Abstract
Practical relevance Inappetence is one of the most common presenting complaints in clinically ill cats requiring hospitalisation. When prolonged, poor food intake can lead to malnutrition and may be associated with impaired metabolic function, immunosuppression, compromised wound healing, and increased morbidity and mortality. It is important to recognise that inappetence or anorexia is always secondary to another condition, and that treatment goals should be targeted at the primary condition. The current emphasis in the nutritional support of hospitalised inappetent cats is to provide more effective means of increasing nutritional intake -for example, by initiating enteral nutrition via feeding tubes - rather than rely solely on traditional approaches such as increasing palatability of foods or using appetite-stimulating drugs.
Clinical challenges Cats that are ill enough to require hospitalisation are at increased risk of becoming malnourished because of the combined catabolic effects of their disease and poor nutritional intake. This article highlights some of the problems encountered in treating inappetent cats and discusses a clinical approach to providing better nutritional support.
Patient group Inappetence and anorexia are associated with a myriad of clinical conditions in cats and can be seen in individuals of any age or breed.
Equipment Provision of nutritional support to cats may involve the use of feeding tubes such as nasooesophageal or oesophagostomy tubes. In cases where enteral nutrition is not feasible (eg, cats with gastrointestinal failure), parenteral nutrition should be considered.
Evidence base Various studies have documented the high prevalence of inappetence or anorexia in clinically ill cats. Additional studies have linked poor food intake in cats with serious sequelae such as immunosuppression and hepatic lipidosis. More recently, techniques for providing more effective nutritional support, such as oesophagostomy tubes, have been clinically evaluated and shown to be associated with minimal complications.

Why is poor nutritional intake a particular problem in cats?
Compared with other animals cats have several metabolic adaptations that impact on their ability to maintain homeostasis in the face of injury, disease and food deprivation; for example, a higher requirement for protein and certain amino acids. 1 Critical illness induces further metabolic alterations in cats that put them at even greater risk of the development of malnutrition and its deleterious effects.
Similar to other species there are important alterations in the body's response to inadequate nutritional intake in a diseased compared with a healthy state. During periods of nutrient deprivation, a healthy animal will primarily lose glycogen and fat (simple starvation). By contrast, sick or traumatised patients will catabolise lean body mass when they are not provided with sufficient calories (stressed starvation). During the initial stages of fasting in the healthy state, glycogen stores are used as the primary source of energy. Within days, a metabolic shift occurs towards the preferential use of stored fat deposits, sparing catabolic effects on lean muscle tissue. In diseased states, the inflammatory response triggers alterations in cytokines and hormone concentrations and rapidly shifts metabolism towards a catabolic state.2,3 Concurrent alterations in lipid, carbohydrate and protein metabolism can affect the animal's nutritional status and the outcome of the condition.
The metabolic changes documented in critically ill cats include a lower circulating concentration of insulin and higher concentrations of glucose, lactate, Cortisol, glucagon, norepinephrine and non-esterified fatty acids.4,5 Glycogen stores are quickly depleted - especially given the cat is a strict carnivore and may have limited glycogen stores in the first instance - and this leads to an early mobilisation of amino acids from muscle stores (up-regulation of proteolysis). As cats undergo continuous gluconeogenesis, the mobilisation of amino acids from muscles is more pronounced than that observed in other species. Unlike other species, cats have a high obligatory rate of protein oxidation and an inability to down-regulate gluconeogenesis or proteolysis in the face of inadequate protein intake.6,7 With continued lack of food intake, energy is almost completely derived from accelerated proteolysis, which in itself is an energy-consuming process. Thus, these animals may preserve fat deposits in the face of lean muscle tissue losses.
Nutritional support should be considered in any ill cat with inadequate food intake for more than 3 days.
The consequences of continued lean body mass losses include negative effects on wound healing, immune function, strength (both skeletal and respiratory) and, ultimately, the overall prognosis. 8 The metabolic derangements that occur in critically ill cats, and the deleterious effects of malnutrition, make the provision of nutritional support to hospitalised cats an essential and vital part of the therapeutic approach.
When to intervene?
In cats, inadequate calorie intake is commonly due to a loss of appetite (hyporexia), an inability to eat or results from the persistent vomiting that accompanies many disease processes. Appetite suppression may be instigated directly by inflammatory cytokines (eg, IL-1β, IL-6, TNF-α) or secondarily by pathological processes (eg, nausea, pyrexia, pain, ileus). 9 Because malnutrition can occur quickly in these animals, it is important to provide nutritional support by either enteral or parenteral routes if oral intake is not adequate.
Identification of overt malnutrition in cats can be challenging because there are no established criteria to define malnutrition in companion animals. As detectable impairment of immune function (eg, decreased lymphocyte counts, reduced CD4+/CD8+) can be demonstrated in healthy cats subjected to acute starvation by day 4, nutritional support should be considered in any ill cat with inadequate food intake for more than 3 days. 10 The need to implement nutritional intervention becomes more urgent when a cat has not fed for more than 5 days.
Goals of nutritional support.
Even in patients with severe malnutrition, the immediate goals of therapy should focus on fluid resuscitation, cardiovascular stabilisation and identification of the primary disease process. As steps are made to address the primary disease, nutritional interventions should be aimed at addressing overt nutritional deficiencies and imbalances. By providing adequate energy substrates, protein, essential fatty acids and micronutrients, the body can support wound healing and tissue repair. A major goal of nutritional support is to minimise metabolic derangements and catabolism of lean body tissue. During hospitalisation, repletion of body weight is not a priority as this will only occur when the animal is recovering from a state of critical illness. The ultimate goal is to have the patient eating adequate amounts of food in its own environment.
