Abstract
Practical relevance:
Orphaned kittens are common and veterinary team members should be prepared to assist owners in providing appropriate care. If a foster queen is not available, the physiologic needs normally provided by the queen, such as warmth, nutrition, elimination, sanitation and social stimulation, must be replaced. Specialized knowledge of physiology and nutritional requirements is necessary for successful management in this age group.
Clinical challenges:
The condition of neonatal kittens can deteriorate rapidly and orphans may have an even higher risk of illness than kittens cared for by a healthy queen. Thus an increased level of veterinary care and monitoring is required. Rapid recognition and correction of problems can only be accomplished through detailed observation and knowledge of developmental milestones. Survival may be dependent on treatment that compensates for the failure of passive transfer of immunity.
Audience:
This review is addressed at veterinarians and all veterinary team members, as well as care-givers such as rescuers and shelter workers.
Evidence base:
The guidance contained in this article is based on a combination of published literature, the author’s personal experience and the experience of colleagues.
Orphaned kittens are those that lack sufficient maternal care for survival during the early pediatric period of life.Veterinarians may be asked to evaluate and treat kittens that have been orphaned through death or illness of the queen, kittens rejected by the queen, or kittens found abandoned. Such kittens require specific care and nutritional support until weaning (Figure 1).
Figure 1.

Queens provide warmth, nutrition, elimination, sanitation and social stimulation for their kittens. These are all needs that must be replaced for orphaned kittens
Housing
The ideal housing for orphaned kittens is an incubator with controlled temperature and humidity, but any warm and safe enclosure will suffice, such as a pet carrier or cardboard box. Orphaned kittens should be kept in a quiet area, away from unsupervised exposure to other pets and children. Hygiene is important for prevention of infectious diseases. Cardboard boxes should be replaced when soiled; enclosures made of other materials should be kept clean. Bedding should be absorbent, soft, warm, and either readily cleaned or disposable (Figure 2). Care-givers and others should wash hands before handling the kittens. At least twice weekly, orphaned kittens should be cleaned gently with a soft moistened wash cloth.
Figure 2.

Bedding should be soft and warm, and either readily washable or disposable. Courtesy of Chantal Bourdon

Neonatal kittens are somewhat poikilothermic. They are unable to regulate their body temperature well until over 4 weeks of age, which is why young kittens huddle together (Figure 3) and also huddle with the queen when she is present. Orphaned kittens do not have the protection of a queen, and may be without littermates too, and so are more sensitive to environmental conditions. Hypothermia is a common cause of illness and death because it is associated with depressed respiration, impaired immune function, bradycardia and ileus. The temperature in the immediate environment of kittens in the first week of life should be 32–34°C (89.5–93°F). The temperature can be gradually lowered to 24°C (75°F) over the next few weeks (Table 1).
Figure 3.

Normal kittens huddle together for warmth in the first 4–6 weeks of life. Courtesy of Janet Wolf
Table 1.
Optimal temperature in the incubator or immediate environment for orphaned kittens
| °C | °F | |
|---|---|---|
| Week 1 | 32–34 | 89.5–93 |
| Week 2 | 27–29 | 81–84 |
| Week 3 | 24–27 | 75–81 |
| Weeks 4–12 | 24 | 75 |
From Gross et al (2010) 2
If a heat source is used in a box or carrier, it should be placed so that a temperature gradient is created, allowing the kittens to move away from the warmest areas when needed. Circulating hot water blankets are an excellent heat source, as are hot water bottles wrapped in clean towels. Heating pads are not recommended as they heat unevenly and may cause burns. Heat lamps provide radiant heat and are safe to use as long as the kittens can move to a cooler part of the enclosure as needed and the temperature at the level of the kittens is monitored. Humidity should be maintained at 55–60% to prevent dehydration and maintain the health of mucous membranes.
Kittens up to 3 weeks of age spend the majority of their time sleeping and are generally quiet when healthy, warm and well fed. A litter of kittens sleeps together until about 4–6 weeks of age, after which they may be observed sleeping separately. Waking time would primarily be spent nursing if with the queen. When stressed by hunger, the absence of the queen, or other reasons, normal kittens will cry and crawl around the nest box, moving the head from side to side in a searching motion. Kittens that cry excessively, are restless, or have poor muscle tone should be examined by a veterinarian promptly.
