Abstract
Practical relevance Phosphorus is retained in chronic kidney disease (CKD), promoting renal secondary hyperparathyroidism and eventually resulting in hyperphosphatemia. Most agree that phosphate retention is a major contributor to the progression of CKD in many species and it is well known that hyperphosphatemia is associated with a significant mortality risk in humans with end-stage renal disease.
Patient group Chronic kidney disease is a common ailment of geriatric cats.
Evidence base There is evidence in cats suggesting that the use of a phosphate-restricted diet in IRIS stage 2–3 disease has a beneficial effect on clinical outcome. However, despite the fact that intestinal phosphate binders are commonly used in veterinary practice for patients with CKD, there have been few published reports focusing on the safety and efficacy of these products in veterinary medicine. No phosphorus binders are licensed as medications for dogs or cats. This article draws on data from clinical trials in humans and studies in cats to discuss treatment goals and options for phosphate retention and hyperphosphatemia in feline CKD.
Clinical significance With careful monitoring of serum phosphate and parathyroid hormone, and implementation of phosphate-restricted dietary management and intestinal phosphate binders, progression of CKD and the degree of hyperparathyroidism in cats may be reduced.
Audience Companion animal and feline practitioners are at the forefront in the management of CKD in cats.

Mechanisms determining serum phosphorus
Phosphorus is one of the most abundant constituents of all tissues and is a major component of bone. Approximately 85% of total body phosphate is present in the skeleton and 14% is intracellular; it is the body's major intracellular anion. Less than 1% of total body phosphate is in the extracellular fluids.1–6 About one-third of extracellular phosphate is inorganic, most of which is unbound. Clinical laboratories typically measure inorganic phosphate in serum.2–5
Serum phosphate levels.
Serum phosphate levels are maintained within a narrow range in health. Young growing animals often have higher levels of serum phosphorus than adults. 27 This may reflect higher levels of growth hormone, which increase renal tubular absorption of phosphorus 6 and, possibly, higher levels of activated vitamin D3. The normal serum phosphorus range of many laboratories encompasses adults and growing animals, which may make it difficult to detect early rises in serum phosphorus above normal.
The typical reference range for phosphorus in the cat is 2.5–6 mg/dl (0.81–1.94 mmol/l). 8
Serum phosphorus concentration depends on the dietary phosphorus intake, the degree of gastrointestinal absorption across the duodenum and jejunum, translocation into intracellular sites, and excretion of phosphorus into the urine. Absorption occurs in the duodenum and jejunum under the control of activated vitamin D3. In an adult animal, the phosphorus that is absorbed from the gastrointestinal tract is eliminated by the kidneys, which play a crucial role in regulating serum phosphorus concentrations. Renal excretion of phosphorus depends on the amount that undergoes glomerular filtration and the amount that is subsequently reabsorbed by the tubules.
Most of the reabsorption of phosphorus occurs in the proximal tubules, an effect that is largely controlled by the expression of type II sodium-dependent phosphate co-transporters on the brush border. Parathyroid hormone (PTH), increasing concentrations of circulating phosphate, chronic metabolic acidosis, and some phosphatonins (fibroblast growth factor 23, secreted frizzled-related protein 4) are known to reduce the expression of this transport system, resulting in increased phosphorus excretion.2,3,9–11 Sodium-dependent phosphate co-transporters are located on the small intestinal brush border membrane as well, and are regulated by activated vitamin D3. Activated vitamin D3 increases influx of phosphate from the intestinal lumen into the mucosa. 12
In the early stages of CKD increased levels of parathyroid hormone can keep serum phosphorus within the reference range.
Phosphate retention and hyperphosphatemia are primarily due to impaired renal phosphate excretion. If renal function is normal, clinically significant hyperphosphatemia seldom develops.6,13,14 Chronic kidney disease (CKD) is the most common cause of hyperphosphatemia in adult cats and dogs (Figs 1 and 2). In the early stages of CKD increased levels of PTH can keep serum phosphorus within the reference range by decreasing expression of the sodium-phosphate transport system in the proximal tubule resulting in increased urine phosphate excretion. This allows for normalization of serum phosphorus at the expense of hyperparathyroidism. However, when the glomerular filtration rate (GFR) decreases to 20% of normal or less, this adaptive mechanism can no longer maintain serum phosphate within the reference range and hyperphosphatemia develops.3,6,14
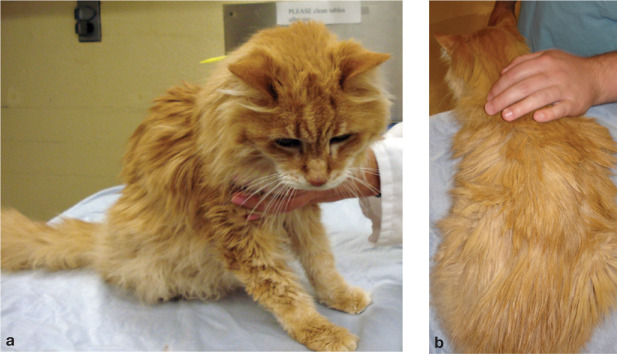
FIG 1.
(a,b) Cat with CKD (IRIS stage 3). Note the cachexia and unkempt coat, due to the effects of moderate uremia
FIG 2.
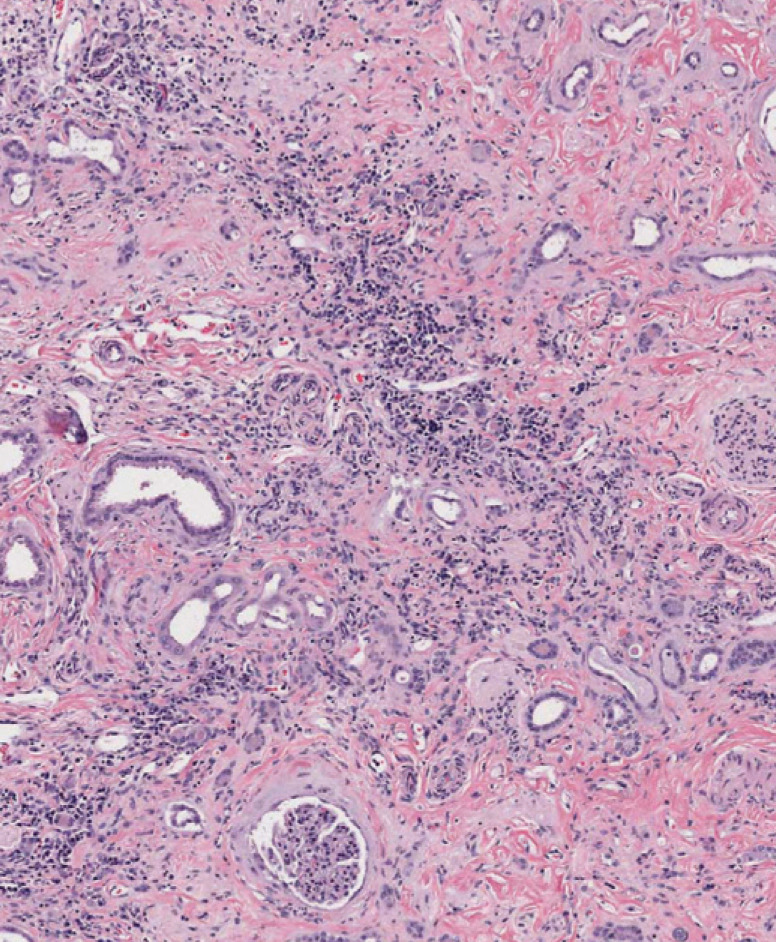
Advanced histopathologic lesion of CKD. Note the large amount of fibrous tissue, loss of nephron mass (tubules and glomeruli), and interstitial inflammation with lymphoplasmacytes. Some tubules are dilated, presumably due to obstruction further down the nephron. Hematoxylin and eosin. Magnification × 8
Pathophysiological consequences of hyperphosphatemia
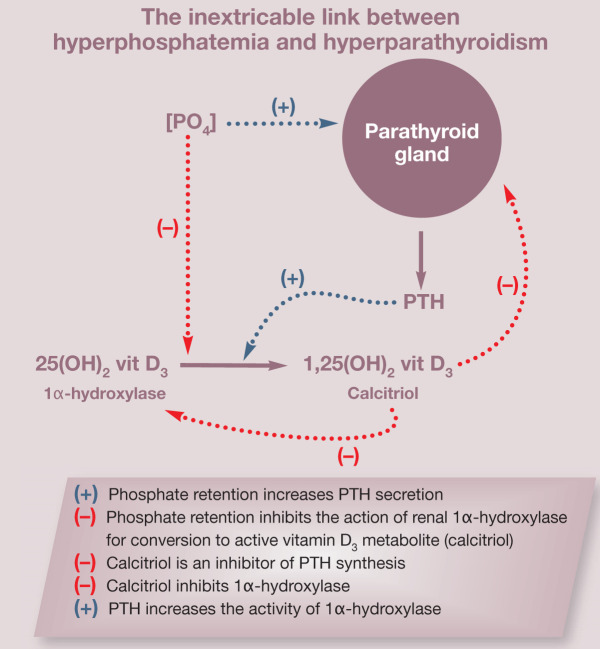
Circulating phosphorus has a complex relationship that affects, and is affected by, PTH, ionized calcium and calcitriol (see box). Hyperphosphatemia can reduce levels of ionized calcium, which in turn stimulates PTH secretion. Phosphate retention inhibits the activity of renal 1α-hydroxylase, the enzyme responsible for the conversion of 25-hydroxy-vitamin D3 (25[OH]2 vit D3) to its active metabolite 1,25-dihydroxyvitamin D3 (1,25[OH]2 vit D3), better known as calcitriol. Calcitriol is important for calcium and phosphorus absorption in the intestine and is an important inhibitor of PTH synthesis. Hyperphosphatemia may also interfere with parathyroid gland responsiveness to calcitriol through decreased vitamin D receptor expression or via a direct action at the vitamin D receptor site. Phosphorus has furthermore been shown to stimulate PTH mRNA synthesis, independent of changes in serum calcium and calcitriol.6,15 As a result of this complex relationship, it is not possible to evaluate phosphorus as a separate entity; hyperparathyroidism is a consistent finding in progressive renal disease.
