis
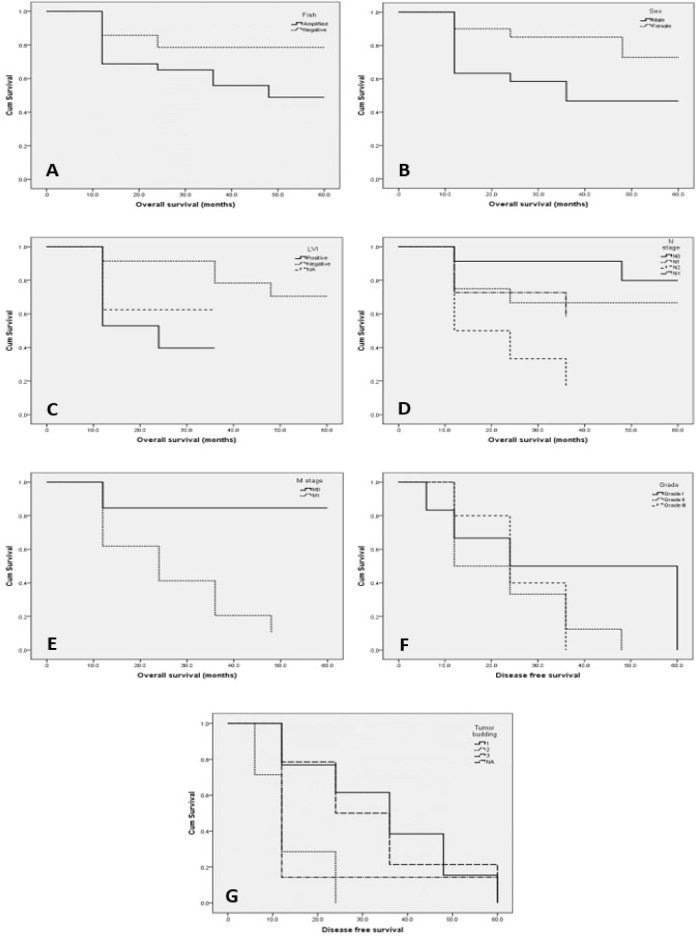
Univariate Kaplan-Meier-survival analysis demonstrated that neither HER2 immunoexpression and FISH amplification showed significant relation with overall survival (OS), disease-free survival (DFS) and progression-free survival (PFS) (HER2 expression; OS, P = 0.113, DFS; P = 0.130 and PFS; P = 0.143) and (HER2 FISH probe; OS, P = 0.102, DFS, P = 0.121 and PFS; P = 0.438). However, HER2 amplified CRCs tended to have a worse survival compared with negative CRCs (Figure 3, A). The median survival time of patients with amplified HER2 was 40 months versus 50 months in patients with negative HER2 expression.
Figure 3.
A, Relation between HER2 FISH probe amplification and OS; B, Relation between sex and OS; C, Relation between lymphovascular emboli and OS; D, Relation between lymph node metastasis and OS; E, Relation between distant metastasis and OS; F, Relation between tumor grade and DFS; G, Relation between tumor budding and DFS.
The univariate analysis of the other parameters examined showed that there was a significant progressive decline in OS with male gender (P = 0.013; Figure 3, B), with presence of LVI (P = 0.006; Figure 3, C), with lymph node metastasis (P = 0.006; Figure 3, D) and also with distant metastasis (P = 0.000; Figure 3, E). The remaining clinicopathological parameters examined, namely: age, tumor site, histologic type, grade, presence of necrosis, tumor budding, poorly differentiated clusters and depth of tumor invasion were not found to be associated significantly with OS (P > 0.05).
There was also a significant progressive decline in DFS with tumor grade III (P = 0.035; Figure 3, F) and high tumor budding (P = 0.007; Figure 3, G). The remaining clinicopathological parameters examined, namely: age, sex tumor site, histologic type, presence of necrosis, lymphovascular emboli, poorly differentiated clusters, depth of tumor invasion, lymph node metastasis and distant metastasis were not found to be associated significantly with OS (P > 0.05).
