Abstract
Practical relevance:
Diabetes mellitus is a common endocrine disorder in feline practice, affecting approximately 1 in 200 cats. The majority of diabetic cats have type 2 diabetes mellitus, which results from a combination of peripheral insulin resistance and a progressive reduction in insulin production.
Clinical challenges:
While usually easy to diagnose, management of diabetes mellitus presents a number of challenges for practitioners and clients alike. Practitioners must decide on diet, insulin type and dose, monitoring method and intensity, and concomitant therapy, which will vary based on individual patient and client needs, and geographic location. Practitioners may also encounter patients with diabetic ketoacidosis or other diabetic complications, and patients with multiple concurrent diseases. Clients may be challenged by the substantial time and financial commitment involved in owning a diabetic cat.
Audience:
Understanding the pathophysiology, optimal treatment protocols and current goals of diabetes management will benefit practitioners managing diabetic cats. This article reviews the most current management plans for feline diabetics. It places particular emphasis on best practice for achieving diabetic remission, which is an attainable goal in the majority of newly diagnosed diabetic cats.
Evidence base:
The information in this article is drawn from the recent human and veterinary literature, including prospective and retrospective studies. The body of prospective clinical data on the use of newer, long-acting insulins (glargine and especially detemir) in cats is limited, but growing.
Prevalence and types of diabetes mellitus
Diabetes mellitus is one of the most common endocrinopathies in feline medicine. In 1990, a retrospective study from 17 North American university hospitals found the prevalence of feline diabetes to be 1 in 400 cases (0.25%); age, male gender, neuter status and increasing body weight were predisposing factors. 1 A retrospective database study found that, by 1999, the prevalence of feline diabetes in North America had increased to 1.24 in 100 cases (1.24%); age, male gender, increasing body weight (males only) and purebred status (females only) were predisposing factors. 2 A 2003 study in feline-only primary accession practices and a 2007 study from the United Kingdom both found a prevalence of feline diabetes of 1 in approximately 200 cases (0.5%).3,4 In 2007, the prevalence of feline diabetes in two feline-only practices in Australia was 7.4 in 1000 cases (0.74%), with a higher prevalence in Burmese cats (2.24%). 5

In the USA, Maine Coon, Russian Blue and Siamese breeds are overrepresented. 6
Pathogenesis of feline diabetes mellitus
The majority of diabetic cats in Western countries have type 2 diabetes mellitus, with insulin resistance plus β-cell dysfunction leading to a relative and, eventually, an absolute deficiency in insulin production. 8
Insulin resistance
Insulin resistance is a condition in which normal amounts of insulin produce a subnormal glucose-lowering response. There are several causes of insulin resistance in cats, as outlined below.
Obesity
Obese cats are nearly four times more likely to develop type 2 diabetes mellitus than ideal weight cats (Figure 1). 9
In ideal weight cats allowed to become obese there is a >50% reduction in insulin sensitivity. 10
Obesity appears to be the most important acquired factor in the development of insulin resistance. 11
Short-term moderate weight gain produces similar adverse effects on mean postprandial blood glucose and insulin concentrations as feeding a diet with 50% of energy from carbohydrate. 12
Insulin resistance induced by obesity appears to be reversible with weight loss. 13
Figure 1.
Obesity and older age (>8 years of age) are the most important risk factors for diabetes
Genetic predisposition
Burmese cats have a higher risk of diabetes mellitus than non-pedigree cats in Australia, New Zealand and the United Kingdom, and this might be associated with a genetic predisposition towards reduced insulin sensitivity (Figure 2).4,5,14–16
Data from recent studies suggest that Burmese cats have a genetic predisposition towards dysregulated lipid metabolism.17–19
Maine Coon, Russian Blue and Siamese cats, as well as domestic shorthair and longhair cats, have a higher prevalence of diabetes mellitus than the general feline population in the USA. 8
Figure 2.

Burmese cats are predisposed to diabetes, and the highest risk is in overweight senior Burmese cats
Physical inactivity
Indoor cats and inactive cats are at increased risk of developing diabetes mellitus, independent of obesity (Figure 3).4,5
Figure 3.

Indoor, inactive cats have an increased risk of diabetes
Male gender
Several studies have shown that male or male neutered cats are more likely to develop diabetes mellitus than female cats.2–4,6,17
Male gender is a risk factor in Burmese cats in the UK, and likely also in Australia.5,14
Increasing age
Medications
Beta-cell dysfunction
It is the combination of insulin resistance plus β-cell dysfunction that gives rise to type 2 diabetes mellitus in cats. Initial β-cell dysfunction is caused by a variety of factors leading to reduced insulin secretion and hyperglycemia. The resultant hyperglycemia is then toxic to the pancreatic β-cells, reducing insulin secretion further.
Initial hyperglycemia
In both healthy cats and cats predisposed to diabetes, the pancreas initially compensates for insulin resistance by increasing insulin output via hyperplasia and hypertrophy of β-cells. 20 However, cats that are predisposed to diabetes eventually develop β-cell dysfunction, and thus fail to compensate for insulin resistance with greater insulin secretion. Failure to produce increased insulin in the face of insulin resistance leads to chronic, persistent hyperglycemia.