Increased levels of serum phosphorus have been associated with increased all-cause mortality, cardiovascular mortality, vascular calcification and valvular calcification in humans with CKD. One human study showed that end-stage renal failure patients with a serum phosphorus level >6.5 mg/dl (>2.10 mmol/l) had a 27% higher mortality risk than patients with a phosphorus level of 2.4–6.5 mg/dl (0.78–2.10 mmol/l); and that patients with a calcium × phosphate (Ca × P) product >72 mg 2 /dl 2 had a 34% higher risk of death compared with those with a Ca × P product between 42 and 52 mg 2 /dl 2 . 16
A complex relationship.
Hyperphosphatemia and renal secondary hyperparathyroidism are common in cats with IRIS stage 3 and 4 CKD, and can be documented in some with IRIS stage 2 disease.
In one retrospective study in cats, the only clinicopathologic variable that was associated with survival in animals with naturally occurring CKD was serum phosphorus. For each 1 unit increase in phosphorus there was an 11.8% increase in the risk of death. 17 In another study, increased plasma phosphate concentration was found to be highly significantly associated with shorter renal survival times. 18
Deleterious effects of phosphate accumulation in cats are most often recognized to be a direct consequence of calcium phosphate precipitation into the tissues (increased Ca × P product). Indirect effects that increase PTH and decrease ionized calcium may also be important. It has been known since the early 1980s that dietary phosphorus restriction provides dramatic benefits to the histologic renal architecture of cats with the remnant kidney model of chronic renal failure. Although renal function was not different over time in cats of this study, those fed a normal maintenance diet had obvious mineralization, fibrosis and mononuclear cell infiltration, whereas the kidneys of cats fed a phosphate-restricted diet had minimal or no changes. Serum phosphorus and PTH concentrations were considerably increased in cats fed the normal phosphate diet compared with those fed the restricted phosphate diet. 19
Hyperphosphatemia and renal secondary hyperparathyroidism are common in cats with IRIS (International Renal Interest Society) stage 3 and 4 CKD and can be documented in some with IRIS stage 2. Barber and Elliott (1998) 20 demonstrated that PTH was increased in 47%, 87% and 100% of 80 cats with CKD categorized, respectively, as compensated (mean serum creatitine 2.6 mg/dl, 229 μmol/l), uremic (mean serum creatinine 3.6 mg/dl, 316 μmol/l) or end-stage (mean serum creatinine 10.3 mg/dl, 909 μmol/l). Overall, 84% of cats with CKD had elevated PTH. Serum phosphorus was increased in approximately 60% of cats with CKD in this same clinical study; 20%, 49% and 100% of cats with compensated, uremic or end-stage CKD, respectively, had elevated serum phosphate. Interestingly, 13% of cats in the study had increased PTH despite normal concentrations of both ionized calcium and serum phosphorus. 20
In a separate investigation by Elliott and colleagues (2000) 21 , a group of 50 cats with CKD (mean serum creatinine near 3 mg/dl, 265 umol/l) was studied for an extended period. Twenty-four of the 50 cats were hyperphosphatemic at the start of the study. Plasma phosphate concentrations at the mid-survival time point increased over baseline in 62% of those eating a normal diet (n = 21) and decreased in 76% of cats eating a phosphate-restricted renal veterinary diet (n = 29). Forty-six of the 50 cats had increased PTH at the start of the study. Parathyroid hormone declined in 69% of cats that were eating the veterinary renal diet at the mid-survival time point, whereas PTH increased in 62% of those eating a maintenance diet. Intestinal phosphate binders were added as a treatment when serum phosphorus remained increased within 4 months of feeding the renal diet in four cats; an additional six cats required the addition of an intestinal phosphate binder throughout the study, making a total of 34% (10/29). Survival time for CKD cats eating the renal diet was considerably longer (633 median and 616 mean days) compared with those eating a maintenance diet (264 median and 383 mean days).
Interestingly, death related to cardiovascular system abnormalities was reported to rank second to renal-related causes in the study by Elliott et al, although detailed micropathology of vascular structures was not provided. 21 It has been shown that humans with end-stage renal disease and a serum phosphate level >6.5 mg/dl (>2.10 mmol/l) have a 41% greater risk of death resulting from coronary artery disease. 22
Metastatic paw calcifications have been reported infrequently in cats with CKD. In a series of five cats with this condition, 23 all had elevated serum phosphorus (2.3–9.4 mmol/l; reference range 0.5–1.6 mmol/l). Only one cat had elevated total calcium (3.4 mmol/l; reference range 2.0–2.75 mmol/l). All cats had a solubility product (calcium × phosphorus) >70 mg 2 /dl 2 . Serum PTH was measured in three of the five cats and was increased in two, one of which had elevated total calcium. Necropsy was performed on one cat and revealed confluent plaques of calcification on the tunica media of the aorta and other main arteries, end-stage kidneys with multifocal tubular calcification, and alveolar emphysema with diffuse calcification of pulmonary arteries and capillaries. In an earlier case report describing a cat with metastatic paw calcifications, which were presumed to be a result of renal secondary hyperparathyroidism, feeding of a protein- and phosphorus-restricted diet resulted in resolution of the paw lesions and hyperparathyroidism approximately 179 days after diagnosis. 24
Treatment approaches
Conventional wisdom and evidence dictates the importance of correcting hyperphosphatemia associated with CKD. Restoration of normophosphatemia is an initial and main goal but phosphorus restriction may be beneficial in reversing existing renal secondary hyperparathyroidism in cats that are not hyperphosphatemic at the time of initial evaluation. Secondary hyperparathyroidism can exist despite normal ionized calcium and serum phosphorus status, as was demonstrated in a study discussed earlier. 20 In another study of cats with IRIS stage 2 or 3 CKD (mean serum creatinine 2.1–4.5 mg/dl, 185.6–397.8 μmol/l), mean serum phosphorus was within the reference range at a time when mean PTH was 25–50% above the upper limit of the reference range. 25 The assumption is made that phosphate retention is occurring in those cats even though serum phosphorus remains normal (because of the hyperparathyroidism) and phosphate restriction may still be beneficial in this normophosphatemic population.
