Abstract
Rationale:
The excessive intake of vitamin A in the form of vitamin concentrate, supplement or vitamin-rich liver can result in hypervitaminosis A in man and animals. Although osteopathologies resulting from chronic vitamin A intoxication in cats are well characterized, no information is available concerning feline hypervitaminosis A-induced liver disease.
Clinical summary:
We report the first case of hepatic stellate cell lipidosis and hepatic fibrosis in a domestic cat that had been fed a diet based on raw beef liver. Radiographic examination revealed exostoses and ankylosis between vertebrae C1 and T7, compatible with deforming cervical spondylosis. Necropsy showed a slightly enlarged and light yellow to bronze liver. Microscopic and ultrastructural analyses of liver tissues revealed diffuse and severe liver fibrosis associated with hepatic stellate cell hyperplasia and hypertrophy. These cells showed immunopositive staining for α-smooth muscle actin and desmin markers. The necropsy findings of chronic liver disease coupled with osteopathology supported the diagnosis of hypervitaminosis A.
Practical relevance:
As in human hepatology, if there is dietary evidence to support increased intake of vitamin A, then hypervitaminosis A should be considered in the differential diagnosis of chronic liver disease in cats.
Case Report
Hypervitaminosis A is caused by excessive consumption of vitamin A, typically as vitamin concentrate, supplement or vitamin-rich liver. Both acute and chronic forms of hypervitaminosis A occur naturally in man and animals, 1 although chronic toxicosis is the more common form in cats. The feline disorder was first described as a debilitating metabolic osteopathology caused by diets based solely on milk and raw ruminant, porcine or avian liver.2–4 The long-term effects of excess vitamin A on the skeleton of the cat are quite distinctive,1,5–7 and characterized by extensive confluent bony exostoses and osteophytes, mainly on the cervicothoracic vertebrae.3,8,9
Although rare, chronic hepatic toxicity associated with hypervitaminosis A has been described in humans; manifestations range from portal hypertension, resulting from hypertrophy and hyperplasia of hepatic stellate cells, to variable degrees of fibrosis and cirrhosis.10,11 The present report is the first, to the best of our knowledge, to describe severe hepatic fibrosis and stellate cell lipidosis induced by chronic hypervitaminosis A in a domestic cat.
Case history
An 8-year-old neutered male domestic shorthair cat was referred to the Veterinary Teaching Hospital in Sao Paulo, Brazil (FMVZ-USP) for investigation of spastic tetraparalysis after a fall of 50 cm some 12 h earlier. The animal lived strictly indoors and was fed exclusively on a home-made diet based on raw beef liver and giblets.
The cat appeared apathetic but in good body condition, and had been experiencing difficulty in ambulation for 3 months prior to the consultation. Physical examination confirmed spastic tetraparalysis without reflexes or deep pain sensation in the limbs, with no changes in cranial nerve function. Osseous crepitation was apparent near the atlantoaxial joint, and severe pain was evident even with sedation (acepromazine 0.2 mg/kg IM) and analgesia (morphine 0.1 mg/kg IM) when manipulation of the cervical and thoracic spine was attempted. Other clinical parameters were within normal limits.
Radiographic evaluation of the cervical and thoracic regions revealed exostosis and fusion of all vertebrae between C1 and T7, with fracture and deviation of the spinal axis around the region between C1 and C2. In view of the severity of the cat’s condition and poor prognosis for recovery, the owner requested euthanasia.
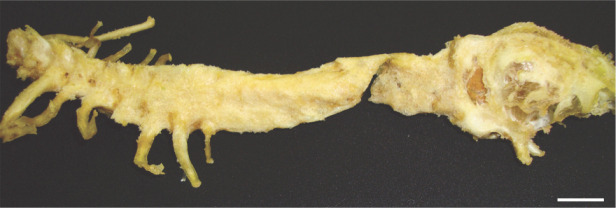
Gross examination on necropsy revealed a slightly enlarged, light yellow liver with small white foci (Figure 1), while the spleen was slightly swollen and pale, and kidneys were tan in color. Chemical and heat maceration techniques were used to remove the soft tissue from the bone structures. Macroscopic evaluation of the skeleton demonstrated extensive and diffuse exostoses involving the occipital bone and the cervical and anterior thoracic vertebrae, with a fracture within the exostosis between C1 and C2 (Figure 2).
Figure 1.