Why do β-cells fail in animals predisposed to diabetes mellitus? The theory of ‘β-cell exhaustion’ is likely oversimplified and incompletely explains the pathogenesis of feline diabetes, especially in the initial stages of β-cell dysfunction. 20 Damage by reactive oxygen species in early and ongoing disease, pancreatic amyloid or toxic oligomer accumulation, and autoimmune destruction are all hypothesised to contribute to the early stages of β-cell failure, with a later contribution from glucose toxicity once blood glucose increases. 20 Pancreatitis is common in cats and may contribute to loss of β-cells. This is not severe enough to cause diabetes in most cats, but in a small minority pancreatitis appears to be associated with diabetes. 21
Glucose toxicity
Once marked β-cell dysfunction occurs, chronic and persistent hyperglycemia ensues. This hyperglycemia is, in turn, toxic to the already dysfunctional β-cells, causing further dysfunction and apoptosis. 22 In humans, this is a result of hyperglycemia-induced disruption of the endoplasmic reticulum, leading to abnormal protein folding and β-cell apoptosis. 23 Resolution of glucose toxicity is the likely explanation for why tight glycemic control increases the probability of remission in diabetic cats. 22
Diagnosis of feline diabetes mellitus
Recent data indicate that the upper cut-point for normal blood glucose concentration in cats is 6.5 mmol/l (117 mg/dl), when measured after overnight hospitalization and withholding of food for 18–24 h. 24 Persistent values above this should be considered pre-diabetic or diabetic. 24
There is, however, currently no accepted lower cut-point for diabetes in cats, and values ≥10–16 mmol/l (180–288 mg/dl) are reported as diagnostic for diabetes in this species.25,26 In a recent study of diabetic cats in remission, 10 mmol/l (180 mg/dl) was used as the lower cut-point for diabetes, and cats with blood glucose ≥7.5 mmol/l (135 mg/dl) and <10 mmol/l (180 mg/dl) were found to be at significantly greater risk of diabetic relapse, consistent with a classification of pre-diabetes (S A Gottlieb, J S Rand and R D Marshall, unpublished data, 2013). Blood glucose concentrations below the renal threshold (approximately 14–16 mmol/l [250–290 mg/dl]) but above the cut-point for diabetes are unlikely to result in clinical signs, and should be classed as subclinical diabetes.27,28 Cats with persistent blood glucose concentrations >6.5 mmol/l (117 mg/dl) require management with a low carbohydrate diet, weight loss, and potentially insulin sensitizers such as darglitazone to prevent deterioration to clinical diabetes. 29
Stress hyperglycemia is an important confounding factor for diagnosis of diabetes and pre-diabetes in some cats. Stress hyperglycemia is usually (but not always) lower than hyperglycemia associated with clinical diabetes (that is, the renal threshold of 14–16 mmol/l [250–290 mg/dl]). Stress-induced hyperglycemia and glucosuria is not persistent over time, and is not accompanied by clinical signs of diabetes. However, concomitant medical conditions such as chronic kidney disease or hyperthyroidism may cause similar signs, confusing diagnosis. Ruling out stress hyperglycemia can be accomplished by measurement of blood and/or urine glucose at home, by hospitalization for up to 24 h or until the stress hyperglycemia resolves (for many cats, a repeat glucose measurement approximately 4 h later is normal), or by measurement of plasma fructosamine.
Diagnosis of clinical diabetes mellitus is made when appropriate signs are accompanied by persistent hyperglycemia and glucosuria. Clinical signs most often include polyuria, polydipsia and weight loss; cats may present with polyphagia, or with inappetence and anorexia, particularly if they are ketoacidotic. 30 In a retrospective study on clinicopathologic abnormalities in 104 diabetic cats, the median pre-treatment blood glucose was 26.6 mmol/l (478 mg/dl) (reference interval 3.9–8.3 mmol/l; 70–150 mg/dl), with a study range of 13–58.7 mmol/l (234–1056 mg/dl). 30 In contrast to stress hyperglycemia, diabetic hyperglycemia is persistent; once hyperglycemia exceeds the renal threshold, glucosuria also will be persistent, and accompanied by typical clinical signs of diabetes.
Elevations in plasma fructosamine (>400 µmol/l), glycated hemoglobin (HbA1c) (reference interval depends on assay used) or plasma betahydroxybutyrate (>1 mmol/l [10 mg/dl]), or the presence of ketonemia or ketonuria can help to confirm diabetes mellitus in a cat with clinical signs of diabetes. Plasma fructosamine, or protein-bound blood glucose, is roughly proportional to the average blood glucose over the preceding 7–14 days. It is commonly used to document persistent hyperglycemia, and to roughly estimate the magnitude of that hyperglycemia. It does not provide information on hourly or daily blood glucose fluctuations. 31 Normal fructosamine does not rule out diabetes if blood glucose is persistently <20 mmol/l (360 mg/dl).
Changing goals in the treatment of feline diabetes mellitus
The goals for treatment of clinical diabetes mellitus have changed in recent years. Traditionally, the goal was to obtain reasonable glycemic control to improve clinical signs of polyuria, polydipsia and weight loss. This was usually pursued using insulin therapy, possibly dietary therapy, and variable blood glucose monitoring, most often with intermittent in-clinic blood glucose curves every 1–2 weeks.
Recently, it has been shown that diabetic remission – that is, euglycemia without insulin therapy or oral hypoglycemic drugs for at least 2 weeks – is achievable for 20% to 80–90% or more of feline diabetics, depending on management and time after diagnosis.8,32–36 Remission is most likely when euglycemia is achieved soon after the diagnosis of diabetes is made; in part, because this breaks the cycle of hyperglycemia-induced β-cell dysfunction leading to insulin deficiency and eventual β-cell loss. 35
Diabetic remission improves quality of life by improving cats’ health, and reducing owners’ time and financial commitments. Therefore, remission is currently the practitioner’s primary goal in the management of feline diabetes.
Insulin therapies
Brief overview of insulin types
Human and veterinary insulins are categorized both by onset and duration of action when given parenterally, and by protein source. Based on onset and duration of action, insulins are short-acting, intermediate-acting and long-acting. Short-acting insulins are generally used in-hospital for intensive glycemic control, while intermediate- and long-acting insulins are used in-hospital and at home for long-term daily management of diabetes. By protein source, insulins are human, biosynthetic ‘human’ analogue (synthetic insulin that is altered from the natural form but retains biological function in humans), bovine or porcine. Bovine insulin is most similar to endogenous feline insulin based on amino-acid sequence. 38
Traditionally, diabetic cats have been treated with a variety of veterinary or human insulins. 39 Veterinary insulins include porcine lente (Vetsulin, Caninsulin; Schering-Plough-Intervet Animal Health/MSD Animal Health), bovine-porcine protamine zinc insulin (PZI-VET; Idexx Laboratories, but no longer available; various compounding pharmacies) and, more recently, protamine zinc human recombinant insulin (ProZinc; Boehringer Ingelheim Vetmedica). 39 Human insulins or insulin analogues reported being used in cats include human origin lente (no longer available), neutral protamine Hagedorn (isophane, NPH, various manufacturers) and, more recently, glargine (Lantus; Sanofi-Aventis) and detemir (Levemir; Novo Nordisk).8,38
In the UK and some other countries, veterinarians are legally required to use insulin registered for veterinary use as their first-line therapy.