PFS was not found to be affected by any clinicopathological parameters. After multivariate analysis using Cox proportional hazard model, distant metastasis (P = 0.002; HR = 6.988; 95% CI, 2.065-23.652) proved to be the only significant independent factor for OS.
Discussion
The HER2 signaling network is known to influence a wide range of cellular processes, including proliferation, motility, and survival [16]. Overexpression of the HER2 receptor is variable in different tumors; as it is detected in 13–20% of human breast cancer [17] but the level and incidence of HER2 overexpression in primary colon tumors appears to be different than those observed in breast cancer. Conflicting data exist about the prevalence of HER2 overexpression in colorectal cancer which ranges from 2.7% to 47.4% [18].
In this work, we studied 60 cases of colorectal carcinoma in South Egypt Cancer Institute in period from 2013 to 2016, HER2 protein expression by immunohistochemistry and gene amplification by fluorescence in situ hybridization, its predictive and prognostic values and relation to DFS, PFS and OS.
Positive HER2 staining (score 2&3) was noticed in 18 and 14 cases respectively (53.3%) and negative staining (score 0&1) in 28 cases (46.7%). Our findings were similar to Shabbir et al. [19] and Kamaland Jalal [20] who detected positive immunostaining in 55% and 53.4% of colorectal cancer cases respectively. However, these results were very different from other researches done by Sawada et al. [21] and Wang et al. [18] who found HER2 positivity in 4.1% and 11.7% of cases respectively.
Possible explanation for this dramatic range in HER2 overexpression positivity across studies may be that antibodies used for staining varied among research groups and also variation in the definition of the positivity pattern either membranous or cytoplasmic. The HER2 antibody used in the study with the highest positive HER2 staining (47.4%) was a polyclonal rabbit antibody. The positive rates of HER2 protein staining in studies using the DAKO HER2 antibody were 2.7% to 15.5%, while it were 8% to 11.4% in the studies using Ventana pathway antibody [18].
HER2 gene amplification was identified in all (18) cases with HER2 IHC scores of 2+ and (14) cases with HER2 IHC scores of 3+, which totally represent 53.7% of all colorectal cancer patients. No tumors with HER2 IHC scores of 1+ showed evidence of HER2 gene amplification by FISH. This indicates that there is a high concordance between HER2 IHC 3+ staining and HER2 gene amplification in colorectal adenocarcinomas. This is similar to Marx et al. [22] who found that 100% of tumors with 3+ HER2 staining showed HER2 gene amplifications and nearly similar to Wang et al. [18] who detected 83% of tumors with 3+ HER2 staining exhibited HER2 gene amplification.
Variations in the antibodies used likely led to differential staining and scoring, contributing to the disagreement in HER2 gene amplification in tumors with 3+ HER2 scores. All these studies, including ours, have shown that HER2 IHC 3+ staining is highly predictive of HER2 gene amplification in colorectal carcinomas.
In contrast, the percentage of tumors with HER2 IHC 2+ staining showing evidence of HER2 gene amplification was highly variable. In our study, HER2 amplification was evident in 100% of tumors, nearly similar to Marx et al. [22], who observed HER2 gene amplification in 75% of tumors with HER2 scores of 2+. In contrast, in the study by Sawada et al. [21] and Wang et al. [18], such amplification was only evident in 36% and 20% of tumors respectively.
In this study, we found a statistically significant association between negative HER2 expression and female patients (P=0.010), low and intermediate tumor budding (P=0.030). No other similar studies in association between HER2 expression and tumor budding were found in the literature. However, tumor budding is an independent adverse prognostic factor in colorectal cancer as it is associated with a higher TNM stage, high tumor grade, the presence of lymphovascular invasion and consequently with lymph node and distant metastases [23, 24]. Therefore, there may be a relation between high tumor budding and positive HER2 expression.
We didn`t find significant relation with site of the tumor, but Seo et al. [25] detected a statistically significant association with tumor location in the rectum (P = 0.033). It has been clearly demonstrated that HER2 gene amplification differs significantly between right/left-sided and rectal carcinomas [26, 27].