When orphaned kittens are housed together, they may try to nurse on each other. Skin trauma or genital trauma, especially of the penis and prepuce, may occur. To prevent injury, it may be necessary to separate the kittens but this must be balanced against the need for socialization.

Figure 4.

Socialization to people and pets is an important part of normal development. Courtesy of Chantal Bourdon
Health management
Care-givers should be educated on the need to maintain good health records (eg, weight gain [see weighing recommendations later], daily food intake, fecal characteristics, behavior, etc). Identification of individuals in a litter is important for monitoring weight and health; if kittens are similar in appearance, different colors of nail polish can be applied to the claws for differentiation. Successful management is dependent on rapid recognition and correction of problems, which can only be accomplished through detailed observation. The condition of neonatal kittens can deteriorate rapidly, so care-givers should be advised to consult a veterinarian promptly when variations from normal or signs of illness are noted.
There are many clinically relevant physiologic differences between young kittens and adult cats. Veterinarians should become familiar with normal physiologic values (Table 2), developmental milestones (Table 3), and normal physical examination findings for young kittens. If the kitten’s birth date is unknown, an estimated age should be established by using body weight and developmental milestones. The normal birth weight for kittens averages 100 g, but there is considerable variation among pedigree breeds (eg, 73 g [Korat] to 116 g or more [Maine Coon]).5,6 Normal values for hematology and serum chemistries vary during the first 8 weeks of life; thus age-specific normal reference intervals should be consulted. 7
Table 2.
Normal physiologic values for neonatal kittens
| Parameter | Normal values |
|---|---|
| Birth weight | Domestic shorthair/longhair: 90–110 g |
| Pedigree breeds: 73 g (Korat) to 116 g or more (Maine Coon) | |
| Rectal temperature | Newborn: 36–37°C (97–98°F) |
| One month: 38°C (100°F) | |
| Heart rate | First 2 weeks: 220–260 beats/min |
| Respiratory rate | Newborn: 10–18 breaths/min |
| One week: 15–35 breaths/min | |
| Urine | Specific gravity: <1.020 |
| Urine output: 2.5 ml/100 g body weight/day | |
| Water requirement | 13–22 ml/100 g body weight/day |
| Caloric requirement | 15–25 kcal ME/100 g body weight/day |
| Stomach capacity | 4–5 ml/100 g body weight |
From Little (2012). 4 ME = metabolizable energy
Table 3.
Developmental milestones in kittens
| Milestone | Age | |
|---|---|---|
| Umbilical cord falls off | 3 days | |
| Voluntary elimination of urine and feces | 3 weeks | |
| Eyes | Eyelids open | 7–10 days (average) |
| Menace/pupillary light reflexes present | 28 days or later | |
| Normal vision | 30 days | |
| Adult iris color | 4–6 weeks | |
| Ears | Ear canals open | 9 days (average) |
| Functional hearing | 4–6 weeks | |
| Locomotion | Crawling | 7–14 days |
| Walking | 14–21 days | |
| Teeth | Deciduous incisors/canines erupt | 3–4 weeks |
| Deciduous premolars erupt | 5–6 weeks | |
From Little (2012) 4
High-risk time points for kitten morbidity and mortality are at birth, in the first 2 weeks of life and around the time of weaning. Orphaned kittens may be at even higher risk of illness than kittens cared for by a healthy queen and therefore require an increased level of veterinary care and monitoring. Veterinary visits every 1–2 weeks may be advisable until weaning.
While rapid identification of illness and prompt intervention are vital to the treatment of sick kittens, the exact cause of illness is not always evident at the time of examination. Therefore, supportive care is very important in this age group. Common problems include hypoglycemia, hypothermia and dehydration. Treatment of these problems is described elsewhere.4,7 Common pathogens in pediatric kittens include those causing respiratory tract disease (eg, feline herpesvirus-1, feline calicivirus, Bordetella bronchiseptica, Mycoplasma species, Chlamydophila felis), diarrhea (eg, panleukopenia virus, coliform bacteria, Tritrichomonas foetus, Giardia species, Isospora species, Ancylostoma species, Toxocara species), neonatal sepsis (eg, Streptococcus species, Staphylococcus species, Escherichia coli, Salmonella species) and systemic infections (eg, feline leukemia virus [FeLV], feline immunodeficiency virus [FIV], feline infectious peritonitis).
