Abstract
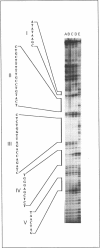
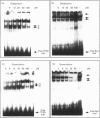
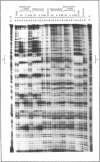
Pharmacological modulation of the interaction between transcription factors and target DNA sequences of cellular and viral genes could have important effects in the experimental therapy of a large variety of human pathologies. For instance, alteration of the DNA/protein interaction might be among the molecular mechanisms of action of DNA-binding drugs, leading to an inhibition of the expression of genes involved in the control of in vitro and in vivo growth of neoplastic cells and virus DNA replication. Natural oligopeptides, such as distamycin, are powerful inhibitors of the interaction between nuclear factors and target DNA sequences and, therefore, have been proposed as compounds retaining antibiotic, antineoplastic and antiviral properties. In this study we performed DNAase I footprinting analysis using a PCR product mimicking a region of the long terminal repeat (LTR) of the human immunodeficiency type 1 (HIV-1) retrovirus. The data obtained suggest that distamycin binds to different regions of the HIV-1 LTR depending on the DNA sequence. Electrophoretic mobility shift assays using both crude nuclear extracts from the Jurkat T-lymphoid cell line and the recombinant proteins transcription factor IID and Sp1 suggest that distamycin differentially inhibits the interaction of these two proteins with their specific DNA target sequences, in good agreement with the results obtained by DNAase I footprinting analysis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad N., Venkatesan S. Nef protein of HIV-1 is a transcriptional repressor of HIV-1 LTR. Science. 1988 Sep 16;241(4872):1481–1485. doi: 10.1126/science.3262235. [DOI] [PubMed] [Google Scholar]
- Barbieri R., Giacomini P., Volinia S., Nastruzzi C., Mileo A. M., Ferrini U., Soria M., Barrai I., Natali P. G., Gambari R. Human HLA-DR alpha gene: a rare oligonucleotide (GTATA) identifies an upstream sequence required for nuclear protein binding. FEBS Lett. 1990 Jul 30;268(1):51–54. doi: 10.1016/0014-5793(90)80970-t. [DOI] [PubMed] [Google Scholar]
- Berg O. G., von Hippel P. H. Selection of DNA binding sites by regulatory proteins. Trends Biochem Sci. 1988 Jun;13(6):207–211. doi: 10.1016/0968-0004(88)90085-0. [DOI] [PubMed] [Google Scholar]
- Braithwaite A. W., Baguley B. C. Existence of an extended series of antitumor compounds which bind to deoxyribonucleic acid by nonintercalative means. Biochemistry. 1980 Mar 18;19(6):1101–1106. doi: 10.1021/bi00547a009. [DOI] [PubMed] [Google Scholar]
- Briggs M. R., Kadonaga J. T., Bell S. P., Tjian R. Purification and biochemical characterization of the promoter-specific transcription factor, Sp1. Science. 1986 Oct 3;234(4772):47–52. doi: 10.1126/science.3529394. [DOI] [PubMed] [Google Scholar]
- Broggini M., Ponti M., Ottolenghi S., D'Incalci M., Mongelli N., Mantovani R. Distamycins inhibit the binding of OTF-1 and NFE-1 transfactors to their conserved DNA elements. Nucleic Acids Res. 1989 Feb 11;17(3):1051–1059. doi: 10.1093/nar/17.3.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratowski S., Hahn S., Guarente L., Sharp P. A. Five intermediate complexes in transcription initiation by RNA polymerase II. Cell. 1989 Feb 24;56(4):549–561. doi: 10.1016/0092-8674(89)90578-3. [DOI] [PubMed] [Google Scholar]
- Byrne B. C., Li J. J., Sninsky J., Poiesz B. J. Detection of HIV-1 RNA sequences by in vitro DNA amplification. Nucleic Acids Res. 1988 May 11;16(9):4165–4165. doi: 10.1093/nar/16.9.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey J. Gel retardation at low pH resolves trp repressor-DNA complexes for quantitative study. Proc Natl Acad Sci U S A. 1988 Feb;85(4):975–979. doi: 10.1073/pnas.85.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaires J. B., Fox K. R., Herrera J. E., Britt M., Waring M. J. Site and sequence specificity of the daunomycin-DNA interaction. Biochemistry. 1987 Dec 15;26(25):8227–8236. doi: 10.1021/bi00399a031. [DOI] [PubMed] [Google Scholar]
- Chaires J. B., Herrera J. E., Waring M. J. Preferential binding of daunomycin to 5'ATCG and 5'ATGC sequences revealed by footprinting titration experiments. Biochemistry. 1990 Jul 3;29(26):6145–6153. doi: 10.1021/bi00478a006. [DOI] [PubMed] [Google Scholar]
- Dervan P. B. Design of sequence-specific DNA-binding molecules. Science. 1986 Apr 25;232(4749):464–471. doi: 10.1126/science.2421408. [DOI] [PubMed] [Google Scholar]
- Dorn A., Affolter M., Müller M., Gehring W. J., Leupin W. Distamycin-induced inhibition of homeodomain-DNA complexes. EMBO J. 1992 Jan;11(1):279–286. doi: 10.1002/j.1460-2075.1992.tb05050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faisst S., Meyer S. Compilation of vertebrate-encoded transcription factors. Nucleic Acids Res. 1992 Jan 11;20(1):3–26. doi: 10.1093/nar/20.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. R., Sansom C. E., Stevens M. F. Footprinting studies on the sequence-selective binding of pentamidine to DNA. FEBS Lett. 1990 Jun 18;266(1-2):150–154. doi: 10.1016/0014-5793(90)81527-u. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambari R., Barbieri R., Nastruzzi C., Chiorboli V., Feriotto G., Natali P. G., Giacomini P., Arcamone F. Distamycin inhibits the binding of a nuclear factor to the -278/-256 upstream sequence of the human HLA-DR alpha gene. Biochem Pharmacol. 1991 Feb 15;41(4):497–502. doi: 10.1016/0006-2952(91)90620-k. [DOI] [PubMed] [Google Scholar]