There are many goals of nutritional support, but two important objectives are to treat malnutrition when it is present and to prevent malnutrition in animals at risk. Whenever possible, the enteral route should be used because it is the safest, most convenient and most physiologically sound method of nutritional support. 11 However, when patients are unable to tolerate enteral feeding (eg, due to vomiting or diarrhoea, cramping, nausea) or unable to utilise nutrients administered enterally (eg, due to severe inflammatory bowel syndrome, intestinal lymphoma), parenteral nutrition should be considered.
Ensuring the successful nutritional management of critically ill patients involves selecting the right patient, making an appropriate nutritional assessment and implementing a feasible nutritional plan.
Assessing the need to feed
As with any intervention, there are risks of complications when initiating nutritional support. Minimising such risks depends on appropriate patient selection and patient assessment. The first step in designing a nutritional strategy for a patient, therefore, is nutritional assessment, which involves making a systematic evaluation of the cat. Nutritional assessment identifies malnourished patients that require immediate nutritional support and also identifies patients at high risk of developing malnutrition, in which pre-emptive nutritional support will be beneficial. 12
Purported indicators of overt malnutrition include recent weight loss of at least 10% of body weight, poor haircoat quality, muscle wasting, signs of poor wound healing, hypoalbuminaemia and lymphopenia (Fig 1). 13 However, these abnormalities are not specific to malnutrition and are not present early in the process. Factors that predispose a patient to malnutrition include anorexia lasting longer than 3 days, serious underlying disease (eg, sepsis, peritonitis, necrotising pancreatitis), and large protein losses (eg, protracted vomiting, diarrhoea or draining wounds).13,14 Nutritional assessment also identifies factors that can impact on the nutritional plan, such as cardiovascular instability, electrolyte abnormalities, hyperglycaemia and hypertriglyceridaemia, or concurrent conditions such as uraemia or hepatic failure. As a main priority of nutritional support centres on avoidance of metabolic complications, identification of abnormalities via nutritional assessment may prompt alterations to the nutritional plan. For example, cats with uraemia may not tolerate high protein diets. Appropriate laboratory analysis should be performed in all patients to assess these parameters.
FIG 1.
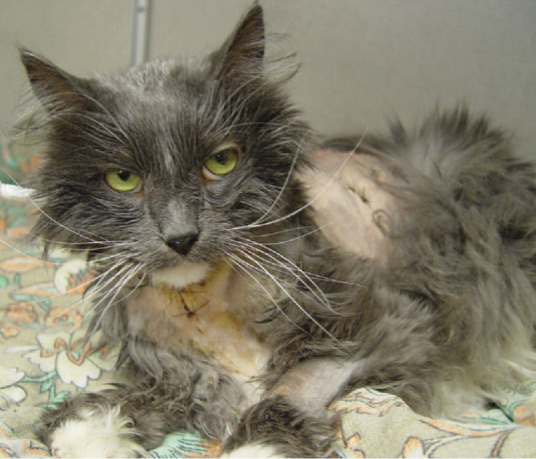
A candidate for nutritional support? Factors such as recent weight loss, poor haircoat and muscle wasting are not specific to malnutrition, hence an appropriate nutritional assessment should be conducted before implementing a nutritional plan
During hospitalisation, repletion of body weight is not a priority as this will only occur when the animal is recovering from a state of critical illness. The ultimate goal is to have the patient eating adequate amounts of food in its own environment.
Before implementation of any nutritional plan, the patient should be adequately resuscitated and have major electrolyte, fluid and acid-base abnormalities addressed.
Implementing a nutritional plan
Proper diagnosis and treatment of the underlying disease is the key to successful nutritional support. Based on the nutritional assessment, a plan is formulated to meet the approximate energy and other nutritional requirements of the patient. The anticipated duration of nutritional support should be determined and factored into the plan. This will largely depend on clinical familiarity with the specific disease process and sound clinical judgement. For example, a cat diagnosed with idiopathic hepatic lipidosis is likely to require long term nutritional support (several weeks) and benefit from tube feeding. Conversely, cats with minor injuries are unlikely to require feeding tubes for nutritional management.
Which route?
For each patient, the best route of nutrition-enteral versus parenteral - should be determined. This decision should be based on the underlying disease and the patient's clinical signs. Important factors to consider include the urgency of commencing nutritional support (eg, a cat showing overt signs of malnutrition or at high risk of developing malnutrition, versus a cat with a serious underlying disease expected to take several weeks to recover) and whether there are any contraindications to a particular mode of nutritional support (eg, cats with protracted vomiting will not tolerate oral or oesophageal feeding, cats with oral or pharyngeal disease may benefit from gastric tube feeding).
Are appetite stimulants useful?
Because inappetence is so common in sick cats, the temptation exists to use appetite stimulants to increase food intake. Unfortunately, appetite stimulants are generally unreliable and seldom result in adequate food intake in critically ill cats. 15 Pharmacological stimulation of appetite is often short-lived and only delays true nutritional support. Loss of appetite is simply a symptom and not a disease process in itself, and therefore the use of pharmacological agents to treat this symptom without addressing the underlying condition is ill-advised. The author does not believe in the use of appetite stimulants in hospitalised patients when there are other, more effective and more appropriate measures of nutritional support, such as placement of feeding tubes.
Loss of appetite is simply a symptom and not a disease process in itself. Therefore, the use of pharmacological agents to treat this symptom without addressing the underlying condition is ill-advised.
Appetite stimulants may be considered in cats that are treated as outpatients (ie, not sick enough to be hospitalised), or in recovering animals (because the primary reason for loss of appetite should ideally be reversed by this time). Appetite stimulants also have negative side effects, such as behavioural changes associated with cyproheptadine and sedation associated with diazepam (oral diazepam can also lead to severe hepatoxicity), and therefore should be used with caution or completely avoided.