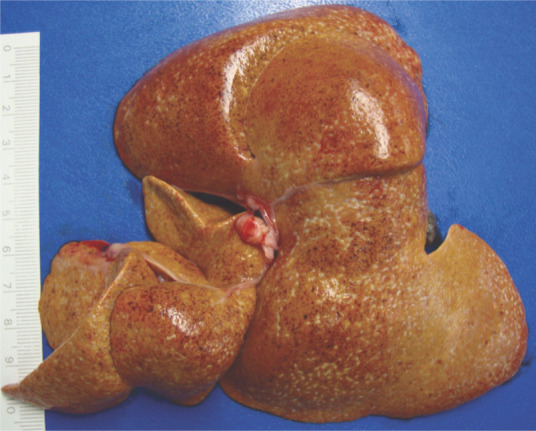
The liver was slightly enlarged and yellowish, with an irregular surface and small linear white foci
Figure 2.
Macerated skeleton showing extensive and diffuse exostoses involving the cervicothoracic vertebrae, and a complete fracture between C1 and C2. Bar = 2 cm
Stomach, intestine, liver, kidneys, spleen, and perihepatic and mesenteric lymph node specimens were fixed (10% formaldehyde for 24 h), embedded in paraffin, sectioned and stained with hematoxylin and eosin and Sirius-red to reveal fibrosis. For transmission electron microscopy, liver fragments were fixed in 2.5% glutaraldehyde, post-fixed with 1% osmium tetroxide, stained with uranyl acetate, dehydrated with acetone, and embedded in epoxy resin. Ultra-thin sections were stained with uranyl acetate–lead citrate and examined and photographed. For immunohistochemical analyses, liver slices were subjected to antigen retrieval in citric acid buffer. Following blockade, slides were incubated overnight with primary antibodies (Keratin 19 [K19; NovoCastra; 1:200], α-smooth muscle actin [α-SMA; Sigma; 1:200], desmin [Dako; 1:100], human alveolar macrophage [HAM-56, Dako; 1:200]), and subsequently with Zymed SuperPicture Poly HRP conjugate, developed in diaminobenzidine solution and counterstained with Harris hematoxylin. For vitamin A measurement, liver fragments were frozen immediately in liquid nitrogen and stored at −80º. Retinol, an active form of vitamin A, was extracted by a liquid–liquid technique and quantified by measurement at an optical absorbance.
In liver sections, stellate cells exhibited marked hyperplasia and hypertrophy, and contained indented dark-staining nuclei without prominent nucleoli, and lipid droplets of various sizes separated by fine cobweb-like strands of cytoplasm. Numerous collections of mononuclear cells were present within distended hepatic sinusoids. A similar population was noted within the medullary cords of the lymph nodes and the splenic cords. These cells exhibited foamy, pink-staining cytoplasm (Figure 3). Sirius-red stain revealed pronounced periportal and moderate centrilobular fibrosis, forming portal-to-portal bridging, with distortion of the normal hepatic architecture. Sinusoidal fibrosis was also noted (Figure 4). Central vein passive congestion and discrete hepatocyte microvesicular degeneration were detected, but iron and amyloid deposits were absent. Portal duct proliferation with K19-positive cells and infiltration of lymphocytes and plasmacytes was observed.
Figure 3.
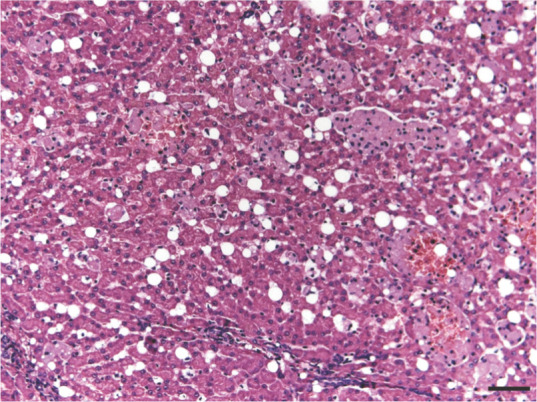
Section of feline liver stained with hematoxylin and eosin showing severe stellate cell lipidosis in the perisinusoidal space. Some groups of reticulohistiocytic cells were also observed. Bar = 100 μm
Figure 4.
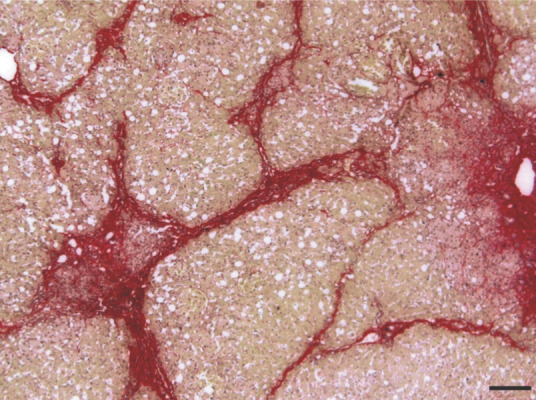
Section of feline liver stained with Sirius-red showing marked periportal and moderate centrilobular fibrosis, forming portal-to-portal bridging, with distortion of the normal hepatic architecture. Bar = 100 μm
Spindle cells along the thick fibrous septa, and most of the lipid-laden stellate cells localized in the perisinusoidal space of Disse, stained strongly positive for α-SMA (Figure 5). Stellate cells were also positive for desmin (Figure 6), while multiple groups of hepatic macrophages (also known as Kupffer cells) were strongly positive for HAM-56 (Figure 7).
