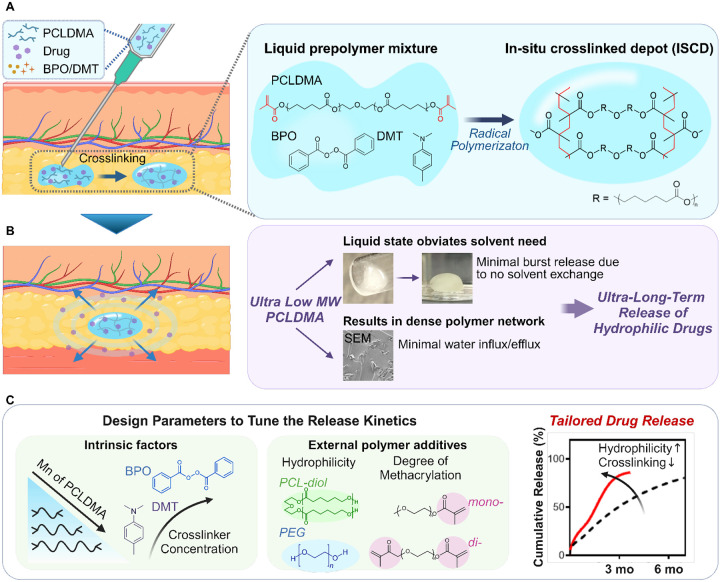
Figure 1. Injectable in situ crosslinked depot (ISCD) platform for sustained release of hydrophilic therapeutics.
A. The main component of ISCD is low molecular weight liquid methacrylated PCL, for example, PCLDMA. The liquid pre-polymer can suspend or dissolve both hydrophilic and hydrophobic drugs and can be easily injected through a standard 18–23 gauge needle. Upon adding an initiator (BPO) and accelerator (DMT) to PCLDMA, the pre-polymer mixture undergoes radical polymerization transitioning from a free-flowing liquid solution to a solid monolithic depot, resulting in physical encapsulation of the drug. B. ISCD has two key features enabling ultra-long-term release of hydrophilic drugs: a solvent-free design and a dense mesh network, both attributed to the ultra-low-molecular weight of the pre-polymer, PCLDMA. The liquid state of the pre-polymer obviates the need for a solvent, minimizing burst release. Cross-linking of the ultra-small chains of the pre-polymer results in a dense network (as shown in the SEM image of an ISCD depot formed in vitro) that limits water influx and efflux, minimizing the drug release rate. C. Design parameters to tailor the ISCD network to tune the drug release kinetics. Modulating the intrinsic factors, including decreasing the concentrations of BPO and DMT, or using higher molecular weight PCLDMA increases drug release. Additionally, adding external polymer additives including polyethylene glycol (PEG) and PCL-diol alongside PCLDMA can enhance the depot’s hydrophilicity, increasing drug release. External additives with different degrees of methacrylation (mono or di) can further tune the drug release. The cumulative release profiles of TAF from two different ISCD depots injected subcutaneously into rats are shown as examples of tailored drug release with varying release rates. Lowering the crosslinking density and increasing the hydrophilicity of the polymer chains achieve faster drug release.