Recently, there has been interest in the use of mirtazepine as an appetite stimulant in cats. There is very limited information regarding the use of this agent in this species. Mirtazepine is a tetracyclic antidepressant that appears to have some antiemetic and appetite-stimulating effects. 16 It also relies on glucuronidation for clearance and, therefore, should be used with caution in cats with hepatic disease. Moreover, side effects associated with mirtazepine include sedation and hypotension, which are highly undesirable in critically ill cats and may necessitate further interventions. 16
As discussed, the enteral route should be considered as the first choice, whenever possible, primarily because it is safer and helps to maintain intestinal structure and function; enteral nutrition is also less expensive than parenteral nutrition. If enteral feeding is not tolerated or the gastrointestinal tract or parts of the gastrointestinal tract must be bypassed, parenteral nutrition should be instituted. In cases where only a portion of the cat's nutritional needs can be met enterally (eg, minimal tolerance to small volumes of enteral feeding), supplemental parenteral nutrition has been demonstrated to be beneficial. 17
The various alterations in metabolic pathways that occur following injury and food deprivation suggest that nutritional support should be introduced gradually and reach target levels in 48–72 h.4,5
How much to feed?
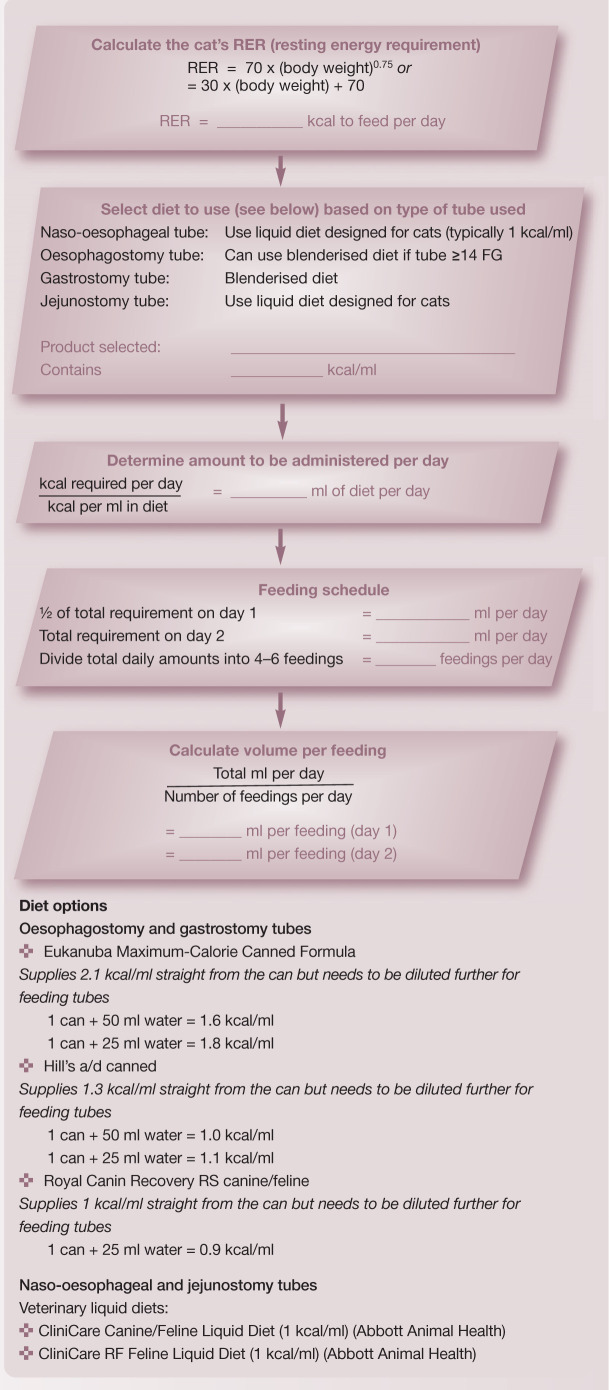
There are various ways to estimate energy requirements in animals. In the clinical setting the easiest and most practical approach is to base estimates on current body weight. As a starting point one can calculate the animal's resting energy requirement (RER), which is the number of calories required for maintaining homeostasis while the animal rests quietly. One of the most common ways to calculate the RER is to use the formula:

Traditionally, the RER was then multiplied by an illness factor of 1.0–2.0 to account for increases in metabolism associated with different conditions and injuries.
Parenteral nutrition.
There are instances where there is such severe gastrointestinal dysfunction that enteral feeding is not feasible and parenteral nutrition should be considered. Recognised indications for parenteral nutrition include intractable vomiting, severe malabsorptive disorders and severe ileus. Cats that may require parenteral nutrition include those with severe inflammatory bowel disease, diffuse intestinal lymphoma and acute necrotising (surgical) pancreatitis. In the initial management stages of these disorders, therapies directed at the underlying aetiology may take time to have an effect, and therefore parenteral nutrition may enable nutrient delivery until gastrointestinal function normalises. For further information about the compounding and administration of parenteral nutrition in cats the reader is referred elsewhere.11,20,21
Recently, there has been less emphasis on these subjective illness factors and current recommendations are to use more conservative estimates (ie, simply calculate the RER) to avoid overfeeding.11,13 Overfeeding can result in metabolic and gastrointestinal complications, hepatic dysfunction, increased carbon dioxide production and weakened respiratory muscles. Of the metabolic complications, the development of hyperglycaemia is most common, and possibly the most detrimental. In two recent studies evaluating parenteral nutrition in cats, the application of illness factors >1.0 was associated with higher complication rates and, possibly even, higher mortality rates.18,19
Currently, the RER is used as an initial estimate of a critically ill patient's energy requirements. It should be emphasised that these general guidelines should be used as starting points, and animals receiving nutritional support should be closely monitored for their tolerance to nutritional interventions. A continuing decline in body weight or body condition should prompt the clinician to reassess and perhaps modify the nutritional plan (eg, increasing the number of calories provided by 25%).