Glargine
Glargine (Lantus; Sanofi-Aventis) is a human analogue insulin with modifications that provide variable solubility at different pHs (Figure 4). Glargine is soluble at a pH of 4.0, at which it is supplied and stored, but in the neutral pH of the body’s blood or subcutaneous tissues it forms microprecipitates, facilitating slow absorption after injection. This results in rapid onset and long duration of action.
Figure 4.
Glargine (Lantus) is a long-acting analogue insulin associated with high remission rates in cats
Several studies have reported outcomes using insulin glargine in healthy and diabetic cats.
Pharmacokinetic and pharmacodynamic studies
Glargine is sometimes described as a ‘peakless’ insulin. However, ‘peakless’ does not mean an absence of a nadir; instead, it is a term that refers to glargine’s glucose utilization rate (the amount of intravenous glucose required to maintain a constant blood glucose concentration after subcutaneous insulin administration). 40 Therefore, it is more appropriate to consider duration of action and nadir when discussing the pharmacodynamics of insulin glargine in cats.
In healthy cats administered one injection of glargine (0.5 U/kg) subcutaneously, mean time to onset of action was 1.3 ± 0.5 h, time to peak action was 5.3 ± 3.8 h, and time to end of action was 11.3 ± 4.5 h. 41 However, variability between cats was high, and day-to-day variability in individual cats has not been studied.
In healthy cats given glargine at either 0.5 U/kg subcutaneously once or 0.25 U/kg subcutaneously twice in 24 h, glargine had a long duration of action of 24 h or more in many cats. However, only the higher 0.5 U/kg dose reliably suppressed blood glucose below baseline in all cats at 12 h, suggesting that twice-daily dosing of glargine may provide better glycemic control than once-daily dosing. 40
Clinical studies
In the first published study using glargine in newly diagnosed and previously treated diabetic cats (n = 13) on a low carbohydrate, high protein diet (Purina DM; Nestlé Purina), once-daily administration at a starting dose of 0.5 U/kg was similarly effective to twice-daily porcine lente (Vetsulin) at 0.5 U/kg. 42 Remission rate for the glargine-treated cats was low, at 17%, likely due to once-daily administration of glargine, and inclusion of diabetic cats with poor control. Poorly controlled diabetics are more likely to have progressed through the stages of β-cell dysfunction towards irreversible glucose toxicity, making remission impossible, and/or have other specific types of diabetes and not type 2.
In two additional small studies involving newly diagnosed and previously treated cats with poor control (n = 5, 32 n = 1243), cats treated twice daily with glargine at a starting dose of 0.5 U/kg achieved low (17%) 32 to moderate (40%) 43 remission rates. In the study with the lower remission rate, only half the cats were fed a low carbohydrate diet. 32
In a hallmark study involving newly diagnosed diabetic cats (n = 24), significantly higher remission rates were achieved in cats treated twice daily with glargine (100%) than with PZI (38%) or porcine lente (25%). All cats were fed a high protein, low carbohydrate diet (Purina DM) and, in contrast to previous studies, had frequent blood glucose curves measured and insulin dose adjustments. 33 Use of twice-daily insulin, inclusion of only newly diagnosed diabetics, diet, and tight monitoring and glycemic control likely contributed to high glargine remission rates in this study. While all insulin types caused occasional subclinical hypoglycemia (blood glucose <3 mmol/l; 54 mg/dl), no glargine-treated cats developed clinical hypoglycemia, compared with one cat each on lente and PZI. It is potentially safer to aim for tight glycemic control with glargine than PZI or lente insulin. In humans, glargine is associated with fewer episodes of clinical hypoglycemia than NPH.44,45
In a second hallmark study, high remission rates were achieved in diabetic cats (n = 55) previously treated predominantly with porcine lente insulin twice daily and a low carbohydrate diet. Cats not in remission were changed to a protocol of twice-daily glargine and intensive home blood glucose monitoring, with frequent dose adjustment by the owners using a dosing protocol aimed at achieving euglycemia. 35 Overall remission rate was 64%, with a significantly higher remission rate for cats started on the protocol within 6 months of diagnosis (84%) compared with later than 6 months after diagnosis (35%). Positive predictors of remission included implementation within 6 months of diagnosis of a protocol aimed at achieving euglycemia (including intensive home monitoring of blood glucose and use of an insulin dosing protocol), a lower maximal insulin dose, and treatment with corticosteroids within 6 months prior to diagnosis of diabetes. Despite subclinical hypoglycemia being detected frequently, clinical hypoglycemia was rare (1 in 55 cats) and mild.
Collectively, these studies suggest that the probability of remission is increased with: twice-daily compared with once-daily glargine administration; twice-daily glargine compared with twice-daily PZI and porcine lente; use in newly diagnosed diabetic cats compared with previously diagnosed and poorly controlled cats; early implementation of treatment; and frequent blood glucose monitoring with use of an insulin dosing protocol aimed at achieving euglycemia.33,40,42
Detemir
Detemir (Levemir; Novo Nordisk) is a human analogue insulin engineered with modifications that allow it to bind albumin with high affinity in the subcutaneous and intravascular spaces, prolonging the insulin’s absorption (Figure 5).38,46 This prolonged absorption gives detemir a long and steady duration of action and less variability in biological activity. 38
Figure 5.

Detemir (Levemir) is a long-acting analogue insulin associated with high remission rates in cats
A small number of studies have investigated insulin detemir in both healthy and diabetic cats.41,47
Pharmacokinetic and pharmacodynamic studies
In healthy cats given detemir at 0.5 U/kg subcutaneously, mean time to onset of action was 1.8 ± 0.8 h, time to peak action was 6.9 ± 3.1 h and time to end of action was 13.5 ± 3.5 h. 41 The pharmacokinetic properties of detemir were generally very similar to glargine. Detemir lasted about 7 h longer than glargine in 3/10 healthy cats in this study, suggesting that there could be clinically relevant differences in duration of action in some cats. If this finding is repeatable in studies on diabetic cats, detemir may be a good insulin choice for cats with inadequate duration of action of glargine.
Clinical studies
To the authors’ knowledge, there has been only one clinical study on the use of insulin detemir in diabetic cats. 47 Using the same protocol as the glargine study, similar remission rates to glargine were achieved in previously treated diabetic cats (n = 18) fed a low carbohydrate diet. 35 Cats not in remission were changed to a protocol of twice-daily detemir and intensive home blood glucose monitoring with frequent dose adjustments aimed at achieving euglycemia.