We also didn`t find significant relation with histologic grade or lymph node metastasis. However, Heppner et al. [28] found statistically significant association of HER2 positivity with nodal status (P =0.033). Conradi et al. [29] reported that HER2 positivity was associated with high grade tumors and positive nodal status. We didn`t find significant relation with depth of tumor invasion or distant metastasis. On the other hand, Wang et al. [18] found a statistically significant association between HER2 gene amplification and tumor depth of invasion and distant metastasis (P = 0.001 and 0.028 respectively).
On the other hand, Marx et al. [22] failed to find an association of positive HER2 status with clinicopathological parameters. All these differences between many studies are probably due to differences in groups analyzed, HER2 testing methods, and tumor biological characteristics. In our study, HER2 positivity had no statistically significant impact on patients’ overall survival, but HER2 positive tumors displayed a tendency to poorer courses, which is in line with Heppner et al. [28]. However, Seo et al. [25] and Wang et al. [18] didn`t detect an association between HER2 expression and survival rates.
In our study, OS showed significant pro¬gressive decline in association with male gender (P = 0.013), presence of LVI (P = 0.006), lymph node metastasis (P = 0.006) and distant metastasis (P = 0.000). This is similar to Chao-Hsien et al. [30] who reported significant decline in OS with lymph node metastasis (P < 0.001) and distant metastasis (P < 0.001). Also, Joachim et al. [31] detected significantly worse median OS in men (P = 0.0394). Finally, Pei et al. [32] reported that presence of lymphovascular emboli was statistically associated with worse OS (P < 0.001).
Unfortunately, we have no detailed information about the different applied therapy regimen, which may be a limitation of the presented study. However, the tendency of poorer overall survival of HER2 positive CRC probably reflects the association with advanced CRC, independent of treatment. In conclusion, HER2 protein overexpression is evident in 53.7% of CRC. Both HER2 IHC scores 2 and 3 are highly correlated with HER2 gene amplification. There is a tendency towards poorer prognosis in amplified HER2 CRC cases.
Recommendation; future studies are recommended for the possibility of benefit of HER2 targeted therapy in certain group of colorectal carcinoma patients.
Acknowledgements
We are grateful for the Research Grant office, Quality Assurance Unite at Faculty of Medicine, Assiut University as the work funded by them; Grant number (2017107/12-003-R1). We also grateful for Surgical Pathology Laboratories, Assuit University Hospital, Faculty of Medicine and South Egypt Cancer Institute, Assuit University as we take from them our speciemens.
Funding sources
This work was funded by the Research Grant office, Quality Assurance Unite at Faculty of Medicine, Assiut University. Grant number (2017107/12-003-R1).
Ethical issue
This research received ethics approval from the Committee of Medical Ethics of Faculty of Medicine, Assuit University, number (17200089).
Data availability
All data generated or analyzed during this study are included in this published article.
Funding
This work was funded by the Research Grant office, Quality Assurance Unite at Faculty of Medicine, Assiut University. Grant number (2017107/12-003-R1).
Conflict of interest
The authors declare that they have no conflict of interest exist.
Author Contribution Statement
Conceptualization: HEB; Data curation: HEB; Formal analysis: HEB, FAT; Investigation: HEB, FAT, THA; Methodology: HEB; Project administration: HEB, HAN.EMA; Resources: HEB, FAT; Supervision: HEB.THA; Validation: HEB; Visualization: HEB; Writing – original draft: HEB, MOM; Writing – review & editing: HEB; Approval of final manuscript: all authors.