While definitive studies determining the actual nutritional requirements of critically ill cats have not been performed, general recommendations can be made. It is currently accepted that cats should be supported with at least 6 g of protein/100 kcal (25–35% of total energy requirements). This is typically met with the use of commercial diets formulated for recovery or convalescence, which tend to have a relatively high fat and protein content. Patients with protein intolerance (eg, hepatic encephalopathy, severe azotaemia) should receive reduced amounts of protein (ie, 4 g protein/100 kcal). 13 Other nutritional requirements will depend on the patient's underlying disease, clinical signs and laboratory parameters.
Instituting enteral feeding
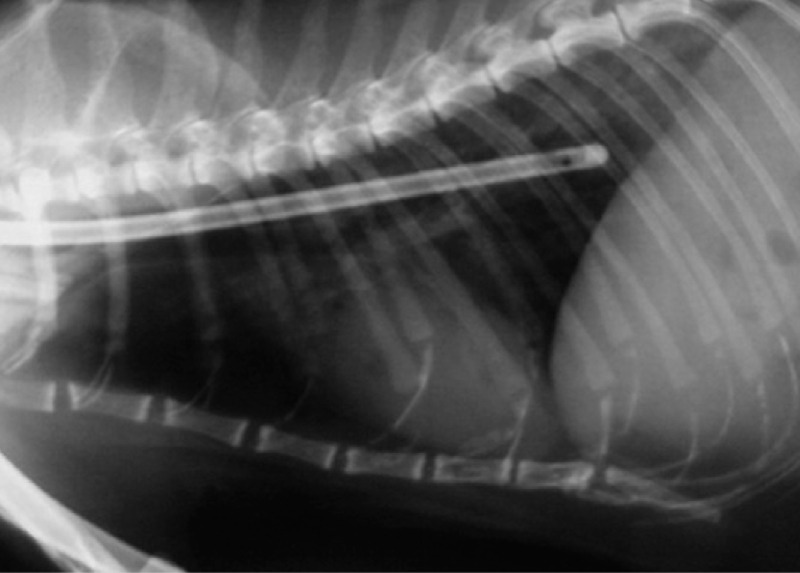
If the enteral route is chosen for nutritional support, the next step is to select the type of feeding tube to be used (see box on page 929). Feeding tubes commonly used in cats include naso-oesophageal, oesophagostomy, gastrostomy and jejunostomy tubes. For nonsurgically placed feeding tubes, such as nasooesophageal or oesophagostomy tubes, confirmation of correct placement via radiography or fluoroscopy is recommended.
Based on the choice of feeding tube and the disease process being treated, an appropriate diet should be selected. For most critically ill cats, an energy-dense, high protein and high fat commercial feline diet is preferred. However, the choice of diet may also depend on the animal's clinical parameters and laboratory results. Diets specially designed for other species (eg, dogs, humans) are not suitable for cats because they often do not meet their unique nutritional needs.
Which feeding tube?
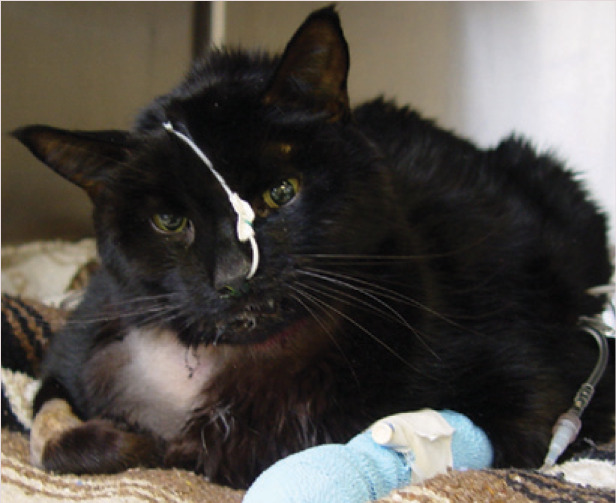
Naso-oesophageal tube
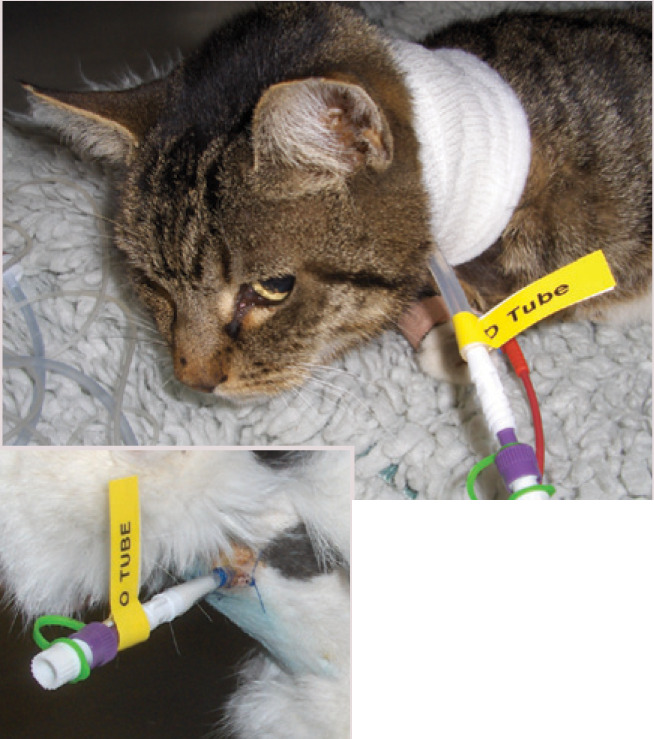
Oesophagostomy tube, (inset) As with all feeding tubes, the exit site should be monitored carefully for irritation or, more significantly, infection
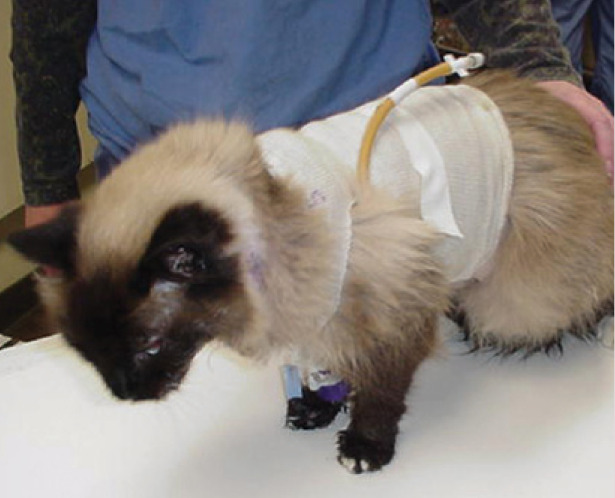
Gastrostomy tube
Feeding tubes have revolutionised the approach to providing nutritional support in critically ill cats. One of their major advantages is that they make it possible for patients to be discharged for home care with good owner compliance. The majority of complications with feeding tubes are considered minor and include tube occlusion and localised irritation at the tube exit site. Occasionally, more serious complications can occur such as infection at the exit site; potentially, complete tube dislodgement could result in peritonitis in the case of a gastrostomy or jejunostomy tube.