Phosphorus restriction in CKD patients with phosphate retention is initiated by feeding a low phosphorus, low protein diet. Compared with an average Western diet, cats consume six times, and dogs five times, as much phosphate on a mg/100 kcal basis when eating grocery store pet foods (Fig 3). Consequently, with such a high starting point, it is difficult to achieve the degree of dietary phosphate restriction advocated for humans with end-stage renal failure. Veterinary renal diets achieve phosphate restriction largely by restriction of dietary proteins that contain phosphorus, especially animal origin proteins. The form of phosphate in the diet influences how much is available for absorption.
FIG 3.
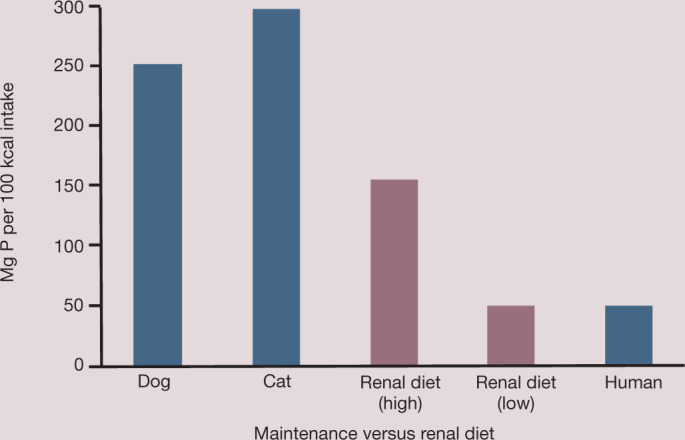
Dietary phosphorus intake of dogs and cats eating commercial or renal therapeutic foods compared with the average Western diet of humans. Note that dogs and cats consume five and six times, respectively, as much phosphorus as the average human, which makes it difficult to achieve adequate dietary phosphorus restriction. Developed by the Nutrition Support Service, The Ohio State University College of Veterinary Medicine (http://vet.osu.edu/nssvet)
Despite similar dietary phosphorus content and phosphorus intake, the amount of phosphorus absorbed across the gut varied considerably in one study of cats due to differences in phosphorus bioavailability. 26 This effect may depend on the source of the phosphorus (ie, organic versus monobasic or dibasic salts of phosphorus) in the diet. Additionally, the degree of intestinal absorption of phosphorus can vary between 60% and 80% 14 of the ingested load in individuals with the same phosphorus intake and level of renal dysfunction. Thus, when there are difficulties in achieving target serum phosphorus or PTH levels during phosphate restriction, this may be due to too high a dietary phosphorus intake, an individual patient's high level of gastrointesintal absorption, or the formulation of the phosphate in the diet.
Dietary modification and intestinal phosphate binders are pivotal interventions to provide optimal phosphorus and PTH control. Dietary phosphorus restriction in CKD was shown to blunt or reverse renal secondary hyperparathyroidism in cats in one study, 21 but not in another. 25 A reduction of dietary phosphorus intake that is proportionate to decreases in GFR will keep serum phosphorus within the reference range without increases in PTH. 27 This can be hard to achieve in the clinical setting. Extremely phosphorus-depleted diets may be unpalatable to cats due to the low levels of protein needed to provide this phosphorus restriction. Diets moderately restricted in phosphorus may provide adequate phosphate control during early stages of CKD; but as kidney disease progresses diet alone is usually not successful in achieving adequate phosphorus control, and phosphorus concentration increases above the reference range or stays in the upper half of the reference range.
Phosphorus restriction may be beneficial in reversing existing renal secondary hyperparathyroidism in cats that are not hyperphosphatemic at the time of initial evaluation.
Goals of dietary phosphorus control
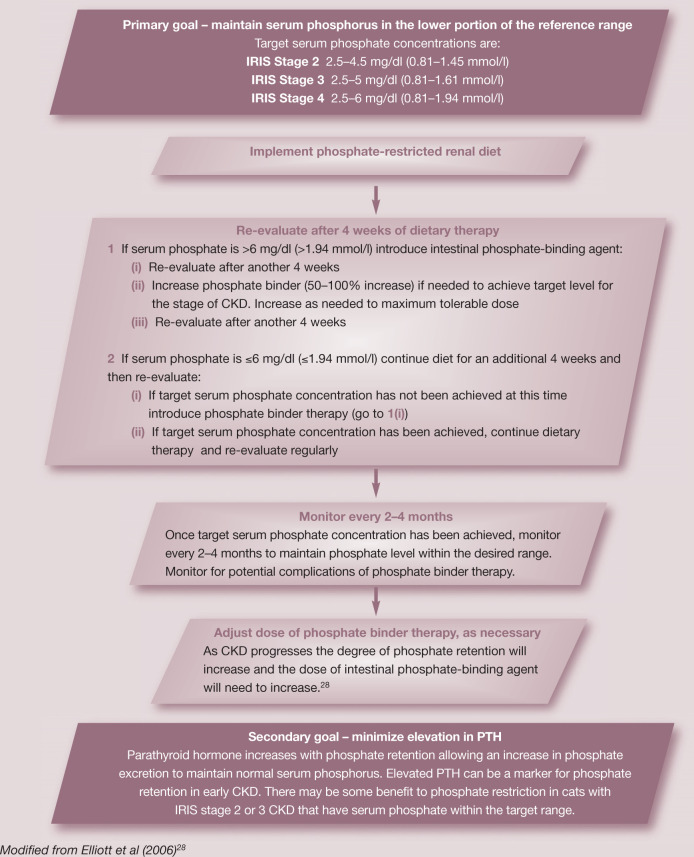
An initial goal is to attempt to return high serum phosphorus concentrations to within the reference range by feeding a phosphate-restricted renal diet. Intestinal phosphate binders (see later) should be added if serum phosphate remains increased after 1 month of consuming the renal diet or if the switch to the renal diet is not accepted by the cat. A serum phosphate concentration in the mid-reference range (<4.5 mg/dl, <1.45 mmol/l) is the recommended target. It is important to measure serum phosphate concentrations in cats with CKD serially, usually monthly, until the target concentration has been achieved and every 2–4 months thereafter if the cat is stable. Serum phosphorus concentration may increase in cats with CKD that increase their food intake following other supportive treatments.
Treatment recommendations for the stable CKD patient.
Intestinal phosphate binders should be added if serum phosphate remains increased after 1 month of consuming a renal diet or if the switch to the renal diet is not accepted by the cat.
Possible adverse effects of phosphate restriction.