Most anthelmintics for kittens are labeled for use from 8 weeks of age, although a few are labeled from 6 weeks depending on the country. Pyrantel pamoate (5–10 mg/kg, PO) is safe and effective for Ancylostoma species and Toxocara species infections, starting as early as 2 weeks of age. Treatment is repeated every 2–3 weeks until 12 weeks of age or until the kitten is old enough to receive another product. Fenbendazole (Panacur; MSD Animal Health) is a broad-spectrum dewormer that is labeled for use in some countries in unweaned kittens (50 mg/kg, PO, q24h for 3 days).
External parasites such as fleas and lice can cause anemia and general debilitation (Figure 5). Many flea control products are available and some are also effective against ear mites and lice. Most products are labeled for use from 8 weeks of age. 8 Exceptions in some countries include lufenuron (Program; Novartis Animal Health, from 6 weeks) and nitenpyram (Capstar; Novartis Animal Health, from 4 weeks/minimum 0.9 kg body weight). Fipronil spray (Frontline; Merial, and others) is labeled for use on kittens from 2 days of age in some countries. For very young kittens, fipronil spray can be applied to a cloth or cotton balls and wiped on the kitten, avoiding the eyes and mucous membranes. Daily flea-combing is a safe option where fipronil spray is not available or not licensed for young kittens. Most flea shampoos are not labeled for use in kittens less than 12 weeks of age. For effective treatment of flea infestations, the environment should also be treated appropriately, as well as all other animals present.
Figure 5.
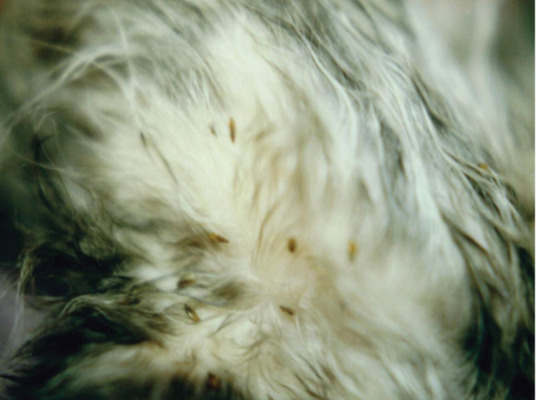
Fleas on the ventral abdomen of an orphaned kitten. Flea-bite anemia may be life-threatening
Ear mites are treated by first gently removing debris from the ear canal with mineral oil to allow topical medications to penetrate. An otic miticide and whole-body flea control product should be used together when possible. Topical 0.01% ivermectin is labeled in some countries for treatment of ear mites in kittens from 4 weeks of age (eg, Acarexx; Boehringer Ingelheim). All affected and in-contact animals should be treated.
Healthy orphaned kittens should be vaccinated similarly to queen-raised kittens. The first dose of injectable or intranasal vaccine against panleukopenia virus, feline herpesvirus-1 and calicivirus can be given as early as 4–6 weeks of age. 9 Attenuated injectable or intranasal vaccines containing panleukopenia virus should not be administered to kittens less than 4 weeks of age due to the risk of cerebellar hypoplasia or clinical disease. In rare circumstances (eg, where the risk of disease is high), a bivalent vaccine against feline herpesvirus-1 and calicivirus may be administered by injection or intranasally to kittens less than 4 weeks of age. 9
Passive transfer of immunity
Mechanisms
Kittens receive almost all their passive immunity against infectious diseases during the first 12–18 h of life with the ingestion of colostrum. Macromolecules such as immunoglobulins are readily absorbed into the blood from the upper small intestine before closure of the intestinal epithelium. There is little or no transplacental transfer of immunoglobulins in the cat because endotheliochorial placentation presents a barrier to antibody transfer. 10 Small amounts of immunoglobulin appear in the serum of fetal and newborn kittens from intestinal absorption of amniotic fluid, which contains small quantities of IgG transferred from maternal blood. 11
The amount of passively acquired immunity a kitten receives is determined by the amount of colostrum ingested, the timing of ingestion and the concentration of immunoglobulin in the colostrum. The kitten’s serum immunoglobulin nadir is reached at 4–6 weeks of age due to catabolism of maternal immunoglobulin and correlates with a period of vulnerability to infection.12–14 Immunoglobulin levels then steadily increase as the kitten’s own adaptive immunity develops.