Complications can be avoided by using the appropriate tube, ensuring proper food selection and preparation, and careful monitoring and owner education.
Comparison of feeding tubes
| Feeding tube | Duration | Advantages | Disadvantages |
|---|---|---|---|
| Naso-oesophageal | Short term (<5 days) | Inexpensive Easy to place No anaesthesia required |
Requires liquid diet Some animals will not eat voluntarily with tube in place |
| Oesophagostomy | Long term | Inexpensive Easy to place Allows calorically dense diets to be fed |
Requires anaesthesia (short term) Cellulitis is a major complication seen |
| Gastrostomy | Long term | Allows calorically dense diets to be fed | Requires anaesthesia and/or surgery, or endoscopy to place PEG (percutaneous endoscopic gastrostomy) tube Tube displacement may result in peritonitis |
| Jejunostomy | Long term | Bypasses stomach and duodenum Can be used in patients with pancreatitis |
Requires anaesthesia and laparotomy For in-hospital use only Requires constant rate infusion Requires liquid diet Tube displacement may result in peritonitis |
The amount of food is then calculated and a specific feeding plan devised (see box on page 930). Generally, feedings are administered every 4–6 h, although by the time of discharge this should be reduced to three to four times a day to facilitate owner compliance. Feeding tubes should be flushed with 5–10 ml of water after each feeding to minimise clogging of the tube.
Commercially available veterinary liquid diets should be used for naso-oesophageal and jejunostomy tube feeding due to the small bore sizes of these catheters (typically <5 French gauge [FG]). Typical ‘semi-solid’ recovery diets are usually unsuitable for small bore tubes because of their tendency to clog these tubes. Jejunostomy tubes are primarily for in-hospital use because they require administration of a liquid diet by continuous rate infusion and this technique also requires more vigilant monitoring. Oesophagostomy and gastrostomy tubes are generally larger (>14 FG) and allow more calorically dense, blenderised diets to be administered. This decreases the volume of food necessary for each feeding. These tubes can be used for long-term enteral feeding.
Mastering the placement of oesophagostomy feeding tubes is essential for the management of critically ill cats.
Formulating a feeding plan.
Placement of oesophagostomy tubes requires minimal equipment, and is a straightforward technique that enables effective nutritional support. In the author's opinion, mastering the placement of oesophagostomy feeding tubes is essential for the management of critically ill cats and this technique can be adopted in almost all practices. Stepwise instructions are given on page 931. Complications associated with oesophagostomy are minimal and client compliance and satisfaction is generally very good.
The maximum volume of food that can be administered safely per feeding to a cat is likely to depend on a combination of individual patient factors and the underlying disease process. A volume of 5–10 ml/kg per feeding is generally well tolerated but this may vary with the individual animal. In cats that are generally healthy but cannot consume food orally (eg, due to a symphyseal jaw fracture), larger volumes of food per feeding (15–20 ml/kg) may be tolerated but this volume must be given slowly over 15–20 mins to allow gastric accommodation, or vomiting may occur. As enteral diets are mostly composed of water (most canned foods comprise >75% water) the amounts of fluids administered parenterally should be adjusted accordingly to avoid volume overload.
Prevention of premature removal of tubes can be achieved by using an Elizabethan collar and by wrapping the tube securely.
Monitoring and reassessment
Body weight should be monitored daily in any hospitalised cat, but especially one receiving nutritional support. It is important that the clinician takes into account fluid shifts in evaluating changes in body weight. The use of the RER as the patient's caloric requirement is merely a starting point. The number of calories provided may need to be increased to keep up with the patient's changing needs, typically by 25% if nutritional support is well tolerated. In patients unable to tolerate the prescribed amounts, the clinician should consider a reduction in enteral nutrition.
Metabolic complications of enteral nutritional support include electrolyte disturbances, hyperglycaemia, volume overload and gastrointestinal signs (eg, vomiting, diarrhoea, cramping, bloating). Occasionally, feeding a chronically debilitated and severely malnourished cat results in a condition known as ‘refeeding syndrome’ (see right). In most cases, severe hypophosphataemia results in muscle weakness and haemolysis. However, severe neurological impairment, hypoventilation, cardiac failure and even death are potential sequelae.22,23 Strategies to reduce the risk of refeeding syndrome include very gradual and conservative provision of nutrition and very low amounts of carbohydrates. 23 Correction of fluid and electrolyte imbalances should take priority over nutritional support in such extreme malnourished cases. Typical monitoring parameters for patients receiving nutritional support include body weight, serum electrolytes, tube patency, appearance of the tube exit site, gastrointestinal signs (eg, vomiting, regurgitation, diarrhoea), and signs of volume overload or aspiration pneumonia. Frequent monitoring should alert the clinician to developing problems.
10 steps to placing an oesophagostomy tube.
Step 1.