Adverse effects of phosphate restriction potentially can occur. Although hypophosphatemia is one possible consequence, it is difficult for this to develop in cats with initially high concentrations of serum phosphorus and a reduced GFR. Hypercalcemia can be encountered when calcium salts are used for intestinal phosphate binding. Hypercalcemia has also been attributed to feeding of phosphate-restricted diets to cats, but this is rare; normocalcemia returned with the feeding of a higher phosphate diet. 29 Adynamic bone disease is a concern during treatment of humans with CKD, as occurs when PTH has been suppressed too much so that healthy bones cannot be maintained 30 - whether this occurs in cats with CKD is not known. Constipation and gastrointestinal effects can occur following use of some intestinal phosphate binders. Absorption of chemicals from the intestinal phosphate binder may occur, with resulting accumulation in the tissues in some instances.3,30
A second goal is to restore PTH to normal levels or to prevent it from increasing, even if serum phosphorus is in the reference range. Further phosphorus restriction with diet and phosphorus binders can be titrated to the effect of lowering PTH if possible. In some instances, PTH cannot be controlled despite dietary intervention and use of intestinal phosphate binders. Other treatments (calcitriol and calcimimetics) may be indicated in these cases. In addition to serial serum phosphate measurements, concurrent serial measurements of PTH and ionized calcium may be considered a gold standard for assessment of sufficient relief of body phosphorus burden and PTH control.
Intestinal phosphate binders
Phosphorus-binding agents (Table 1) are given orally to trap phosphorus in the gut and increase insoluble phosphate salt excretion in the feces. Phosphate binders work because the cation in the binder combines with dietary phosphate, producing insoluble, non-absorbable, phosphate compounds. 3
TABLE 1.
Dose rates for intestinal phosphate binder therapy in cats
| Intestinal phosphate binder | Dose |
|---|---|
| Aluminum hydroxide (Alternagel; Johnson & Johnson-Merck) 600 mg/5 ml | 30 mg/kg PO q8h; 45 mg/kg PO q12h (give with meal) |
| Calcium carbonate (Tums; GlaxoSmithKline) regular strength 500 mg/tablet | 30 mg/kg PO q8h; 45 mg/kg PO q12h (give with meal) |
| Sevelamer hydrochloride (Renagel; Genzyme) 400 mg tablets | 33–54 mg/kg PO q8h; 50–80 mg/kg PO q12h (give with meal) |
| Chitosan and calcium carbonate (Epakitin/Ipakitine; Vétoquinol) | 1 g/10 lb twice daily with food |
| Lanthanum carbonate (Fosrenol; Shire Pharmaceuticals) 500 mg chewable tablets | 12.5–25 mg/kg/day PO 6.25–12.5 mg/kg PO q12h starting dose (give with meal, do not swallow tablet whole) |
| Lanthanum carbonate octahydrate (Renalzin; Bayer HealthCare) Not available in USA | 2 ml applied to cat's food once or twice daily |
PO = orally
Despite the fact that intestinal phosphate binders are commonly used in veterinary practice for patients with CKD, there have been few published reports focusing on the safety and efficacy of these products in veterinary medicine. No phosphorus binders are licensed as medications for dogs or cats. Recently, two intestinal phosphorus-binding products have been approved as feed supplements: Epakitin (Vétoquinol USA) and Renalzin (Bayer HealthCare). The phosphorus binder contained in the latter, Lantharenol, is approved in various parts of the world for use as a food additive to restrict intestinal absorption of phosphorus in adult cats with CKD, but not yet in the United States.
Aluminum salts
Aluminum (aluminium) salts are the most widely used phosphate binders in cats. Aluminum-based phosphate binding agents (aluminum hydroxide, aluminum carbonate) are highly effective in lowering serum phosphate levels, forming insoluble and non-absorbable aluminum phosphate precipitates in the intestinal lumen. 6
Aluminum salts were the preferred intestinal phosphate binder for use in humans with end-stage renal failure from the 1970s, when the importance of phosphorus control in patients with renal failure was first emphasized, until approximately the mid-1980s.6,14,30
The gastrointestinal tract is relatively impermeable to aluminum, although, under certain circumstances, aluminum can be absorbed causing an increase in concentration in the blood and tissue. Normally excess aluminum is excreted by the kidneys, and aluminum intoxication is not seen in people with normal renal function. 30 In humans with CKD, significant aluminum may be retained in the body, especially the bones, leading to osteomalacia, adynamic bone disease, microcytic anemia and encephalopathy.6,14,32–34 As a result, the use of aluminum salts has been limited in human medicine.
The National Kidney Foundation Dialysis Outcomes Quality Initiative (NKF-DOQI) Guidelines in the USA recommend that aluminum salts are only used in human renal failure patients that have a serum phosphorus concentration >7.0 mg/dl (>2.26 mmol/l), and then only in the short term (<4 weeks), and only for one course of treatment, thereafter to be replaced by other phosphate binders. There is no known safe dose of aluminum salts for humans with CKD. The NKF-DOQI Guidelines recommend 3-monthly monitoring of serum aluminum concentration in those human patients receiving aluminum-containing medications (with baseline <20 μg/l). 35
Detrimental effects of aluminum-based phosphate binders, as described above in humans, have not been systematically evaluated in small animal patients and are rarely clinically appreciated. As cats with CKD can live for years on treatment, concerns regarding aluminum accumulation and long-term safety deserve more study. A recent case series described probable aluminum intoxication in two dogs with renal failure, one acute and the other chronic. The dogs exhibited severe neuromuscular abnormalities 62 and 65 days after initiation of therapy with aluminum hydroxide. The dosage at the time abnormalities were seen was 126 mg/kg/day and 200 mg/kg/day and both dogs had elevated serum aluminum levels (0.52 and 0.318 ppm, respectively; reference range 0.008–0.012). Both dogs were treated with, and responded favorably within 48 h to, deferoxamine, an aluminum chelating agent. 36
Phosphorus binder therapy.
Intestinal phosphate binders work best when given with meals or within 2 h of feeding to maximize their binding of dietary phosphorus. 31 Due to varying effects of intestinal phosphate binders on the absorption of other drugs, it is advisable to give any other medications 1 h before or 3 h after an intestinal phosphate binder is administered. The dose of any phosphate binder should be based on the meal size (phosphorus intake) and the prevailing serum phosphorus level for each CKD patient; the dose is titrated to effect. 31
Despite concerns regarding toxicity, and stringent use guidelines in humans, aluminum salts remain the most commonly prescribed intestinal phosphate binders in veterinary medicine as they are very effective and are inexpensive. Aluminum hydroxide or aluminum carbonate is used at an initial dosage of 30 mg/kg q8h or 45 mg/kg q12h, given with food. Constipation is the most common side effect encountered during treatment with aluminum phosphate binders. Lactulose treatment may help to alleviate constipation but may also contribute to dehydration due to extra fluid loss in the stool.
Sucralfate (a complex of aluminum hydroxide and sulfated sucrose) has been used empirically by some clinicians as a phosphate binder, although there are no reports of its use for such in cats or dogs.
Calcium salts
Calcium-based phosphate binders took the place of aluminum salts in humans with end-stage renal disease following reports of aluminum accumulation and toxicity. They lower serum phosphorus via binding in the gastrointestinal tract, but are not as effective as aluminum salts, having a lower affinity for phosphorus. Thus, effective binding of dietary phosphorus requires large doses of calcium, often enough to induce hypercalcemia in humans.14,37 The use of calcium-based phosphate binders can be associated with adverse effects during treatment in humans with end-stage renal disease: hypercalcemia from absorption of calcium from the gastrointestinal tract, increased incidence of adynamic bone disease due to oversuppression of PTH production as a result of chronic hypercalcemia, and increased incidence of soft tissue and vascular calcification from chronic hypercalcemia and increased Ca × P product. 23 Soft tissue and vascular calcifications have been associated with significant morbidity and mortality in children and adults with end-stage renal disease.30,38,39
The most commonly used calcium-based phosphate binders are calcium carbonate and calcium acetate. Calcium carbonate can be used in cats at a starting dosage of 30 mg/kg q8h or 45 mg/kg q12h, given with food. Calcium carbonate binds phosphorus best in an acidic environment (approximately pH 5) and binding capacity is reduced in the neutral pH range. 40 This is relevant as many patients with CKD receive inhibitors of gastric acid secretion, potentially reducing the ability of calcium carbonate to bind phosphorus. Calcium acetate can bind phosphate over a wide range of pH, 40 has about twice the phosphate-binding capacity of calcium carbonate and, as such, can be used at a lower dosage, and has been shown to cause less hypercalcemia than calcium carbonate when activated vitamin D metabolites are not also being used.41,42 Doses of 20, 30 or 40 mg/kg given with each meal approximate doses of calcium acetate recommended for humans with dialysis-dependent CKD.