Failure of passive transfer
Failure of passive transfer of immunity (FPT) is an important problem in calves, lambs and foals, and is associated with an increased risk of illness and death due to bacterial and viral infections of the gastrointestinal and respiratory tracts. FPT is diagnosed in large animal species when post-nursing serum IgG concentrations are less than 400 mg/dl. 12 Commercial colostrum supplements and replacements are available for the neonates of large animal species.
Although no studies on a correlation between FPT and susceptibility to infection in kittens have been published, neonatal sepsis is one of the most common causes of kitten mortality in the first few weeks of life. Kittens with uncorrected FPT start to produce immunoglobulins at about 2 weeks of age and have immunoglobulin levels similar to colostrum-treated kittens by 6 weeks. Colostrum-deprived kittens are therefore most vulnerable to infection from birth to about 6 weeks of age.
If a full history is not available for an orphaned kitten or litter, it may be difficult to know if FPT has occurred. Patient-side immunoglobulin G (IgG) assays are available for detection of FPT in calves and foals, but at the time of writing no patient-side assays are available for kittens. Definitive diagnosis is by measurement of serum IgG concentration at a reference laboratory that offers the assay. One study evaluating surrogate markers for FPT in kittens found that adequacy of passive transfer correlated only with serum alkaline phosphatase activity, and then only for the first 2 days of life. 15
A common recommendation for orphaned kittens at risk of FPT is to foster on to a lactating queen. However, newborn colostrum-deprived kittens fostered on to surrogate queens in the milk phase of lactation do not acquire sufficient levels of immunoglobulins. 12 Colostrum-deprived kittens fed milk replacer also fail to acquire sufficient levels of immunoglobulins. 12 Milk replacers for kittens may be advertised as containing colostrum, but there is no evidence that the ingredient provides any immunity.
Methods of correction
Correction of FPT can be accomplished by intraperitoneal or subcutaneous injection of adult cat serum containing adequate amounts of pathogen-specific antibodies (from natural exposure or vaccination) harvested from a cat of compatible blood type that is free from infectious diseases. Donor cats should be at low risk for infectious diseases and test negative for FeLV and FIV; even then, disease transmission is still possible since some infected cats will test negative on routine screening.
The blood and serum collection, processing, administration and storage processes must be performed under sterile conditions to avoid bacterial contamination. To harvest blood, sedation of the donor cat may be required. The area over the jugular vein is shaved and prepared for aseptic venepuncture. Blood is collected into sterile clot tubes without additives and allowed to clot at room temperature for 20 mins before centrifugation to separate serum. A sterile needle and syringe are used to remove the serum from the tubes while avoiding harvesting red blood cells. Serum can be administered immediately or refrigerated for a few days.
In one study, administering 15 ml serum/100 g body weight (divided into three doses over 24 h) either intraperitoneally or subcutaneously achieved serum IgG concentrations of over 900 mg/dl (range 910–2200 mg/dl) in colostrum-deprived kittens. 13 Although the minimum serum IgG concentration for protection of neonatal kittens against disease is unknown, recommendations in large animal species suggest IgG concentrations greater than 400–800 mg/dl are sufficient. Serum IgG concentrations in colostrum-deprived kittens given adult serum parenterally declined to a nadir between 3 and 5 weeks of age, similar to colostrum-fed kittens. 13
This protocol requires collection of large volumes of blood under sterile conditions and may be impractical, especially for treating more than one kitten. However, serum can be harvested when the opportunity arises and stored frozen at −20°C (−4°F) in single dose aliquots (5 ml) for up to 1 year, as long as it is not repeatedly thawed. Serum aliquots are warmed to 37°C (98.6°F) immediately before administration. Approximately 30 ml of whole blood must be harvested to provide 15 ml of serum to treat one kitten. One unit of whole blood (60 ml) can supply serum for two kittens.
Whether smaller amounts of adult cat serum administered parenterally may be effective in correcting FPT is not known. Also it is not known if oral administration of adult cat serum would provide adequate immunity to colostrum-deprived kittens. Even if effective, the oral route of administration could only be used in the first 18 h of life, before gut closure. The amount of serum that could be fed orally would be limited by the stomach capacity of the neonate and may interfere with adequate intake of milk replacer.
A commercially available equine IgG product licensed for treatment of FPT in foals was evaluated in kittens with FPT in an effort to find a more practical solution. 16 While treated kittens achieved adequate serum concentrations of the equine IgG when it was administered subcutaneously (but not orally), the equine antibodies failed to promote bacterial phagocytosis by feline neutrophils in vitro. Therefore, equine IgG cannot be recommended for treatment of FPT in kittens.