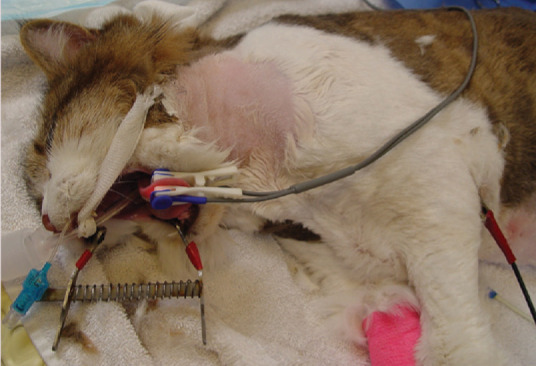
Proper placement of an oesophagostomy feeding tube (O tube) requires the distal tip to be placed in the distal oesophagus at a level no further than the ninth intercostal space. This may require premeasurement of the tube. Rather than cutting the distal tip and creating a sharp edge, the exit hole should be elongated using a small blade
Step 2.
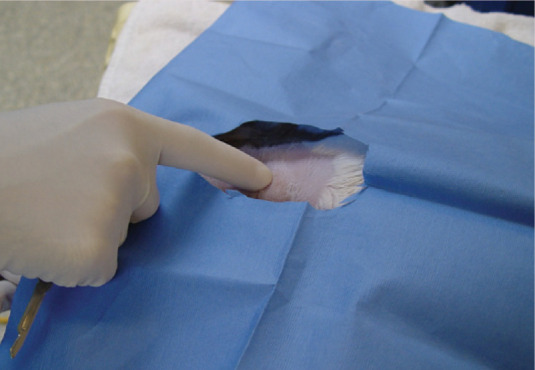
The patient should be anaesthetised and preferably intubated. While in right lateral recumbency, the left side of the neck should be clipped and a routine surgical scrub performed
Step 3.
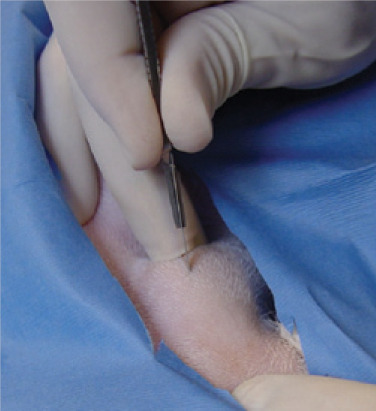
A curved Rochester-Carmalt clamp is placed into the mouth and down the oesophagus to the mid-cervical region. The jugular vein should be identified and avoided
Step 4.
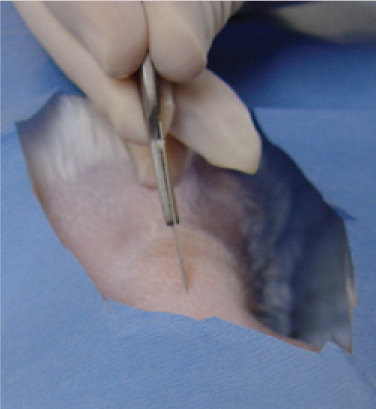
The tip of the Carmalt clamp is then pushed dorsally, elevating the oesophagus towards the skin. Having confirmed the location of the tip of the instrument by palpating over the skin, an incision is made through the skin onto the tip of the Carmalt in the oesophagus. The mucosa of the oesophagus is relatively more difficult to incise than the skin
Step 5.
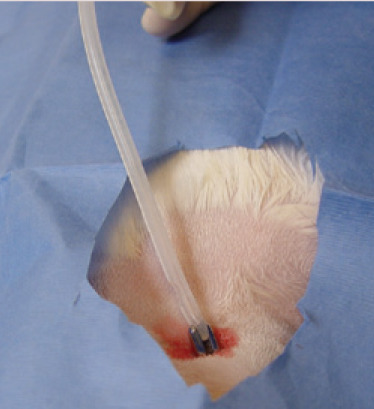
The tip of the instrument is then forced through the incision, which can be slightly enlarged with the blade to allow the tips of the Carmalt to be opened and the O tube to be grasped
Step 6.
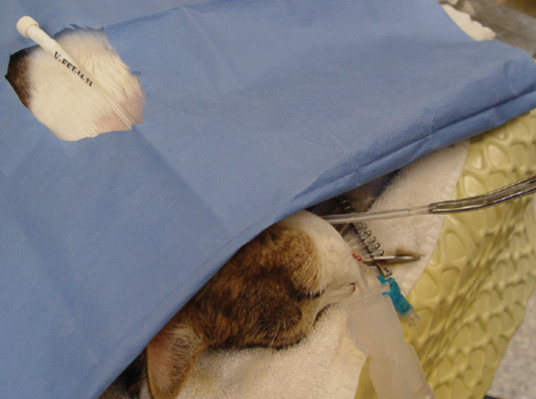
The Carmalt is then clamped closed and pulled from the oral cavity
Step 7.
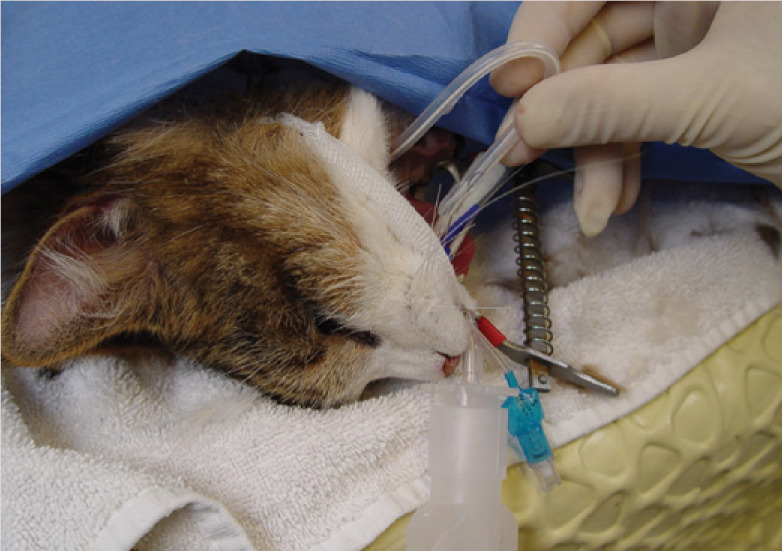
The tips of the Carmalt are disengaged and the tip of the O tube is curled back into the mouth and fed into the oropharynx. As the curled tube is pushed into the oesophagus, the proximal end is gently pulled simultaneously. This results in a subtle ‘flip’ as the tube is redirected within the oesophagus. The tube should easily slide back and forth a few millimetres, confirming that the tube has straightened
Step 8.