In addition to serial serum phosphate measurements, concurrent serial measurements of PTH and ionized calcium may be considered a gold standard for assessment of sufficient relief of body phosphorus burden and PTH control.
Cats should be monitored for the development of hypercalcemia whenever calcium-containing phosphorus binders are used.
Cats should be monitored for the development of hypercalcemia whenever calcium-containing phosphorus binders are used. Non-calcium containing salts are preferred in animals receiving calcitriol or activated vitamin D metabolites as a treatment for CKD, to lessen the risk of the development of hypercalcemia. Another class of phosphate binder should be selected for cats with calcium-containing nephrolithis or ureteroliths, and cats that have CKD and idiopathic hypercalcemia.
Sevelamer
Sevelamer hydrochloride (Renagel; Genzyme Corporation) and the very recently US Food and Drug Administration approved sevelamer carbonate (Renvela; Genzyme Corporation) are intestinal phosphorus binders marketed for use in human CKD patients on dialysis.43,44 Sevelamer is an organic polymer that does not contain aluminum or calcium and is not absorbed from the gastrointestinal tract (being excreted entirely in the feces). These compounds are exchange resins that bind dietary phosphorus and release the counter-ion chloride (sevelamer hydrochloride)43,45 or carbonate (sevelamer carbonate). 44 Many human clinical studies have demonstrated the ability of sevelamer hydrochloride to lower serum phosphorus and PTH levels, and control Ca × P product in dialysis patients compared with calcium-containing phosphate binders.46–48 Their effects on cats and dogs with clinical renal failure, however, have not been reported. However, we have occasionally used sevelamer with success as a phosphate binder in both dogs and cats with CKD.
Sevelamer hydrochloride is hydrophilic and sevelamer carbonate is hygroscopic, but both are insoluble in water. We have not noted major effects on hydration from the hygroscopic effects of this drug but our experience is limited. Pills should be given intact and will expand in water. Sevelamer may be associated with gastrointestinal side effects including constipation and, at extremely high dosages in dogs (six to 100 times the recommended dosage in humans), it may be associated with impaired absorption of folic acid and vitamins K, D and E.43,44 Since it is administered as an acid salt there is concern regarding worsening of metabolic acidosis. Sevelamer hydrochloride has been reported to decrease bicarbonate levels and exacerbate metabolic acidosis in hemodialysis patients.49,50 As a result, sevelamer carbonate was manufactured with the added benefit of providing a carbonate buffer.
An additional benefit of sevelamer is its ability to bind and sequester bile acids, resulting in a favorable lipid profile. Reductions in low-density lipoprotein and total cholesterol have been shown in human studies with the use of sevelamer.46–48 Lipid abnormalities in cats with CKD have not been well studied. However, in one study describing clinicopathologic abnormalities in cats with CKD, hypercholesterolemia was a common finding. 51
Renagel is available in 400 and 800 mg tablets and Renvela is available in 800 mg tablets.43,44 Sevelamer hydrochloride can also be compounded into a suspension. 52 The recommended starting dose in adult humans ranges from 800–1600 mg according to serum phosphorus level. The dose is then titrated in order to achieve the desired serum phosphorus level (<5.5 mg/dl, <1.78 mmol/l).43,44 Sevelamer hydrochloride has been used effectively in children with end-stage renal failure; reported dosages are extrapolated from adult doses, ranging from an initial dose of 100–121 mg/kg/day divided every 8 h and titrated to a final dose of 130–163 mg/kg/day divided every 8 h.53,54 These doses may be applied to small animal patients with careful monitoring for side effects and serial serum phosphate measurements with titration of the dose as needed.
Chitosan
Epakitin (Vétoquinol USA) is marketed as a veterinary feed supplement in the USA, and Ipakitine is the equivalent product in Europe. These contain the adsorbent chitosan (8% crab and shrimp shell extract), 10% calcium carbonate and 82% lactose, and are designed to reduce gastrointestinal phosphorus absorption and to lower urea nitrogen due to effects of reduced protein digestibility. One short-term study of a small number of normal and CKD cats showed a reduction in protein and phosphorus digestibility, along with decreases in blood urea nitrogen (BUN) and serum phosphorus, in cats eating a normal maintenance diet supplemented with the chitosan and calcium carbonate product. 55
In that study, the diet of 10 normal cats was supplemented for 21 days with Ipakitine at a dose of 1 g/5 kg body weight. Compared with control cats fed the same diet, the apparent digestibility was reduced for crude protein, crude fiber and gross energy. The apparent digestibility of phosphorus was reduced by over 50% and that of calcium by over 100% during the treatment period. Urinary excretion of phosphorus and calcium did not change during treatment. In six cats diagnosed with CKD, based on increased BUN and clinical signs, fed the same diet and supplement as the normal cats for 35 days, a significant reduction in BUN and serum phosphorus was detected at day 35 (mean initial BUN 85.6 mg/dl [14.3 mmol/l], mean 35-day BUN 61.2 mg/dl [10.2 mmol/l; mean initial phosphorus 5.2 mg/dl [1.7 mmol/l], mean 35-day phosphorus 3.4 mg/dl [1.1 mmol/l]). Both BUN and serum phosphorus were reduced in each cat; serum creatinine did not change. 55
Results of two studies suggest that Epakitin/Ipakitine could be an alternative to prescription renal veterinary diets, allowing some cats to continue on their regular diet while still reducing the risk of progression of CKD associated with total body phosphorus burden.
Another longer term study showed the ability of a chitosan and calcium carbonate intestinal phosphate binder to significantly decrease serum phosphorus and plasma PTH levels when added to a maintenance diet for cats with CKD. 56 Ten experimental cats with greater than 90% reduction in renal mass were fed a maintenance diet or a maintenance diet plus intestinal phosphate binder (Epakitin) for 56 days. The phosphate binder was given at a dosage of 1 g for each meal to cats weighing <5 kg and 2 g for each meal to cats weighing >5 kg. The cats fed the maintenance diet and intestinal phosphorus binder showed significantly lower serum phosphorus (5.14 ± 0.11 mg/dl, 1.66 ± 0.036 mmol/l) and plasma PTH concentrations at the end of the trial period compared with cats fed the maintenance diet alone (serum phosphorus 5.55 ± 0.11 mg/dl, 1.79 ± 0.036 mmol/l).
In a second phase of the same study, six of the cats with IRIS stage 1 or 2 CKD were fed a maintenance diet only for 3 months, the diet and phosphate binder (Epakitin) (0.21 ± 0.02 g/kg q12h) for the following 9 months, then the maintenance diet only for another 3 months. Mean serum phosphorus and PTH concentrations of these cats were significantly lower at months 6 and 9 while being fed the maintenance diet and phosphate binder. These values were compared with measurements taken at the end of 3 months and in the last 3 months of the study period when the maintenance diet alone was fed. 56
The results of these two studies suggest that this supplement could be an alternative to prescription renal veterinary diets, thereby allowing some cats to continue on their regular diet while still reducing the risk of progression of CKD associated with total body phosphorus burden. We have, however, observed the development of hypercalcemia in a few CKD cats treated with Epakitin, probably as a consequence of the calcium carbonate.