Cats had an overall remission rate of 67%, with a trend towards a higher remission rate for those that entered the protocol within 6 months of diagnosis (81%) versus later than 6 months after diagnosis (42%). 47 Cats on detemir required a lower maximal insulin dose than those on glargine. Overall, however, the starting dose, outcomes and pharmacodynamics of detemir were very similar to those of glargine.
Given the paucity of clinical data on the use of detemir in diabetic cats, and the similarities in pharmacokinetic and pharmacodynamic data between glargine and detemir in healthy cats, current recommendations are to utilize and monitor detemir in cats as you would glargine.
Insulin degludec
Degludec (Novo Nordisk) is an ultra-long-acting basal insulin with a longer duration of action than glargine or detemir in humans. 48 At the time of publication, degludec is approved for use in human diabetics in Japan, the European Union and Mexico (Novo Nordisk company announcement, 10 February 2013). It is also being trialled combined with a fixed dose of liraglutide, a GLP-1 receptor agonist. Currently there are no published data on the use of degludec in cats.
How to use glargine and detemir in diabetic cats
To begin a diabetic cat on glargine or detemir:
Start with a dose of 0.5 U/kg of ideal body weight q12h if the blood glucose is >20 mmol/l (360 mg/dl), and 0.25 U/kg q12h if the blood glucose is lower. Do not start with a total dose higher than 3 U/cat, regardless of body weight. All doses should be based on ideal body weight in kilograms.
Monitor the blood glucose q3–4 h for the first 3 days of insulin therapy, to assess for hypoglycemia. Monitoring before each insulin injection, especially the evening injection, is important, because for many cats blood glucose is lower at night than in the morning. Additional samples can then be spaced q2–4h, if monitoring in-hospital. If home monitoring, samples should be taken before each insulin injection, with additional samples when the owner comes home from work and before they go to bed (Figures 6–8).
If no blood glucose monitoring is being performed in the first 3 days after starting insulin, begin with 1 U/cat and increase based on blood glucose curves and dose adjustment protocols.
Figure 6.
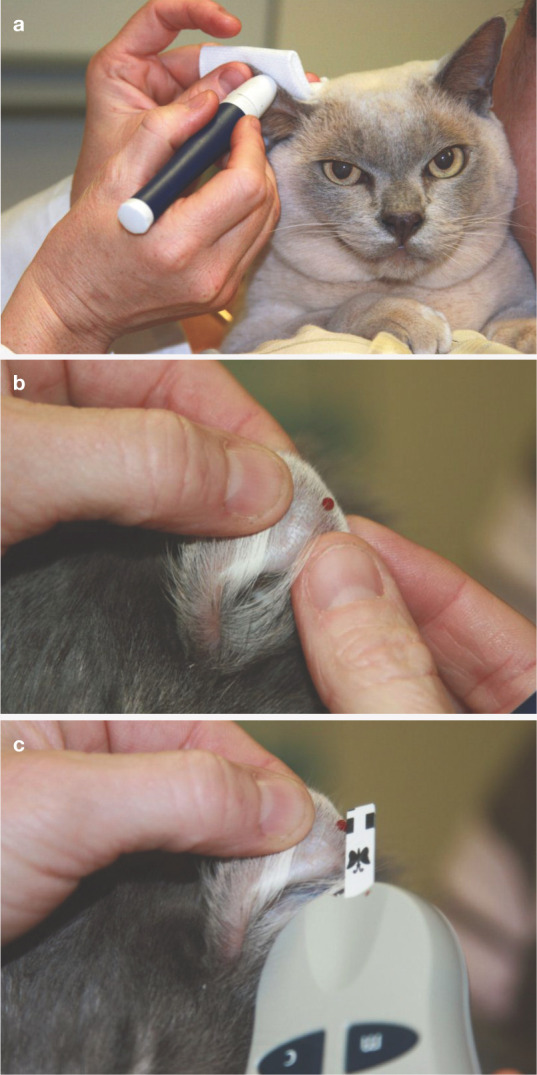
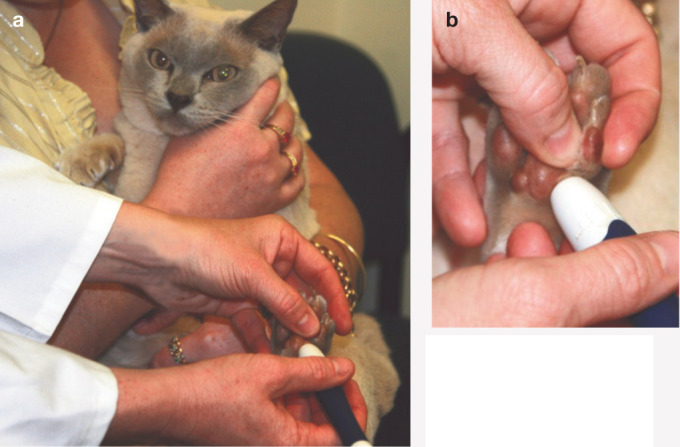
(a) Use of a lancet (AlphaTRAK; Abbott) to obtain blood from the pinna of a cat. (b) Drop of blood on the ear margin. (c) Measuring blood glucose using a meter calibrated for feline blood (AlphaTRAK; Abbott). Such meters, which require only a small volume of blood (eg, 0.3 μl), facilitate successful home blood glucose monitoring in cats. Courtesy of M Reeve-Johnson
Figure 7.
(a,b) Use of a lancet to obtain a blood sample from the edge of a foot pad. Courtesy of M Reeve-Johnson
Figure 8.