- Gaynor R. Cellular transcription factors involved in the regulation of HIV-1 gene expression. AIDS. 1992 Apr;6(4):347–363. doi: 10.1097/00002030-199204000-00001. [DOI] [PubMed] [Google Scholar]
- Herbst R. S., Boczko E. M., Darnell J. E., Jr, Babiss L. E. The mouse albumin enhancer contains a negative regulatory element that interacts with a novel DNA-binding protein. Mol Cell Biol. 1990 Aug;10(8):3896–3905. doi: 10.1128/mcb.10.8.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. Structure and function of transcription factors. Semin Cancer Biol. 1990 Feb;1(1):5–17. [PubMed] [Google Scholar]
- Kageyama R., Pastan I. Molecular cloning and characterization of a human DNA binding factor that represses transcription. Cell. 1989 Dec 1;59(5):815–825. doi: 10.1016/0092-8674(89)90605-3. [DOI] [PubMed] [Google Scholar]
- Kakkis E., Riggs K. J., Gillespie W., Calame K. A transcriptional repressor of c-myc. Nature. 1989 Jun 29;339(6227):718–721. doi: 10.1038/339718a0. [DOI] [PubMed] [Google Scholar]
- Lewin B. Oncogenic conversion by regulatory changes in transcription factors. Cell. 1991 Jan 25;64(2):303–312. doi: 10.1016/0092-8674(91)90640-k. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Montecucco A., Fontana M., Focher F., Lestingi M., Spadari S., Ciarrocchi G. Specific inhibition of human DNA ligase adenylation by a distamycin derivative possessing antitumor activity. Nucleic Acids Res. 1991 Mar 11;19(5):1067–1072. doi: 10.1093/nar/19.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastruzzi C., Menegatti E., Pastesini C., Cortesi R., Esposito E., Spano M., Biagini R., Cordelli E., Feriotto G., Gambari R. DNA binding activity and inhibition of DNA-protein interactions. Differential effects of tetra-p-amidino-phenoxyneopentane and its 2'-bromo derivative. Biochem Pharmacol. 1992 Nov 17;44(10):1985–1994. doi: 10.1016/0006-2952(92)90101-n. [DOI] [PubMed] [Google Scholar]
- Neidle S., Pearl L. H., Skelly J. V. DNA structure and perturbation by drug binding. Biochem J. 1987 Apr 1;243(1):1–13. doi: 10.1042/bj2430001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M. G., Tanese N., Pugh B. F., Tjian R. Functional domains and upstream activation properties of cloned human TATA binding protein. Science. 1990 Jun 29;248(4963):1625–1630. doi: 10.1126/science.2363050. [DOI] [PubMed] [Google Scholar]
- Pugh B. F., Tjian R. Mechanism of transcriptional activation by Sp1: evidence for coactivators. Cell. 1990 Jun 29;61(7):1187–1197. doi: 10.1016/0092-8674(90)90683-6. [DOI] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Voulalas P. J., Franza B. R., Jr, Curran T. Fos and Jun bind cooperatively to the AP-1 site: reconstitution in vitro. Genes Dev. 1988 Dec;2(12B):1687–1699. doi: 10.1101/gad.2.12b.1687. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Schmidt M. C., Berk A. J., Baltimore D. Transcriptional activation by Sp1 as directed through TATA or initiator: specific requirement for mammalian transcription factor IID. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4509–4513. doi: 10.1073/pnas.87.12.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm R. A., Das G., Herr W. The ubiquitous octamer-binding protein Oct-1 contains a POU domain with a homeo box subdomain. Genes Dev. 1988 Dec;2(12A):1582–1599. doi: 10.1101/gad.2.12a.1582. [DOI] [PubMed] [Google Scholar]
- Tong-Starksen S. E., Luciw P. A., Peterlin B. M. Human immunodeficiency virus long terminal repeat responds to T-cell activation signals. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6845–6849. doi: 10.1073/pnas.84.19.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis L., Reinberg D. Transcription by RNA polymerase II: initiator-directed formation of transcription-competent complexes. FASEB J. 1992 Nov;6(14):3300–3309. doi: 10.1096/fasebj.6.14.1426767. [DOI] [PubMed] [Google Scholar]
- Wingender E. Compilation of transcription regulating proteins. Nucleic Acids Res. 1988 Mar 25;16(5):1879–1902. doi: 10.1093/nar/16.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoumpourlis V., Kerr D. J., Spandidos D. A. Doxorubicin stimulates transcription from the human immunodeficiency virus long terminal repeat sequences. Cancer Lett. 1991 Feb;56(2):181–185. doi: 10.1016/0304-3835(91)90094-x. [DOI] [PubMed] [Google Scholar]
- Zoumpourlis V., Patsilinacos P., Kotsinas A., Maurer H. R., Lenas P., Spandidos D. A. Cisplatin stimulates the expression from the human immunodeficiency virus long terminal repeat sequences in human fibroblasts. Anticancer Drugs. 1990 Oct;1(1):55–58. doi: 10.1097/00001813-199010000-00010. [DOI] [PubMed] [Google Scholar]