Abbreviations
CRC: colorectal cancer
TNM: Tumor Node Metastasis
HER2: human epidermal growth factor receptor 2
EGFR: epidermal growth factor receptor
IHC: immunohistochemistry
FISH: fluorescent in situ hybridization
WHO: World Health Organization
AJCC: American Joint Committee on Cancer
ITBCC: International Tumor Budding Consensus Conference (ITBCC)
PBS: phosphate buffered saline
HERACLES: HER2 Amplification for Colorectal Cancer Enhanced Stratification
DAPI: 4,6-diamidino-2-phenylindole
CEP: centromere enumeration probe
SPSS: Statistical Package for Social Sciences
LVI: lymphovascular invasion
OS: overall survival
DFS: disease free survival
PFS: progression free survival
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.El-Deek HEM, Ahmed AM, Mohammed RAA. Aberration of nrf2bach1 pathway in colorectal carcinoma; role in carcinogenesis and tumor progression. Ann Diagn Pathol. 2019;38(9):138–44. doi: 10.1016/j.anndiagpath.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Iqbal N, Iqbal N. Human epidermal growth factor receptor 2 (her2) in cancers: Overexpression and therapeutic implications. Mol Biol Int. 2014;2014(6):852748. doi: 10.1155/2014/852748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu JL, Hung MC. The role of her2, egfr, and other receptor tyrosine kinases in breast cancer. Cancer Metastasis Rev. 2016;35(4):575–88. doi: 10.1007/s10555-016-9649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early her2-positive breast cancer (neosphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 6.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of her2-positive advanced gastric or gastro-oesophageal junction cancer (toga): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 7.Farzand S, Siddique T, Saba K, Bukhari MH. Frequency of her2/neu overexpression in adenocarcinoma of the gastrointestinal system. World J Gastroenterol. 2014;20(19):5889–96. doi: 10.3748/wjg.v20.i19.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C, Liu DR, Ye LY, Huang LN, Jaiswal S, Li XW, et al. Her-2 overexpression and survival in colorectal cancer: A meta-analysis. J Zhejiang Univ Sci B. 2014;15(6):582–9. doi: 10.1631/jzus.B1300258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blok EJ, Kuppen PJ, van Leeuwen JE, Sier CF. Cytoplasmic overexpression of her2: A key factor in colorectal cancer. Clin Med Insights Oncol. 2013;7(6):41–51. doi: 10.4137/CMO.S10811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richman SD, Southward K, Chambers P, Cross D, Barrett J, Hemmings G, et al. Her2 overexpression and amplification as a potential therapeutic target in colorectal cancer: Analysis of 3256 patients enrolled in the quasar, focus and piccolo colorectal cancer trials. J Pathol. 2016;238(4):562–70. doi: 10.1002/path.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 who classification of tumours of the digestive system. Histopathology. 2020;76(2):182–8. doi: 10.1111/his.13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brierley JD, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. John Wiley & Sons; 2017. [Google Scholar]
- 13.Lugli A, Kirsch R, Ajioka Y, Bosman F, Cathomas G, Dawson H, et al. Recommendations for reporting tumor budding in colorectal cancer based on the international tumor budding consensus conference (itbcc) 2016. Mod Pathol. 2017;30(9):1299–311. doi: 10.1038/modpathol.2017.46. [DOI] [PubMed] [Google Scholar]
- 14.Valtorta E, Martino C, Sartore-Bianchi A, Penaullt-Llorca F, Viale G, Risio M, et al. Assessment of a her2 scoring system for colorectal cancer: Results from a validation study. Mod Pathol. 2015;28(11):1481–91. doi: 10.1038/modpathol.2015.98. [DOI] [PubMed] [Google Scholar]
- 15.Ruschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, et al. Her2 testing in gastric cancer: A practical approach. Mod Pathol. 2012;25(5):637–50. doi: 10.1038/modpathol.2011.198. [DOI] [PubMed] [Google Scholar]