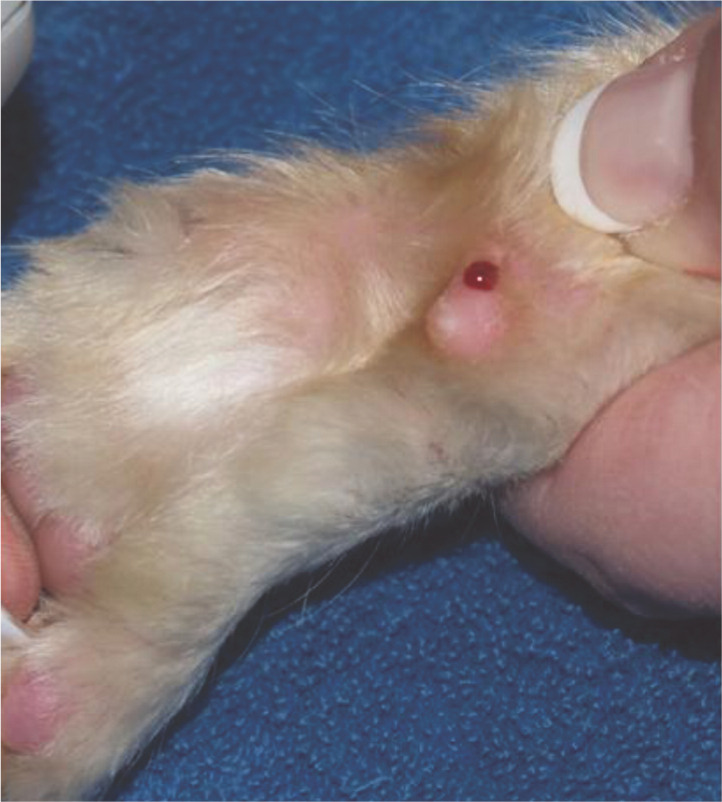
A drop of blood being obtained from the pisiform pad following use of a lancing device designed for home monitoring. Reproduced with permission from S. Ford. In: Rand JS (ed). Clinical Endocrinology of Companion Animals. Wiley-Blackwell, 2013; 16: 169–190

In the first week of therapy:
Do not increase the insulin dose in the first week of therapy unless there is little response to insulin after 2–3 days and blood glucose is being monitored daily, as discussed above.
Decrease the insulin dose if the pre-insulin blood glucose is ≤10 mmol/l (180 mg/dl), nadir blood glucose is <6 mmol/l (108 mg/dl), or clinical signs of hypoglycemia occur.
The nadir, or lowest blood glucose measurement, can be found or approximated by checking a pre-insulin blood glucose, and then checking blood glucose q3–4 h until the next insulin injection.
Thereafter:
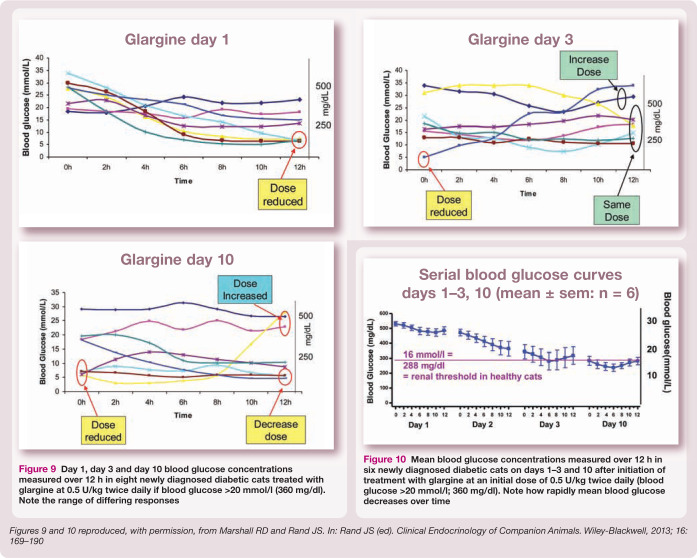
Perform weekly at-home or in-hospital blood glucose curves, with blood glucose measurement q3–4 h for 12 h or longer. Adjust insulin dose weekly as indicated by dose adjustment protocols (Figures 9 and 10).
Alternatively, once the approximate timing of the nadir is known, owners may measure blood glucose two or three times each day at home. Measurements should be taken pre-insulin in the morning, around the time of the nadir, and pre-insulin in the evening. Insulin can then be adjusted every week, as indicated by dose adjustment protocols
Continue for approximately 4–6 months, with weekly assessment of glycemic control and dose adjustment, if indicated. The majority of cats will be regulated or in remission by that time.
- Insulin dose adjustment is based on:
- – Daily or weekly serial blood glucose measurements;
- – Food and water intake;
- – Clinical signs of hypoglycemia.
Figure 9.
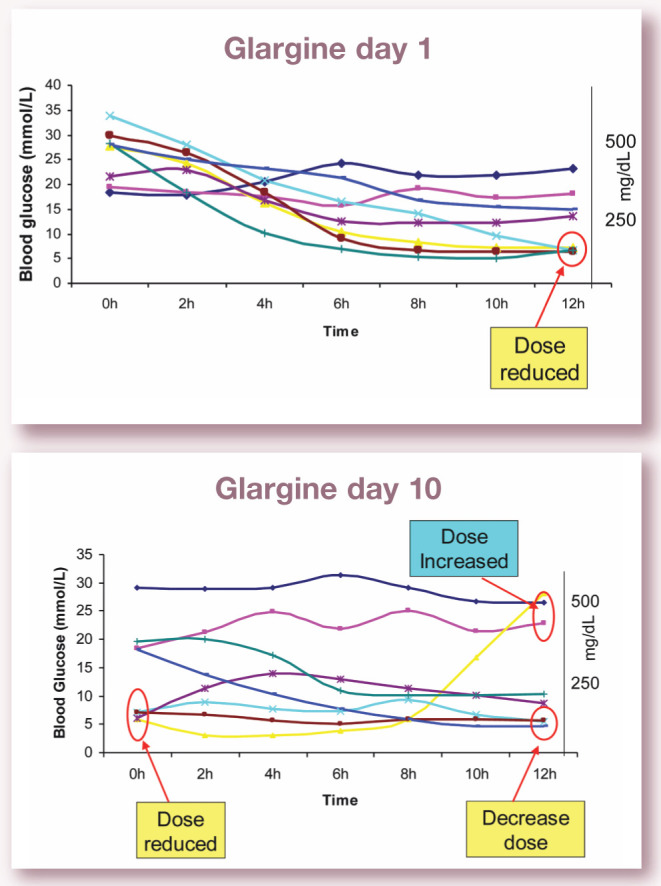
Day 1, day 3 and day 10 blood glucose concentrations measured over 12 h in eight newly diagnosed diabetic cats treated with glargine at 0.5 U/kg twice daily if blood glucose >20 mmol/l (360 mg/dl). Note the range of differing responses
Figure 10.