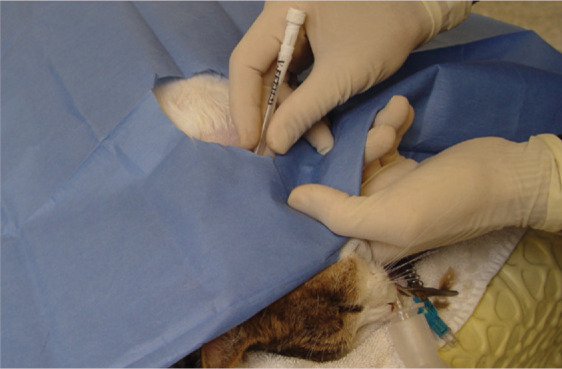
The oropharynx is visually inspected to confirm that the tube is no longer present within it
Step 9.
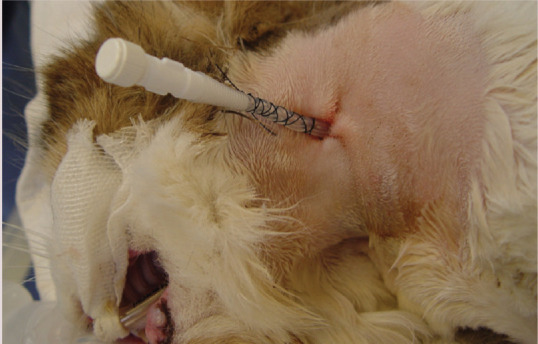
The incision site should be briefly re-scrubbed before a purse-string suture is placed followed by a Chinese finger trap suture, further securing the tube in place. A light wrap is then applied to the neck
Step 10.
Correct O tube placement is confirmed using either radiography or fluoroscopy
MULTIMEDIA.
A video recording showing an oesophageal tube being placed in a cat is included in the online version of this article at doi: 10.1016/j.jfms.2009.09.013
With continual reassessment, the clinician can determine when to transition the patient from assisted feeding to voluntary consumption of food. The discontinuation of nutritional support should only begin when the patient can consume approximately its RER without much coaxing.
What leads to refeeding syndrome?
The major shifts in electrolytes observed in refeeding syndrome relate to the resurgence of insulin following administration of carbohydrates. In severe malnourished states, insulin levels are extremely low and ATP synthesis is minimal. Following administration of carbohydrates, insulin instigates electrolyte shifts and cellular processes, such as synthesis of ATP, until phosphorus is depleted, resulting in the clinical manifestations of refeeding syndrome. 23
Case notes.
Tabitha, a 7-year-old spayed female domestic longhair cat, presents with a 4-week history of progressive weakness, inappetence and weight loss.

Pertinent history Tabitha has no history of other medical problems and is mostly housed indoors in a multi-cat household. She has always had a good appetite and is being fed a light commercial dry diet to prevent further weight gain, as she was deemed on her previous visits to be overweight. Her owners have recently relocated and Tabitha has spent more time hiding than her fellow cats.
Physical examination Tabitha is depressed but responsive, and estimated to be 7% dehydrated based on skin tenting and mucous membrane evaluation. Icteric sclera, skin and mucous membranes are evident. Excessive drooling is noted. She weighs 4.7 kg and has a body condition score (BCS) of 4/9 (compared with previous weight of 6.1 kg and BCS 6/9), and there is obvious muscle wasting over the spine. Thoracic auscultation reveals a heart rate of 260 bpm, respiratory rate of 36 breaths per minute, a grade 2/6 systolic murmur and normal rhythm. Peripheral pulses are deemed weak and thready. Abdominal palpation reveals cranial organomegaly but no pain is detected.
Further assessment Pertinent laboratory findings include a PCV of 21%, total protein 67 g/l, ALT 290 U/l, AST 119 U/l, ALP 395 U/L, total bilirubin 106 umol/l and potassium 2.5 mmol/l. Venous blood gas analysis is consistent with metabolic acidosis and hyperlactataemia. Coagulation times and GGT are within normal limits. An enlarged hyperechoic liver is seen on abdominal ultrasonography and a fine-needle aspirate reveals severe vacuolar changes within hepatocytes. A diagnosis of hepatic lipidosis is made.
-
WHAT IS YOUR ASSESSMENT?
-
Why would simply treating Tabitha with appetite stimulants be inappropriate?
Appetite stimulants do not address the primary problem.
Appetite stimulants do not reliably result in adequate food intake necessary to reverse disease.
Appetite stimulants can result in undesirable side effects in critically ill cats.
All of the above.
-
Placement of an oesophagostomy tube is planned for nutritional support. Before performing such a procedure, what measures should be taken in Tabitha's case?
Administer parenteral nutrition until she is restored to her normal body weight.
Provide supportive therapy until liver enzymes and bilirubin return to normal.
Address cardiovascular stabilisation, rehydration and correction of major acid-base abnormalities and electrolyte disturbances prior to general anaesthesia.
Force-feed a high calorie diet and reassess the need to place a feeding tube.
-
Answers and discussion
(d) For all of these reasons, the use of appetite stimulants is not recommended for the treatment of inappetence in critically ill cats. Appetite stimulants may have a role in recovering cats in which the underlying disease is successfully treated.