Nutritional support
Foster queen ± supplemental feeding
A lactating foster queen is the best option for feeding orphaned kittens as this provides not only ideal nutrition, but also maternal care. Fostered kittens would be expected to have lower morbidity and mortality rates than hand-raised kittens and are likely to have normal social development. Most lactating queens readily accept additional kittens. Ideally, orphaned kittens should be fostered on to a queen whose own kittens are less than 2 weeks older to avoid competition due to size discrepancy.
Partial hand-raising is a strategy employed when a foster queen is available but has older kittens of her own, or when there are too many kittens for the foster queen to raise alone. Partial hand-raising can also be used when a queen is unable to nutritionally support her own litter due to illness, poor milk production, or when the litter is larger than average for the breed. In those situations, it may be possible to leave the kittens with the queen for maternal care and socialization and provide supplemental feeding by hand.

Queen’s milk provides complete nutrition for kittens, as well as non-nutritive factors that enhance digestion, development and immunity (both systemic and local). The nutrient composition of queen’s milk changes during lactation so that provision of nutrients to nursing kittens adapts to support normal growth and development. 17 For example, as lactation progresses, energy, protein, lactose, calcium and phosphorus levels increase. The unique features of queen’s milk mean that milk of other species is not suitable for nursing kittens without considerable adjustment. For example, cow’s milk is lower in protein and fat than queen’s milk, and is a poor source of taurine. Goat’s milk has no nutritional benefits compared with cow’s milk. 2
Hand-raising
What to feed and how much?
If a lactating foster queen is not available, orphaned kittens should be fed a commercial milk replacer that approximates the composition of queen’s milk. Homemade formulas are best reserved for short-term or emergency use. In addition to appropriate nutrient composition, milk replacers should be highly digestible (>90%) to avoid the need to feed large quantities and to avoid diarrhea. 2 The osmolality should approximate that of queen’s milk (329 mOsm/kg) to prevent hyperosmolar diarrhea and delayed gastric emptying. 2 Milk replacers with inadequate arginine content have been associated with cataract formation. 18 The manufacturer’s directions for mixing, storage and feeding quantities should be followed. Strict hygiene is necessary, and if milk replacer must be reconstituted, no more than a 24 h supply should be prepared at a time. The reconstituted milk replacer can be divided into individual feedings and refrigerated until use. Milk replacer left at room temperature should be discarded after 1 h.
The daily energy and water requirements of orphaned kittens should be calculated as accurately as possible and adjusted as needed (see box). Kittens should be weighed q12h in the first 2 weeks of life, q24h until weaned, and then once or twice weekly until about 8 weeks of age to ensure nutrition is adequate to support growth. If the kitten has been starved or is inappetent, feedings for the first 24–48 h should be at 50% of the calculated daily requirement. Over the next few feedings, the volume can be increased. The amount of milk replacer fed should be adjusted to achieve normal weight gain (10–15 g/day).
Typically, faster growth rates are seen in kittens fed commercial milk replacer compared with kittens receiving queen’s milk. 18 Poor weight gain or failure to thrive should prompt the care-giver to seek veterinary attention. The feeding plan should be reviewed to ensure the quality of the milk replacer is adequate, the product is being prepared and stored properly, and the feeding amounts are appropriate. It may be necessary to change to a different product since the commercially available milk replacers differ in nutrient composition. The nutrient content of some commercial milk replacers compared with queen’s milk has been published. 2

Feeding methods
Various feeding methods for orphans have been used, including nursing bottles and orogastric tubes. Spoons and eye droppers should be avoided as aspiration may occur. The choice of method will be dependent on factors such as available equipment, the number and vitality of the kittens, and the experience of the care-giver. Hypothermic kittens (rectal temperature less than 35°C [95°F]) should be slowly rewarmed before feeding as hypothermia causes ileus and increases the risk of aspiration pneumonia. All equipment used for feeding orphaned kittens should be properly cleaned after use by washing well or boiling. Milk replacer should be warmed to 35–38°C (95–100°F) by immersing the container in a warm water bath. Microwaving milk replacer may cause overheating or uneven heating.