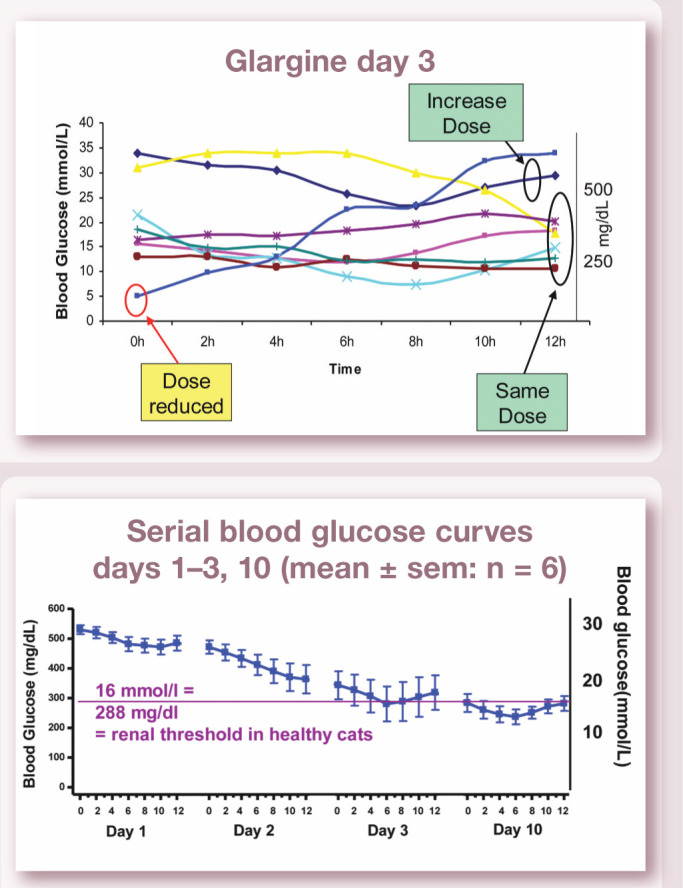
Mean blood glucose concentrations measured over 12 h in six newly diagnosed diabetic cats on days 1–3 and 10 after initiation of treatment with glargine at an initial dose of 0.5 U/kg twice daily (blood glucose >20 mmol/l; 360 mg/dl). Note how rapidly mean blood glucose decreases over time
Table 1 presents a guide for beginning glargine or detemir therapy in diabetic cats. We recommend using a small-sample volume (0.3 µl) feline-calibrated glucometer (eg, AlphaTRAK glucometer; Abbott) and aiming for a blood glucose nadir in the normal range; that is, 3.5–6.5 mmol/l (65–117 mg/dl) at home or 4–9 mmol/l (72–160 mg/dl) on the day of admission to the veterinary clinic. Note that, on average, blood glucose is 2–3 mmol/l (36–54 mg/dl) higher in healthy cats on admission to the clinic compared with cats hospitalized over night. In some cats, stress can result in blood glucose measurements >16 mmol/l (288 mg/dl).
Table 1.
Parameters for changing insulin dosage when using glargine or detemir in diabetic cats and monitoring once a week in the initial stabilization phase 49
| Parameter used for dosage adjustment | Change in dose |
|---|---|
| • Begin with 0.5 U/kg ideal body weight q12h if blood glucose >20 mmol/l (360 mg/dl) or 0.25 U/kg q12h if blood glucose is lower. Do not exceed a total dose of 3U/cat/injection regardless of body weight
• Do not increase in first week unless minimal response to insulin occurs, but decrease if necessary • If no blood glucose monitoring is occurring in first week, begin with 1 U/cat q12h |
|
| • If pre-insulin blood glucose concentration is >12 mmol/l (216 mg/dl) provided nadir is not in hypoglycemic range
or • If nadir blood glucose concentration is >10 mmol/l (180 mg/dl) |
Increase by 0.251U depending on magnitude of dose (greater or less than 3 U/cat) and how close blood glucose is to these glucose cut-points |
| • If pre-insulin blood glucose concentration is 1012 mmol/l (180216 mg/dl) provided nadir is not in hypoglycemic range
and/or • Nadir blood glucose concentration is in normal range, that is, 3.56.5 mmol/l (65117 mg/dl) at home, or 49 mmol/l (72160 mg/dl) on day of admission to clinic |
Maintain same dose |
| • If nadir glucose concentration is 3.54 mmol/l (6572 mg/dl) | Use water drunk, urine glucose and next pre-insulin glucose concentration to determine if insulin dose is decreased or maintained |
| • If pre-insulin blood glucose concentration is <10 mmol/l (180 mg/dl)
and/or • If nadir blood glucose concentration is <3.5 mmol/l (65 mg/dl) |
Reduce by 0.251U depending on magnitude of dose (greater or less than 3 U/cat) and how close blood glucose is to 10 mmol/l (180 mg/dl)
or If pre-insulin blood glucose concentration is <10 mmol/l (180 mg/dl) and total dose is 0.5 U q24h, stop insulin and check for diabetic remission |
| If clinical signs of hypoglycemia are observed | Reduce dose by 50% |

Managing diabetic remission
Cats entering diabetic remission will have normal water intake (provided other causes of polydipsia such as chronic kidney disease are absent), may show signs of hypoglycemia, and will have decreasing pre-insulin or nadir blood glucose despite unchanged insulin therapy.
If pre-insulin blood glucose is ≤10 mmol/l (180 mg/dl) or the nadir is below the normal blood glucose range, decrease the dose by 0.25–1 U per injection. Decrease the insulin dose by less (0.25–0.5 U per injection) if on a low insulin dose (<3 U per injection) or if the blood glucose is close to these glucose cut-points.
Recheck blood glucose weekly and slowly decrease the insulin dose every 1–2 weeks if the indicators for a dose reduction are met, until the cat is on 0.5 U twice daily, and then decrease to 0.5 U once daily.
If the clinical and laboratory evidence still support remission after 2 weeks on 0.5 U once daily, withhold insulin while monitoring the patient’s blood glucose for the day. If the patient remains euglycemic without insulin therapy, diabetic remission is likely, but it is essential that this is confirmed by at least weekly blood glucose measurements (more is better) for a minimum of 2 weeks.
Remission is achievable in 80–90% or more of feline diabetics following rapid and intensive blood glucose control using glargine or detemir.

Key points
Diabetes mellitus is a common endocrine disorder in cats, affecting approximately 1 in every 200 cats.
The majority of cats have type 2 diabetes mellitus, involving insulin resistance plus a relative or absolute deficiency in insulin production by the pancreas.