Lanthanum salts
Lanthanum carbonate (Fosrenol; Shire Pharmaceuticals) is another newly developed non-aluminum and non-calcium containing intestinal phosphate binder and is indicated for use in human patients with end-stage renal failure to reduce serum phosphorus. Lanthanum is a rare earth metal that can be found in trace amounts in the body. Free lanthanum cations become available following exposure of lanthanum carbonate to the acidic environment of the stomach and highly insoluble lanthanum phosphate complexes develop.
Very little lanthanum is absorbed across the gastrointestinal tract. 57 Only 0.00005% of an oral dose of lanthanum carbonate given to a healthy canine population was absorbed, versus 0.05–0.1% for aluminum. 58 Lanthanum also accumulates to a far lesser extent following absorption compared with aluminum (lanthanum undergoes extensive hepatic excretion whereas aluminum is excreted mostly by the kidneys). Lanthanum appears to have minimal toxicity in humans, but it has only been in use for a short time. 59 Toxicity studies performed in dogs show that lanthanum increases in many tissues (especially the gastrointestinal tract, bone and liver) during treatment. Tissue levels remained detectable for longer than 6 months in dogs following discontinuation of treatment. 57
Lanthanum carbonate has been shown to be as efficacious as calcium carbonate in lowering serum phosphorus levels in human patients with end-stage renal failure. 60 The recommended initial total daily dose for people is between 750 and 1500 mg. The dose may be titrated every 2–3 weeks until an acceptable phosphate level is reached, with most human patients requiring a total daily dose of between 1500 and 3000 mg. Intact tablets should not be swallowed, but tablets may be crushed to aid in chewing. 57 Extrapolating from humans (and based on an average human weight of 60 kg) initial daily doses of Fosrenol for use in cats range from 12.5–25 mg/kg. However, doses of 35–50 mg/kg/day are often needed since commercial cat foods contain more phosphate proportionally than an average human consumes daily.
Reports of the use of lanthanum carbonate in cats are emerging. In an early study, four groups of eight normal European shorthair cats were fed a canned maintenance food supplemented with a lanthanum-based phosphate binder at 0, 0.3, 1.0 or 3.0 g/kg of food as fed (1.6–16 g/kg of standard dry food) for 2 weeks. Phosphorus excretion into feces increased while phosphorus excretion into urine decreased in a dose-related manner; serum phosphorus did not differ between dose groups. Food intake did not change during treatment. 61
In another study, four groups of nine normal European shorthair cats were fed a wet veterinary renal diet with lanthanum carbonate octahydrate added at 0, 1.5, 4.5 or 7.5 g/kg of complete food. Similar to the normal cats receiving maintenance food and lanthanum treatment described in the previous study, veterinary food intake was not changed among treatment groups, apparent phosphorus digestibility decreased, intestinal phosphorus absorption was decreased due to increased fecal phosphorus excretion and urinary phosphorus excretion was decreased. Serum phosphorus did not change between treatment groups. 62
In 2007, based on previous reports of efficacy and safety in cats, the European Food Safety Authority (EFSA) approved lanthanum carbonate octahydrate (Lantharenol; Bayer HealthCare) as a feed additive for adult cats in order to decrease intestinal phosphate absorption. The approved dose was 1500–7500 mg/kg of complete feed. 63 Renalzin (Bayer HealthCare AG) is the proprietary name for the delivery system of Lantharenol. In addition to Lantharenol, Renalzin also contains kaolin, for uremic toxin-binding effects, and vitamin E, for its antioxidant effects, but the benefits of these other compounds have not yet been demonstrated. The product comes in liquid form, with a pump to deliver the appropriate dose to food. 64
In a recent study, 10 experimental cats that had undergone subtotal nephrectomy were fed wet cat food supplemented with Lantharenol for 2 weeks at 0.3–3 g/kg of wet food (1.77–17.7 g/kg of complete food). 65 All were asymptomatic, mildly azotemic and normophosphatemic following renal mass reduction. Food intake was not altered in the cats and a dose-dependent decrease in phosphorus availability was demonstrated. Urinary phosphorus excretion was increased, contrary to the decreased urinary phosphorus excretion seen in normal cats. 65 This may be due to excretion of phosphate from cellular stores that accumulated during renal failure.
More recently still, nine cats with subtotal nephrectomy were fed a standard feline maintenance diet supplemented with Renalzin (5 mg/kg original moist feed) for 6 months. Serum urea, creatinine and phosphorus values were significantly improved and pH was increased, from starting values, after 2 months of Renalzin administration. These parameters, however, tended to deteriorate towards the end of the 6-month trial period, possibly due to a progressive decline in kidney function. Renalzin was tolerated by all cats and did not affect body weight. 66
In a dose tolerance study 10 normal European shorthair cats were fed an escalating dose of Lantharenol. An initial 125 mg/kg body weight was given and every 14 days the dose was doubled. A dose of 1 g/kg body weight was well tolerated by all cats. However, at 2 g/kg, repeated vomiting of food occurred in all cats; this resolved 2 days after discontinuation of dosing, and recurred on re-challenge with 2 g/kg Lantharenol. A dose of 1 g/kg body weight corresponds to a concentration of 84 g Lantharenol per kg complete feed. Given the approved feed concentration range of 1.5–7.5 g/kg complete feed, Lantharenol has a safety margin of 10. 68
Finally, 23 cats with CKD (decreased urinary specific gravity, increased BUN and serum creatinine) were enrolled on an 8-week study comparing the effects of a veterinary renal diet (nine cats) or a maintenance diet supplemented with 400–600 mg Lantharenol per day (14 cats). The Renalzin-treated group showed an improvement in serum phosphorus control, overall clinical status, and behavioral scores for quality of life compared with cats fed the veterinary renal diets. Due to an unintended but relevant group difference at randomization and enrolment into the study - the Renalzin group comprised a higher proportion of animals with hyperphosphatemia as well as impaired quality of life and overall clinical status - a comparison of the effects of Renalzin and the renal diet in this study became impossible, and further testing is required. 67 Nevertheless, the evidence suggests that Renalzin, similar to Epakitin/Ipakitine, may be beneficial in cats on regular maintenance diets.
Evidence suggests that Renalzin, similar to Epakitin/Ipakitine, may be beneficial in cats on regular maintenance diets.
KEY POINTS.
Chronic kidney disease is the most common cause of phosphate retention and hyperphosphatemia in adult cats.
Hyperphosphatemia is a major contributor to the progression of CKD and studies in cats suggest that dietary phosphorus restriction plays a beneficial role in the management of CKD.
Intestinal phosphate binders, which decrease the amount of phosphorus available for intestinal absorption, provide another means to combat phosphate retention/hyperphosphatemia and renal secondary hyperparathyroidism.
Although intestinal phosphate binders are regularly used in the management of CKD in cats, and a new generation of such agents has become available, additional studies need to be performed to ascertain their benefits and long term complications.
Aluminum toxicity is a concern with the use of aluminum-based phosphate binders in cats with CKD. This may be more important in cats than in dogs due to the long average survival times in cats with CKD, which place them at greater risk of aluminum exposure and accumulation.
Contrary to conventional wisdom, some phosphate binders may exert benefits in cats with CKD eating normal maintenance diets rather than a special renal diet.
Which intestinal phosphate binder is best for use in cats has yet to be determined.