- 16.Achalla LSV, Shinde RK, Jogdand S, Vodithala S. Review of the role of her2/neu in colorectal carcinomas. Cureus. 2022;14(5):e25409. doi: 10.7759/cureus.25409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rakha EA, Pinder SE, Bartlett JM, Ibrahim M, Starczynski J, Carder PJ, et al. Updated uk recommendations for her2 assessment in breast cancer. J Clin Pathol. 2015;68(2):93–9. doi: 10.1136/jclinpath-2014-202571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang XY, Zheng ZX, Sun Y, Bai YH, Shi YF, Zhou LX, et al. Significance of her2 protein expression and her2 gene amplification in colorectal adenocarcinomas. World J Gastrointest Oncol. 2019;11(4):335–47. doi: 10.4251/wjgo.v11.i4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shabbir A, Mirza T, Khalid AB, Qureshi MA, Asim SA. Frequency of her2/neu expression in colorectal adenocarcinoma: A study from developing south asian country. BMC Cancer. 2016;16(1):855 . doi: 10.1186/s12885-016-2912-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamal LA, Jalal JA. Immunohistochemical expression of her2/neu in colorectal carcinoma in erbil city, kurdistan region. Zanco J Med Sci. 2019;23(3):421–8. [Google Scholar]
- 21.Sawada K, Nakamura Y, Yamanaka T, Kuboki Y, Yamaguchi D, Yuki S, et al. Prognostic and predictive value of her2 amplification in patients with metastatic colorectal cancer. Clin Colorectal Cancer. 2018;17(3):198–205. doi: 10.1016/j.clcc.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Marx AH, Burandt EC, Choschzick M, Simon R, Yekebas E, Kaifi JT, et al. Heterogenous high-level her-2 amplification in a small subset of colorectal cancers. Hum Pathol. 2010;41(11):1577–85. doi: 10.1016/j.humpath.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 23.van Wyk HC, Park J, Roxburgh C, Horgan P, Foulis A, McMillan DC. The role of tumour budding in predicting survival in patients with primary operable colorectal cancer: A systematic review. Cancer Treat Rev. 2015;41(2):151–9. doi: 10.1016/j.ctrv.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Rogers AC, Winter DC, Heeney A, Gibbons D, Lugli A, Puppa G, et al. Systematic review and meta-analysis of the impact of tumour budding in colorectal cancer. Br J Cancer. 2016;115(7):831–40. doi: 10.1038/bjc.2016.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seo AN, Kwak Y, Kim D-W, Kang S-B, Choe G, Kim WH, et al. Her2 status in colorectal cancer: Its clinical significance and the relationship between her2 gene amplification and expression. PloS One. 2014;9(5):e98528. doi: 10.1371/journal.pone.0098528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salem ME, Weinberg BA, Xiu J, El-Deiry WS, Hwang JJ, Gatalica Z, et al. Comparative molecular analyses of left-sided colon, right-sided colon, and rectal cancers. Oncotarget. 2017;8(49):86356–68. doi: 10.18632/oncotarget.21169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loree JM, Bailey AM, Johnson AM, Yu Y, Wu W, Bristow CA, et al. Molecular landscape of erbb2/erbb3 mutated colorectal cancer. J Natl Cancer Inst. 2018;110(12):1409–17. doi: 10.1093/jnci/djy067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heppner B, Behrens H, Balschun K, Haag J, Krüger S, Becker T, et al. Her2/neu testing in primary colorectal carcinoma. Br J Cancer. 2014;111(10):1977–84. doi: 10.1038/bjc.2014.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conradi LC, Styczen H, Sprenger T, Wolff HA, Rodel C, Nietert M, et al. Frequency of her-2 positivity in rectal cancer and prognosis. Am J Surg Pathol. 2013;37(4):522–31. doi: 10.1097/PAS.0b013e318272ff4d. [DOI] [PubMed] [Google Scholar]
- 30.Chao-Hsien L, Cheng S-C, Hong-Yi T, Chang S-C, Ching C-Y, Shu-Fen W. The risk factors affecting survival in colorectal cancer in taiwan. Iran J Public Health. 2018;47(4):519. [PMC free article] [PubMed] [Google Scholar]
- 31.Joachim C, Macni J, Drame M, Pomier A, Escarmant P, Veronique-Baudin J, et al. Overall survival of colorectal cancer by stage at diagnosis: Data from the martinique cancer registry. Medicine. 2019;98:35. doi: 10.1097/MD.0000000000016941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pei Q, Zhu H, Tan F, Yu N, Zhou Z, Zhou Y, et al. Intravascular emboli is an independent risk factor for the prognosis of stage iii colorectal cancer patients after radical surgery. Oncotarget. 2016;7(35):57268. doi: 10.18632/oncotarget.11266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Funding
This work was funded by the Research Grant office, Quality Assurance Unite at Faculty of Medicine, Assiut University. Grant number (2017107/12-003-R1).