- Goals for the treatment of type 2 diabetes mellitus have changed in recent years:
- – Previous goals were to achieve reasonable control of blood glucose and clinical signs of polyuria, polydipsia, polyphagia and weight loss;
- – Current goals are to achieve rapid, intensive glycemic control, facilitating diabetic remission.
Newer, long-acting insulins, such as glargine and detemir, lead to rapid achievement of glycemic control in diabetic cats, increasing the probability of remission.
Glargine or detemir treatment, along with a low carbohydrate diet and intensive blood glucose monitoring, are currently the optimal management strategies for feline diabetes mellitus.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this article.
The authors do not have any potential conflicts of interest to declare.
SAGE cannot grant permission for re-use of images. For re-use of images only, please contact the author.
Contributor Information
Carly Anne Bloom, The Animal Medical Center, 510 East 62nd Street, New York, NY, 10065, USA email: Carly.Bloom@amcny.org.
Jacquie Rand, School of Veterinary Science, University of Queensland, Gatton, Queensland 4343, Australia email: j.rand@uq.edu.au.
References
- 1. Panciera DL, Thomas CB, Eicher SW, Atkins CE. Epizoological patterns of diabetes mellitus in cats: 333 cases (1980–1986). J Am Vet Med Assoc 1990; 197: 1504–1508. [PubMed] [Google Scholar]
- 2. Prahl A, Guptill L, Glickman NW, Tetrick M, Glickman LT. Time trends and risk factors for diabetes mellitus in cats presented to veterinary teaching hospitals. J Feline Med Surg 2007; 9: 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baral RM, Rand JS, Catt MJ. Prevalence of feline diabetes mellitus in a feline private practice. J Vet Intern Med 2003; 17: 433–434. [Google Scholar]
- 4. McCann TM, Simpson KE, Shaw DJ, Butt JA, Gunn-Moore DA. Feline diabetes mellitus in the UK: the prevalence within an insured cat population and a questionnaire-based putative risk factor analysis. J Feline Med Surg 2007; 9: 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lederer R, Rand JS, Jonsson NN, Hughes IP, Morton JM. Frequency of feline diabetes mellitus and breed predisposition in domestic cats in Australia. Vet J 2009; 179: 254–258. [DOI] [PubMed] [Google Scholar]
- 6. Lund E. Epidemiology of feline diabetes mellitus. Vet Focus 2011; 21: 17–18. [Google Scholar]
- 7. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010; 562–569.20067953 [Google Scholar]
- 8. Rand JS, Marshall RD. Diabetes mellitus in cats. Vet Clin North Am Small Anim Pract 2005; 35: 211–224. [DOI] [PubMed] [Google Scholar]
- 9. Scarlett JM, Donoghue S. Associations between body condition and disease in cats. J Am Vet Med Assoc 1998; 212: 1725–1731. [PubMed] [Google Scholar]
- 10. Appleton DJ, Rand JS, Sunvold GD, Priest J. Dietary chromium tripicolinate supplementation reduces glucose concentrations and improves glucose tolerance in normal-weight cats. J Feline Med Surg 2002; 4: 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Backus RC, Cave NJ, Ganjam VK, Turner JBM, Biourge VC. Age and body weight effects on glucose and insulin tolerance in colony cats maintained since weaning on high dietary carbohydrate. J Anim Physiol Anim Nutrit 2010; 94: E318–E328. [DOI] [PubMed] [Google Scholar]
- 12. Coradini M, Rand JS, Morton JM, Rawlings JM. Effects of two commercially available feline diets on glucose and insulin concentrations, insulin sensitivity and energetic efficiency of weight gain. Brit J Nutrit 2011; 106: S64–S77. [DOI] [PubMed] [Google Scholar]
- 13. Biourge V, Nelson RW, Feldman EC, Willits NH, Morris JG, Rogers QR. Effect of weight gain and subsequent weight loss on glucose tolerance and insulin response in healthy cats. J Vet Intern Med 1997; 11: 86–91. [DOI] [PubMed] [Google Scholar]
- 14. Rand JS, Bobbermien LM, Hendrikz JK, Copland M. Over representation of Burmese cats with diabetes mellitus. Aust Vet J 1997; 75: 402–405. [DOI] [PubMed] [Google Scholar]
- 15. Wade C, Gething M, Rand JS. Evidence of a genetic basis for diabetes mellitus in Burmese cats [Abstract]. J Vet Intern Med 1999; 13: 269. [Google Scholar]
- 16. O’Leary C, Duffy D, Gething M, McGuckin C, Rand J. Investigation of diabetes mellitus in Burmese cats as an inherited trait: a preliminary study. N Z Vet J 2013; 61: 354–358. [DOI] [PubMed] [Google Scholar]
- 17. Lee P, Mori A, Coradini M, Mori N, Sagara F, Yamamoto I, Rand JS, Arai T. Potential predictive biomarkers of obesity in Burmese cats. Vet J 2012; 195: 221–227. [DOI] [PubMed] [Google Scholar]
- 18. Kluger EK, Hardman C, Govendir M, Baral RM, Sullivan DR, Snow D, et al. Triglyceride response following an oral fat tolerance test in Burmese cats, other pedigree cats and domestic crossbred cats. J Feline Med Surg 2009; 11: 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kluger EK, Caslake M, Baral RM, Govendir M. Preliminary post-prandial studies of Burmese cats with elevated triglyceride concentrations and/or presumed lipid aqueous. J Feline Med Surg 2010; 12: 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rand JS. Pathogenesis of feline diabetes. Vet Clin North Am Small Anim Pract 2013; 43: 221–231. [DOI] [PubMed] [Google Scholar]
- 21. Caney SM. Pancreatitis and diabetes in cats. Vet Clin North Am Small Anim Pract 2013; 43: 303–317. [DOI] [PubMed] [Google Scholar]
- 22. Zini E, Osto M, Franchini M, Guscetti F, Donath MY, Perren A, et al. Hyperglycaemia but not hyperlipidaemia causes beta cell dysfunction and beta cell loss in the domestic cat. Diabetologia 2009; 52: 336–346. [DOI] [PubMed] [Google Scholar]