References
- 1.Guyton AC, Hall JE. Textbook of medical physiology. 10th edn. Philadelphia: WB Saunders. 2000. [Google Scholar]
- 2.Schropp DM, Kovacic J. Phosphorous and phosphate metabolism in veterinary patients. J Vet Emerg Crit Care 2006; 17:127–34. [Google Scholar]
- 3.Dibartola SP, Willard MD. Disorders of phosphorous: hypophosphatemia and hyperphosphatemia. In: Dibartola SP, ed. Fluid, electrolyte, and acid-base disorders in small animal practice. 3rd edn. Missouri: Elsevier. 2006: 195–209. [Google Scholar]
- 4.Shiber JR, Mattu A. Serum phosphate abnormalities in the emergency department. J Emerg Med 2002; 23: 395–400. [DOI] [PubMed] [Google Scholar]
- 5.Subramanian R, Khardori R. Severe hypophosphatemia: pathophysiologic implications, clinical presentations, and treatment. Medicine 2000; 79: 1–8. [DOI] [PubMed] [Google Scholar]
- 6.Albaaj F, Hutchinson AJ. Hyperphosphatemia in renal failure, causes, consequences and current management. Drugs 2003; 63: 577–96. [DOI] [PubMed] [Google Scholar]
- 7.Chew DJ, Meuten DJ. Disorders of calcium and phosphorous metabolism. Vet Clin North Am Small Anim Pract 1982; 12: 411–38. [DOI] [PubMed] [Google Scholar]
- 8.Bates JA. Phosphorus: a quick reference. Vet Clin North Am Small Anim Pract 2008; 38: 471–75. [DOI] [PubMed] [Google Scholar]
- 9.Rose BD, Post TW. Clinical physiology of acid-base and electrolyte disorders. 5th edn. Boston, MA: McGraw-Hill, 2001. [Google Scholar]
- 10.Tenenhouse HS. Regulation of phosphorous homeostasis by the type IIa Na/phosphate cotransporter. Annu Rev Nutr 2005; 25:197–214. [DOI] [PubMed] [Google Scholar]
- 11.Berndt T, Kumar R. Phosphatonins and the regulation of phosphate homeostasis. Annu Rev Physiol 2007; 69: 341–59. [DOI] [PubMed] [Google Scholar]
- 12.Murer H, Hildmann B. Transcellular transport of calcium and inorganic phosphate in the small intestinal epithelium. Am J Physiol Gastrointest Liver Physiol 1981; 240: G 409–16. [DOI] [PubMed] [Google Scholar]
- 13.Crook M. Phosphate: an abnormal anion? Br J Hosp Med 1994; 52:200–4. [PubMed] [Google Scholar]
- 14.Bellasi A, Kooienga L, Block GA. Phosphate binders: new products and challenges. Hemodial Int 2006; 10: 225–34. [DOI] [PubMed] [Google Scholar]
- 15.Lewin E, Huan J, Plgaard K. Parathyroid growth and suppression in renal failure. Semin Dial 2006; 19: 238–45. [DOI] [PubMed] [Google Scholar]
- 16.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorous and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 1998; 31: 607–17. [DOI] [PubMed] [Google Scholar]
- 17.Boyd LM, Langston C, Thompson K, Zivin K, Imanishi M. Survival in cats with naturally occurring chronic kidney disease (2000–2002). J Vet Intern Med 2008; 22: 1111–17. [DOI] [PubMed] [Google Scholar]
- 18.King JN, Tasker S, Gunn-Moore DA, Strehlau G, BENRIC Study Group Prognostic factors in cats with chronic kidney disease. J Vet Intern Med 2007; 21: 906–16. [PubMed] [Google Scholar]
- 19.Ross LA, Finco DR, Crowell WA. Effect of dietary phosphorous restriction on the kidneys of cats with reduced renal mass. Am J Vet Res 1982; 43: 1023–26. [PubMed] [Google Scholar]
- 20.Barber PJ, Elliott J. Feline chronic renal failure: calcium homeostasis in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract 1998; 39:108–16. [DOI] [PubMed] [Google Scholar]
- 21.Elliott J, Rawlings MJ, Markwell PJ, Barber PJ. Survival of cats with naturally occurring chronic renal failure: effect of dietary management. J Small Anim Pract 2000; 41: 235–42. [DOI] [PubMed] [Google Scholar]
- 22.Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK. Association of elevated serum PO4, Ca × PO4 product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 2001; 12: 2131–138. [DOI] [PubMed] [Google Scholar]
- 23.Bertazzolo W, Toscani L, Calcaterra S, Crippa L, Caniatti M, Bonfanti U. Clinicopathological findings in five cats with paw calcification. J Feline Med Surg 2003; 25:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson HA, Barber PJ. Resolution of metastatic calcification in the paws of a cat with successful dietary management of renal hyperparathyroidism. J Small Anim Pract 1998; 39: 495–97. [DOI] [PubMed] [Google Scholar]
- 25.Ross SJ, Osborne CA, Kirk CA, Lowry SR, Koehler LA, Polzin DJ. Clinical evaluation of dietary modification for treatment of spontaneous chronic kidney disease in cats. J Am Vet Med Assoc 2006; 229: 949–57. [DOI] [PubMed] [Google Scholar]
- 26.Finco DR, Barsanti JA, Brown SA. Influence of dietary source of phosphorus on fecal and urinary excretion of phosphorus and other minerals by male cats. Am J Vet Res 1989; 50: 263–66. [PubMed] [Google Scholar]
- 27.Slatopolsky E, Caglar S, Gradowska L, et al. On the prevention of secondary hyperparathyroidism in experimental chronic renal disease using ‘proportional reduction’ of dietary phosphorous intake. Kidney Int 1972; 2:147–51. [DOI] [PubMed] [Google Scholar]
- 28.Elliott J, Brown SA, Cowgill LD, et al. Phosphatemia management in the treatment of chronic kidney disease, a round table discussion. 2006. January 10; http://www.vetoquinol.ca/documents/Quoi%20de%20neuf/Articles/Round%20table%20discussion.pdf (accessed April 8, 2009).