Bottle feeding satisfies the kitten’s need to suckle (Figure 6). Vigorous orphans with a good suck reflex may be bottle-fed in sternal recumbency with the head elevated, simulating a normal nursing position. The slit in the nipple should be made large enough to allow a drop of milk to slowly form when the bottle is held upside down. A drop of milk is expressed from the bottle on to the kitten’s tongue to help initiate feeding. Milk should never be forced out of the bottle while it is in the kitten’s mouth, to avoid aspiration. Care should be taken to ensure no air is ingested while feeding. Bottle-fed kittens control their own food intake, which can prevent over-feeding. However, bottle feeding is a time-consuming method, especially when more than one kitten must be fed.
Figure 6.
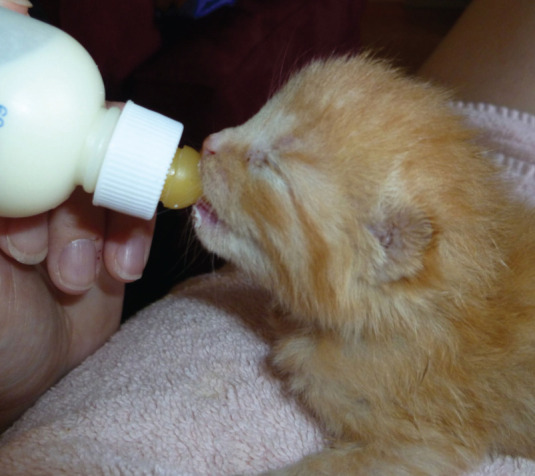
Orphaned kittens with a good suck reflex can be bottle-fed. Courtesy of Chantal Bourdon
Figure 7.
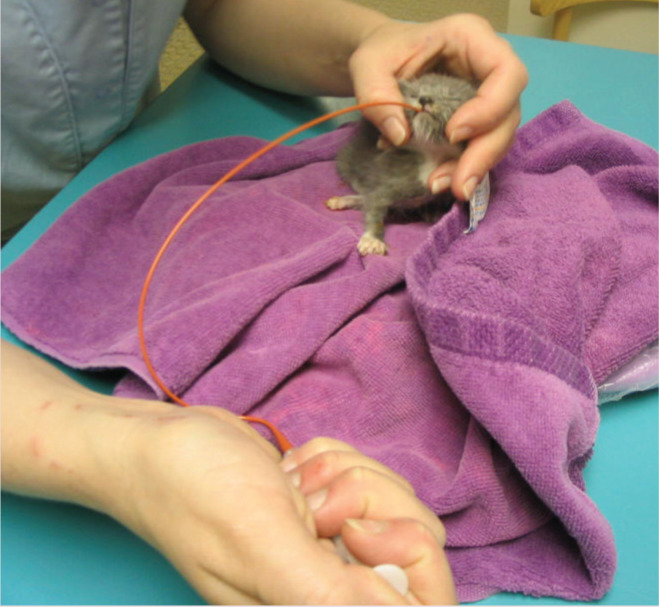
Kittens can be easily fed with a gastric tube. They should be positioned in sternal recumbency with the head elevated to simulate a normal nursing position
Normothermic, well-hydrated kittens can also be fed with an orogastric tube (see box). 19 Tube feeding is more efficient than bottle feeding if more than one kitten must be hand-raised.
Frequency of feeds
The daily volume of milk replacer should be divided into feeds given q2–4h during the first week of life, and then q4–6h until weaning. Common mistakes to avoid include feeding cold milk replacer, feeding too fast and over-feeding.
Elimination
Kittens less than 3 weeks of age cannot eliminate urine and feces voluntarily. Defecation and micturition is stimulated by the queen licking the kitten’s perineum (Figure 8). Orphans younger than 3 weeks of age must have the anogenital area stimulated after every feeding to induce defecation and micturition.
Figure 8.

Until about 3 weeks of age, elimination of feces and urine must be stimulated by the queen licking the kitten’s perineum
Hematuria may be associated with urinary tract infection and pigmenturia with neonatal isoerythrolysis. Dark yellow urine may indicate dehydration as normal urine specific gravity is typically 1.020 or lower in the first few weeks of life. Kittens fed queen’s milk or milk replacer have pasty yellow to light brown stools. Kittens eating solid food have soft formed stools.