- 23. Back SH, Kaufman RJ. Endoplasmic reticulum stress and type 2 diabetes. Ann Review Biochem 2012; 81: 767–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reeve-Johnson MK, Rand JS, Vankan D, Anderson S, Marshall RD, Morton JM. Diagnosis of prediabetes in cats: cutpoints for impaired fasting glucose and impaired glucose tolerance in cats 8 years and older using ear or paw samples and a portable glucose meter calibrated for cats [Abstract]. Proceedings of the American College of Veterinary Internal Medicine Forum, 2013. [Google Scholar]
- 25. Link KRJ, Rand JS. Reference values for glucose tolerance and glucose tolerance status in cats. J Am Vet Med Assoc 1998; 213: 492–496. [PubMed] [Google Scholar]
- 26. Rios L, Ward C. Feline diabetes mellitus: diagnosis, treatment, and monitoring. Compend Contin Educ Vet 2008; 30: 626–639. [PubMed] [Google Scholar]
- 27. Kruth SA, Cowgill LD. Renal glucose transport in the cat [Abstract]. Proceedings of the American College of Veterinary Internal Medicine Forum, 1982, p 78. [Google Scholar]
- 28. Reusch C. Feline diabetes mellitus. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine. 7th ed. St Louis: Saunders Elsevier, 2010, pp 1796–1816. [Google Scholar]
- 29. Hoenig M, Ferguson DC. Effect of darglitazone on glucose clearance and lipid metabolism in obese cats. Am J Vet Res 2003; 64: 1409–1413. [DOI] [PubMed] [Google Scholar]
- 30. Crenshaw KL, Peterson ME. Pretreatment clinical and laboratory evaluation of cats with diabetes mellitus: 104 cases (1992–1994). J Am Vet Med Assoc 1996; 209: 943–949. [PubMed] [Google Scholar]
- 31. Link KRJ, Rand JS. Changes in blood glucose concentration are associated with relatively rapid changes in circulating fructosamine concentrations in cats. J Feline Med Surg 2008; 10: 583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boari A, Aste G, Rocconi F, Dalessandri A, Vita S. Glargine insulin and high-protein–low-carbohydrate diet in cats with diabetes mellitus [Abstract]. Vet Res Comm 2008; 32 (Suppl 1): S243–S245. [DOI] [PubMed] [Google Scholar]
- 33. Marshall RD, Rand JS, Morton JM. Treatment of newly diagnosed diabetic cats with glargine insulin improves glycaemic control and results in higher probability of remission than protamine zinc and lente insulins. J Feline Med Surg 2009; 11: 683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sieber-Ruckstuhl N, Kley S, Tschuor F, Zini E, Ohlerth S, Boretti F, et al. Remission of diabetes mellitus in cats with diabetic ketoacidosis. J Vet Intern Med 2008; 22: 1326–1332. [DOI] [PubMed] [Google Scholar]
- 35. Roomp K, Rand J. Intensive blood glucose control is safe and effective in diabetic cats using home monitoring and treatment with glargine. J Feline Med Surg 2009; 11: 668–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zini E, Hafner M, Osto M, Franchini M, Ackermann M, Lutz TA, et al. Predictors of clinical remission in cats with diabetes mellitus. J Vet Intern Med 2010; 24: 1314–1321. [DOI] [PubMed] [Google Scholar]
- 37. Bennett N, Greco DS, Peterson ME, Kirk C, Mathes M, Fettman MJ. Comparison of a low carbohydrate–low fiber diet and a moderate carbohydrate–high fiber diet in the management of feline diabetes mellitus. J Feline Med Surg 2006; 8: 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Scott-Moncrieff JC, Moore GE, Coe J, Lynn RC, Gwin W, Petzold R. Characteristics of commercially manufactured and compounded protamine zinc insulin. J Am Vet Med Assoc 2012; 240: 600–605. [DOI] [PubMed] [Google Scholar]
- 39. Gilor C, Graves TK. Synthetic insulin analogs and their use in dogs and cats. Vet Clin North Am Small Anim Pract 2010; 40: 297–307. [DOI] [PubMed] [Google Scholar]
- 40. Marshall RD, Rand JS, Morton JM. Insulin glargine has a long duration of effect following administration either once daily or twice daily in divided doses in healthy cats. J Feline Med Surg 2008; 10: 488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gilor C, Ridge TK, Attermeier KJ, Graves TK. Pharmacodynamics of insulin detemir and insulin glargine assessed by an isoglycemic clamp method in healthy cats. J Vet Intern Med 2010; 24: 870–874. [DOI] [PubMed] [Google Scholar]
- 42. Weaver KE, Rozanski EA, Mahony OM, Chan DL, Freeman LM. Use of glargine and lente insulins in cats with diabetes mellitus. J Vet Intern Med 2006; 20: 234–238. [DOI] [PubMed] [Google Scholar]
- 43. Hall TD, Mahony O, Rozanski EA, Freeman LM. Effects of diet on glucose control in cats with diabetes mellitus treated with twice daily insulin glargine. J Feline Med Surg 2009; 11: 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fulcher GR, Gilbert RE, Yue DK. Glargine is superior to NPH for improving glycated hemoglobin and fasting blood glucose during intensive insulin therapy. Intern Med J 2005; 35: 536–542. [DOI] [PubMed] [Google Scholar]
- 45. Fonseca V, Bell DS, Berger S, Thomson S, Mecca TE. A comparison of bedtime insulin glargine with bedtime neutral protamine hagedorn in patients with type 2 diabetes: subgroup analysis of patients taking once-daily insulin in a multicenter, randomized, parallel group study. Am J Med Sci 2004; 328: 274–280. [DOI] [PubMed] [Google Scholar]
- 46. Owens DR, Bolli GB. Beyond the era of NPH insulin – long-acting insulin analogs: chemistry, comparative pharmacology and clinical application. Diabetes Techn Therapeut 2008; 10: 333–349. [DOI] [PubMed] [Google Scholar]
- 47. Roomp K, Rand JS. Evaluation of detemir in diabetic cats managed with a protocol for intensive blood glucose control. J Vet Intern Med 2012; 14: 566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. American Diabetes Organization. A vote (and a warning) for a new insulin. In: Diabetes forecast: the healthy living magazine. www.diabetesforecast.org. February 2013. (accessed 23 November 2013).
- 49. Rand JS. Feline diabetes mellitus. In: Rand JS. (ed). Clinical endocrinology of companion animals. Ames, Iowa: Wiley-Blackwell; 2013, pp 169–190. [Google Scholar]