- 29.Barber PJ, Rawlings JM, Markwell PJ, Elliott J. Effect of dietary phosphate restriction on renal secondary hyperparathyroidism in the cat. J Small Anim Pract 1999; 40: 62–70. [DOI] [PubMed] [Google Scholar]
- 30.Loghman-Adham M. Safety of new phosphate binders for chronic renal failure. Drug Saf 2003; 26:1093–115. [DOI] [PubMed] [Google Scholar]
- 31.Schiller L, Santa A, Sheikh M, et al. Effect of the time of administration of calcium acetate on phosphorous binding. N Engl J Med 1989; 320: 1110–13. [DOI] [PubMed] [Google Scholar]
- 32.Russo LS, Beale G, Sandron S, Ballinger WE. Aluminum intoxication in undialysed adults with chronic renal failure. J Neurol Neurosurg Psychiatry 1992; 55: 697–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mallulche HH. Aluminum and bone disease in chronic renal failure. Nephrol Dial Transplant 2002; 17 (suppl 2): 21–4. [DOI] [PubMed] [Google Scholar]
- 34.Cannata-Andia JB, Fernandez-Martin JL. The clinical impact of aluminum overload in renal failure. Nephrol Dial Transplant 2002; 17 (suppl 2): 9–12. [DOI] [PubMed] [Google Scholar]
- 35.Eknoyan G, Levin A, Levin N, et al. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42 (suppl 3): S1–201. [PubMed] [Google Scholar]
- 36.Segev G, Bandt C, Francey T, Cowgill LD. Aluminum toxicity following administration of aluminum-based phosphate binders in 2 dogs with renal failure. J Vet Intern Med 2008; 22: 1432–35. [DOI] [PubMed] [Google Scholar]
- 37.Malluche HH, Mawad H. Management of hyperphosphatemia of chronic kidney disease: lessons from the past and future directions. Nephrol Dial Transplant 2002; 17: 1170–75. [DOI] [PubMed] [Google Scholar]
- 38.Parfitt AM. Soft tissue calcification in uremia. Arch Intern Med 1996; 124: 544–56. [PubMed] [Google Scholar]
- 39.Milliner DS, Zinsmeister AR, Lieberman E, Landing B. Soft tissue calcification in pediatric patients with end-stage renal disease. Kidney Int 1990; 38: 931–36. [DOI] [PubMed] [Google Scholar]
- 40.Sheikh MS, Maguire JA, Emmett M, et al. Reduction of dietary phosphorous absorption by phosphorous binders, a theoretical, in vitro, and in vivo study. J Clin Invest 1989; 83: 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janssen MJA, Van Der Kuy A, Ter Wee PM, Van Boven WPL. Aluminum hydroxide, calcium carbonate and calcium acetate in chronic intermittent hemodialysis patients. Clin Nephrol 1996; 45: 111–19. [PubMed] [Google Scholar]
- 42.Delmez JA, Tindira CA, Windus DW, et al. Calcium acetate as a phosphorous binder in hemodialysis patients. J Am Soc Nephrol 1992; 3: 96–102. [DOI] [PubMed] [Google Scholar]
- 43.Renagel [package insert]. Cambridge, MA: Genzyme Corporation; November 2007. [Google Scholar]
- 44.Renvela [package insert]. Cambridge, MA; Genzyme Corporation; November 2007. [Google Scholar]
- 45.Burke SL, Slatopolsky EA, Goldberg DI. Renagel®, a novel calcium-and aluminum-free phosphate binder, inhibits phosphate absorption in normal volunteers. Nephrol Dial Transplant 1997: 12; 1640–44. [DOI] [PubMed] [Google Scholar]
- 46.Goldberg DI, Dillon MA, Slatopolsky EA, et al. Effect of RenaGel, an non-absorbed calcium- and aluminum-free phosphate binder, on serum phosphorous, calcium, and intact parathyroid hormone in end-stage renal disease patients. Nephrol Dial Transplant 1998; 13: 2303–10. [DOI] [PubMed] [Google Scholar]
- 47.Bleyer AJ, Burke SK, Dillon M, et al. A comparison of the calcium-free phosphate binder sevelamer hydrochloride with calcium acetate in the treatment of hyperphosphatemia in hemodialysis patients. Am J Kidney Dis 1999; 33: 694–701. [DOI] [PubMed] [Google Scholar]
- 48.Slatopolsky EA, Furke SK, Dillon MA. RenaGel®, a nonabsorbed calcium- and aluminum-free phosphate binder, lowers serum phosphorous and parathyroid hormone. Kidney Int 1999; 55: 299–307. [DOI] [PubMed] [Google Scholar]
- 49.Oka Y, Myazaki M, Takatsu S, et al. Sevelamer hydrochloride exacerbates metabolic acidosis in hemodialysis patients, depending on the dosage. Ther Apher Dial 2007; 11: 107–113. [DOI] [PubMed] [Google Scholar]
- 50.Vlahakos DV, Retsa K, Kalogeropoulou S, Katsoudas S, Bacharaki D, Agroyannis B. Chronic acid-base perturbations in hemodialysis patients treated with sevelamer hydrochloride: a two-year follow-up study. Artif Organs 2007; 31: 892–95. [DOI] [PubMed] [Google Scholar]
- 51.Dibartola SP, Rutgers HC, Zack PM, Tarr MJ. Clinicopathologic findings associated with chronic renal disease in cats: 74 cases (1973–1984). J Am Vet Med Assoc 1987; 190: 1196–1202. [PubMed] [Google Scholar]
- 52.McElhiney LF. Sevelamer suspension in children with end-stage renal disease. Int J Pharm Compound 2007; 11: 20–4. [PubMed] [Google Scholar]
- 53.Mahdavi H, Kuizon BD, Gales B, Wang H, Elashoff RM, Salusky IB. Sevalmer hydrochloride: an effective phosphate binder in dialyzed children. Pediatr Nephrol 2003; 18: 1260–64. [DOI] [PubMed] [Google Scholar]
- 54.Storms LE, Chicella MF, Dice JE. Sevelamer therapy for pediatric end-stage renal disease. Pharmacotherapy 2006; 26: 410–13. [DOI] [PubMed] [Google Scholar]
- 55.Wagner E, Schwendenwein I, Zentek J. Effects of a dietary chitosan and calcium supplement on calcium and phosphorous metabolism in cats. Berl Munch Tierarztl Wochenschr 2004; 117: 310–15. [PubMed] [Google Scholar]
- 56.Brown SA, Rickertsen M, Sheldon S. Effects of an intestinal phosphorous binder on serum phosphorous and parathyroid hormone concentrations in cats with reduced renal function. Intern J Appl Res Vet Med 2008; 6: 155–60. [Google Scholar]
- 57.Fosrenol [package insert]. Wayne, PA: Shire US Inc; April 2008. [Google Scholar]
- 58.Albaaj F, Hutchison AJ. Lanthanum carbonate (Fosrenol®): a novel agent for the treatment of hyperphosphatemia in renal failure and dialysis patients. Int J Clin Pract 2005; 59: 1091–96. [DOI] [PubMed] [Google Scholar]
- 59.Persey VP, Behets GJ, Bervoets AR, De Broe ME, D'Haese PC. Lanthanum: A safe phosphate binder. Semin Dial 2006; 19: 195–99. [DOI] [PubMed] [Google Scholar]
- 60.Hutchinson AJ, Maes B, Vanwalleghem J, et al. Efficacy, tolerability and safety of lanthanum carbonate in hyperphsophatemia: a 6-month randomized, comparative trial versus calcium carbonate. Nephron Clin Pract 2005; 100: c8–c19. [DOI] [PubMed] [Google Scholar]
- 61.Schmidt B, Delport P, Spiecker-Hauser U. Bay 78–1887, a novel lanthanum-based phosphate binder decreases intestinal phosphorous absorption in cats. J Vet Pharmacol Ther 2006; 29 (suppl 1): 206–7. [Google Scholar]
- 62.Spiecker-Hauser U, Kraemer F, Epe C, Schmidt B. Efficacy of Lantharenol to reduce intestinal phosphorous absorption from feline renal diet. Proceedings of the 11th European Society of Veterinary and Comparative Nutrition congress; 2007. Nov 2–3; Leipzig, Germany, 2007: 133.
- 63.European Food Safety Authority Safety and efficacy of Lantharenol (Lanthanum carbonate octahydrate) as a feed additive for cats according to Regulation (EC) No 1831/2003. Opinion of the Scientific Panel on Additives and Products or Substances used in Animal Feed, Sept 18, 2007. http://www.efsa.europa.eu/EFSA/efsa_locale-1178620753812_1178645767641.htm (accessed March 8, 2009).
- 64.Nacken K. Renalzin®. Bayer HealthCare backgrounder, 2008. http://www.animalhealth.bayerhealthcare.com/fileadmin/presslounge/att/Renalzin%20Backgrounder.pdf (accessed March 14, 2009).
- 65.Schmidt B, Spieker-Hauser U, Murphy M. Efficacy and safety of Lantharenol on phosphorous metabolism in cats with chronic kidney disease. Proceedings of the 26th Annual ACVIM Forum; 2008. June 4–7; San Antonio, Texas, USA. J Vet Intern Med 2008; 22: 798. [Google Scholar]
- 66.Schmidt BH, Murphy M. A study on the long-term efficacy of Renalzin® (Lantharenol® suspension 20%) in cats with experimentally induced chronic kidney disease. Vet Clin Pathol. In Press 2009. [Google Scholar]
- 67.Schmidt B, Adler K, Hellmann K. The use of Renalzin, a new intestinal phosphate binder, in feline chronic renal failure. Proceeding of the Vetoalp 2008. Conference; 2008 March 31; Chamonix, France, 2008: 69.
- 68.Schmidt B, Spiecker-Hauser U. Overdose acceptance and tolerance of Lantharenol in adult healthy cats. J Vet Pharmacol Ther. In Press 2009. [Google Scholar]