Management of common problems
Diarrhea is a common problem in kittens fed milk replacer and may be due to over-feeding or an inappropriate milk replacer. Kittens with diarrhea should always be examined for intestinal parasites and treated with a broad spectrum dewormer. Diarrhea can be treated by temporarily reducing the amount fed and by diluting the formula by 50% with water or oral electrolyte solution for a few feedings. Anecdotally, probiotics labeled for use in cats have been used in unweaned kittens to treat diarrhea. Kittens with diarrhea must be kept clean and monitored for perineal dermatitis and rectal prolapse.
Constipation is another common problem in kittens fed milk replacer. It may be associated with feeding a product that is too concentrated so that the kitten’s daily water needs are not being met. It may also be a consequence of inadequate perineal stimulation after feedings in kittens that are not yet able to eliminate voluntarily. Treatments include small volume warm water or lactulose enemas and oral lactulose. Stimulant laxatives and liquid paraffin (mineral oil) are not recommended.
If diarrhea or constipation is persistent, a different milk replacer should be tried.
Weaning
Even the best milk replacers have the potential to cause problems, so orphaned kittens should be weaned as soon as practical. At 3–4 weeks of age, kittens can be taught to drink milk replacer from a shallow saucer (Figure 9). Many kittens learn to ingest food by first stepping it in (Figure 10) and cleaning themselves, so the weaning process is often messy!
Figure 9.

Starting at 3–4 weeks of age, kittens can be taught to drink milk replacer from a saucer
Figure 10.
Kittens first learn to ingest food by walking in it and licking it from their fur. Courtesy of Chantal Bourdon
Solid food can be introduced by mixing a small amount of canned food with milk replacer and offering it for 30 mins at a time, several times a day. Food warmed to 38°C (100°F) may be more acceptable. Once the kitten has learned to eat from a saucer, the amount of milk replacer fed can be slowly decreased until only solid food is being ingested. The weaning food should be a highly digestible canned diet formulated for kitten growth that is offered several times daily (Figure 11). Flat dishes, such as saucers or paper plates, are the easiest for kittens to eat from. By 5–6 weeks of age, kittens are able to chew dry food and should be eating 30% or more of their daily caloric requirement in solid food. A small amount of a dry kitten growth diet can be fed ad libitum in addition to meals of canned diet. Clean fresh water in a wide shallow bowl should also be provided. Weaning is usually completed by 6–9 weeks of age.
Figure 11.

The first solid food for weaning should be a canned diet formulated for kitten growth and development
Litter should be made available from about 4 weeks of age. A litter box with low sides and 2.5 cm (1 inch) or less of non-clumping litter or shredded newspaper should be provided (Figure 12). After each feeding, as well as after play time or naps, the kitten should be placed in the litter box to encourage elimination. Confining kittens to a smaller space during this time period may facilitate the process of litter training. The litter box should be kept clean, and situated away from food and water.
Figure 12.

A shallow litter box with shredded newspaper or non-clumping litter should be provided from about 4 weeks of age. Courtesy of Chantal Bourdon
Weaning is a stressful period in a kitten’s life as three factors come together: independent feeding, exposure to the greater world and waning maternal antibodies. This is why weaning is a high-risk time for morbidity and mortality as outbreaks of diarrhea and disease are common. Attention to nutrition and wellness care, as well as good husbandry and hygiene, is important.
Post-weaning, kittens should continue to gain about 100 g/week until 5 months of age, although male kittens will start to be larger than female kittens. Impaired growth rate is a very common sign of illness or malnutrition and should always be investigated.
Footnotes
Funding: The author received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this article.
The author does not have any potential conflicts of interest to declare.
Key Points
Under normal circumstances, kittens are dependent on the queen for nutrition and care from birth to weaning.
Veterinarians may be asked to evaluate and treat kittens that have been orphaned through death or illness of the queen, rejection by the queen or abandonment. Such kittens require specific care and nutritional support until weaning.
If a foster queen is not available, the care-giver must replace physiologic needs normally provided by the queen, such as warmth, nutrition, elimination, sanitation and social stimulation.
Successful management can be accomplished with knowledge of the particular needs of these often-fragile pediatric patients.
Veterinarians and veterinary team members should be prepared to provide care-givers with the knowledge and support they require.