(c) Even in severely malnourished cats, before nutritional interventions are performed, patients should be cardiovascularly stable and have major acid-base and electrolyte abnormalities addressed. This is to reduce the risk of metabolic complications following institution of nutritional support.
KEY POINTS.
While critically ill cats are often not regarded as being in urgent need of nutritional support given their more pressing other problems, the severity of their injuries, their altered metabolic condition and the necessity for frequent fasting (eg, for diagnostic or surgical procedures) place these patients at high risk of becoming malnourished during hospitalisation.
The unique metabolic peculiarities of cats make them particularly prone to energy and protein catabolism, and nutritional support in this species may be more pertinent during critical illness.
Proper identification of patients in need of nutritional support, and careful planning and execution of a nutritional plan, can be key factors in the successful recovery of these cats.
References
- 1.MacDonald ML, Rogers QR, Morris JG. Nutrition of the domestic cat, a mammalian carnivore. Annu Rev Nutr 1984; 4: 521–62. [DOI] [PubMed] [Google Scholar]
- 2.Mitchel KE. Nitrogen metabolism in critical care patients. Vet Clin Nutr 1998; (suppl): 20–22. [Google Scholar]
- 3.Sakurai Y, Zhang X, Wolfe RR. Short-term effects of tumor necrosis factor on energy and substrate metabolism in dogs. J Clin Invest 1993; 91: 2437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown B, Mauldin GE, Armstrong J, et al. Metabolic and hormonal alterations in cats with hepatic lipidosis. J Vet Intern Med 2000; 14: 20–6. [DOI] [PubMed] [Google Scholar]
- 5.Chan DL, Freeman LM, Rozanski EA, et al. Alterations in carbohydrate metabolism in critically ill cats. J Vet Emerg Crit Care 2006; 16: S7–S13. [Google Scholar]
- 6.Rogers QR, Morris JG, Freedland RA. Lack of hepatic enzymatic adaptation to low and high levels of dietary protein in the adult cat. Enzyme 1977; 22: 348–56. [DOI] [PubMed] [Google Scholar]
- 7.Green AS, Ramsey JJ, Villaverde C, Asami DK, Alfreda Wei, Fascetti AJ. Cats are able to adapt protein oxidation to protein intake provided their requirement for dietary protein is met. J Nutr 2008; 138: 1053–60. [DOI] [PubMed] [Google Scholar]
- 8.Remillard RL. Nutritional support in critical care patients. Vet Clin North Am Small Anim Pract 2002; 32: 1145–64. [DOI] [PubMed] [Google Scholar]
- 9.Perboni S, Inui A. Anorexia in cancer: role of feeding-regulatory peptides. Philos Trans R Soc Lond B Biol Sci 2006; 361: 1281–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freitag KA, Saker KE, Thomas E, Kalmitsky J. Acute starvation and subsequent refeeding affect lymphocyte subsets and proliferation in cats. J Nutr 2000; 130: 2444–49. [DOI] [PubMed] [Google Scholar]
- 11.Freeman LM, Chan DL. Total parenteral nutrition. In DiBartola SP, ed. Fluid, electrolyte, and acid-base disorders in small animal practice. 3rd edn. St Louis: Elsevier, 2006: 584–601. [Google Scholar]
- 12.Buffington T, Holloway C, Abood A. Nutritional assessment. In Buffington T, Holloway C, Abood S, eds. Manual of veterinary dietetics. St Louis: WB Saunders, 2004: 1–7. [Google Scholar]
- 13.Chan DL. Nutritional requirements of the critically ill patient. Clin Tech Small Anim Pract 2004; 19: 1–5. [DOI] [PubMed] [Google Scholar]
- 14.Thatcher CD. Nutritional needs of critically ill patients. Compend Contin Educ Pract Vet 1996; 18: 1303–11. [Google Scholar]
- 15.Delaney SJ: Management of anorexia in dogs and cats. Vet Clin North Am Small Anim Pract 2006; 36: 1243–49. [DOI] [PubMed] [Google Scholar]
- 16.Plumb DC. Plumb's veterinary drug handbook. 6th edn. Ames: Blackwell Publishing Professional, 2008. [Google Scholar]
- 17.Chan DL, Freeman LM, Labato MA, Rush JE. Retrospective evaluation of partial parenteral nutrition in dogs and cats. J Vet Intern Med 2002; 16: 440–45. [DOI] [PubMed] [Google Scholar]
- 18.Pyle SC, Marks SL, Kass PH. Evaluation of complications and prognostic factors associated with administration of total parenteral nutrition in cats: 75 cases (1994–2001). J Am Vet Med Assoc 2004; 255: 242–50. [DOI] [PubMed] [Google Scholar]
- 19.Crabb SE, Chan DL, Freeman LM. Retrospective evaluation of total parenteral nutrition in cats: 40 cases (1991–2003). J Vet Emerg Crit Care 2006; 16: S21–S26. [Google Scholar]
- 20.Thomovsky E, Reniker A, Backus R, Mann FA, Dodam JR. Parenteral nutrition: uses, indications, and compounding. Compend Contin Educ Pract Vet 2007; 29: 76–85. [PubMed] [Google Scholar]
- 21.Thomovsky E, Backus R, Reniker A, Mann FA, Dodam JR. Parenteral nutrition: formulation, monitoring, and complications. Compend Contin Educ Pract Vet 2007; 29: 88–103. [PubMed] [Google Scholar]
- 22.Justin RB, Hoenhaus AE: Hypophosphatemia associated with enteral alimentation in cats. J Vet Intern Med 1995; 9: 228–33. [DOI] [PubMed] [Google Scholar]
- 23.Armitage-Chan EA, O'Toole T, Chan DL. Management of prolonged food deprivation, hypothermia and refeeding syndrome in a cat. J Vet Emerg Crit Care 2006; 16: S34–S41. [Google Scholar]