References
- 1. Macintire DK. Pediatric intensive care. Vet Clin North Am Small Anim Pract 1999; 29: 971–988. [DOI] [PubMed] [Google Scholar]
- 2. Gross KL, Becvarova I, Debraekeleer J. Feeding nursing and orphaned kittens from birth to weaning. In: Hand M, Thatcher C, Remillard R, Roudebush P, Novotny R. (eds). Small animal clinical nutrition. 5th ed. Topeka, KS: Mark Morris Institute, 2010, pp 415–427. [Google Scholar]
- 3. Overall K, Rodan I, Beaver B, Carney H, Crowell-Davis S, Hird N, et al. Feline behavior guidelines from the American Association of Feline Practitioners. J Am Vet Med Assoc 2005; 227: 70–84. [DOI] [PubMed] [Google Scholar]
- 4. Little SE. Pediatrics. In: Little SE. (ed). The cat: clinical medicine and management. St Louis: Elsevier Saunders, 2012, pp 1228–1251. [Google Scholar]
- 5. Moik K, Kienzle E. Birth weight and postnatal growth of pure-bred kittens. Br J Nutr 2011; 106 suppl 1: S32–34. [DOI] [PubMed] [Google Scholar]
- 6. Sparkes AH, Rogers K, Henley WE, Gunn-Moore DA, May JM, Gruffydd-Jones TJ, et al. A questionnaire-based study of gestation, parturition and neonatal mortality in pedigree breeding cats in the UK. J Feline Med Surg 2006; 8: 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Little S. Feline pediatrics: how to treat the small and the sick. Compend Contin Educ Vet 2011; 33: E1–E6. [PubMed] [Google Scholar]
- 8. Siak M, Burrows A. Flea control in cats. New concepts and the current armoury. J Feline Med Surg 2013; 15: 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Richards JR, Elston TH, Ford RB, Gaskell RM, Hartmann K, Hurley KF, et al. The 2006 American Association of Feline Practitioners Feline Vaccine Advisory Panel report. J Am Vet Med Assoc 2006; 229: 1405–1441. [DOI] [PubMed] [Google Scholar]
- 10. Casal M, Jezyk P, Giger U. Transfer of colostral antibodies from queens to their kittens. Am J Vet Res 1996; 57: 1653–1658. [PubMed] [Google Scholar]
- 11. Yamada T, Nagai Y, Matsuda M. Changes in serum immunoglobulin values in kittens after ingestion of colostrum. Am J Vet Res 1991; 52: 393–396. [PubMed] [Google Scholar]
- 12. Claus MA, Levy JK, MacDonald K, Tucker SJ, Crawford PC. Immunoglobulin concentrations in feline colostrum and milk, and the requirement of colostrum for passive transfer of immunity to neonatal kittens. J Feline Med Surg 2006; 8: 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levy J, Crawford P, Collante W, Papich M. Use of adult cat serum to correct failure of passive transfer in kittens. J Am Vet Med Assoc 2001; 219: 1401–1405. [DOI] [PubMed] [Google Scholar]
- 14. MacDonald K, Levy JK, Tucker SJ, Crawford PC. Effects of passive transfer of immunity on results of diagnostic tests for antibodies against feline immunodeficiency virus in kittens born to vaccinated queens. J Am Vet Med Assoc 2004; 225: 1554–1557. [DOI] [PubMed] [Google Scholar]
- 15. Crawford P, Levy J, Werner L. Evaluation of surrogate markers for passive transfer of immunity in kittens. J Am Vet Med Assoc 2006; 228: 1038–1041. [DOI] [PubMed] [Google Scholar]
- 16. Crawford P, Hanel R, Levy J. Evaluation of treatment of colostrum-deprived kittens with equine IgG. Am J Vet Res 2003; 64: 969–975. [DOI] [PubMed] [Google Scholar]
- 17. Adkins Y, Zicker SC, Lepine A, Lonnerdal B. Changes in nutrient and protein composition of cat milk during lactation. Am J Vet Res 1997; 58: 370–375. [PubMed] [Google Scholar]
- 18. Remillard RL, Pickett JP, Thatcher CD, Davenport DJ. Comparison of kittens fed queen’s milk with those fed milk replacers. Am J Vet Res 1993; 54: 901–907. [PubMed] [Google Scholar]
- 19. Macintire DK. Pediatric fluid therapy. Vet Clin North Am Small Anim Pract 2008; 38: 621–627, xii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kirk C. New concepts in pediatric nutrition. Vet Clin North Am Small Anim Pract 2001; 31: 369–392. [DOI] [PubMed] [Google Scholar]