Figure 5.
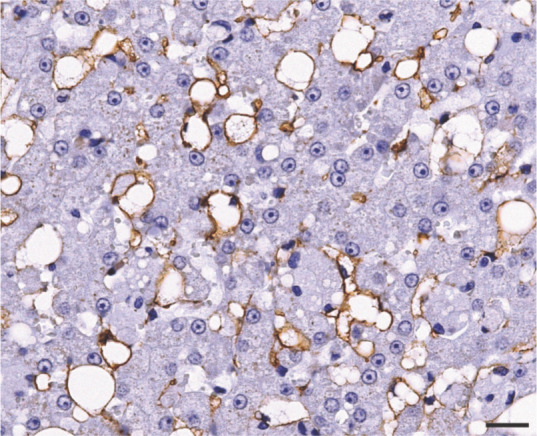
Immunohistochemical analysis for α-smooth muscle actin showing positive staining in lipid-laden hepatic stellate cells localized in the perisinusoidal space. Bar = 20 μm
Figure 6.
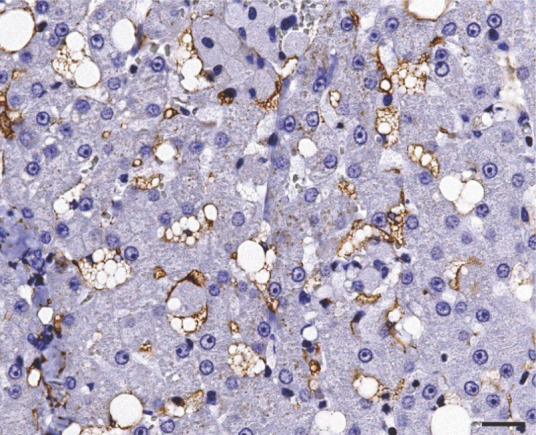
Immunohistochemical analysis for desmin showing positive staining in hepatic lipid-laden stellate cells. Bar = 20 μm
Figure 7.
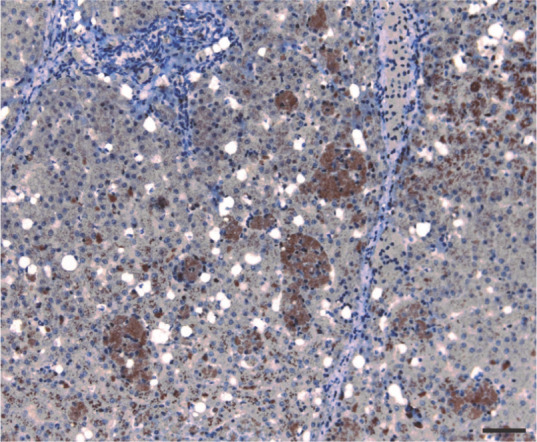
Multiple groups of reticulohistiocytic cells showing strong positive immunohistochemical reaction for human alveolar macrophage. Bar = 40 μm
Transmission electron microscopy showed stellate cells with lipid deposits and large foamy cytoplasm containing small vacuoles of varying sizes. The nuclei were dense, darkly stained and often irregular in shape (Figure 8). Groups of hepatic macrophages exhibited multiple parallel intracytoplasmic cholesterol crystals with angular conformations of varying regularity (Figure 9). The hepatic vitamin A concentration was 894.6 µg/g wet liver. Renal tubular lipidosis without fibrosis and mild chronic lymphoplasmacytic enteritis were also observed.
Figure 8.
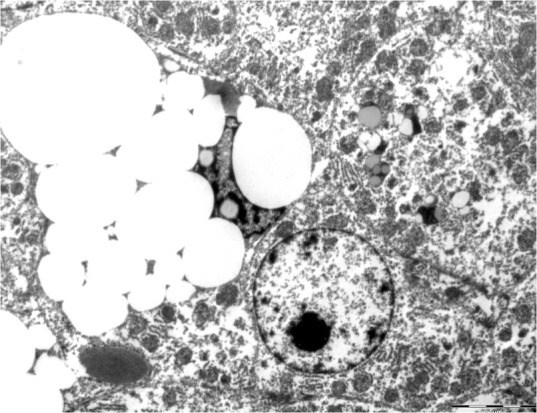
Transmission electron micrograph of feline liver showing stellate cells with several cytoplasmic vacuoles of various sizes. Bar = 5 μm
Figure 9.
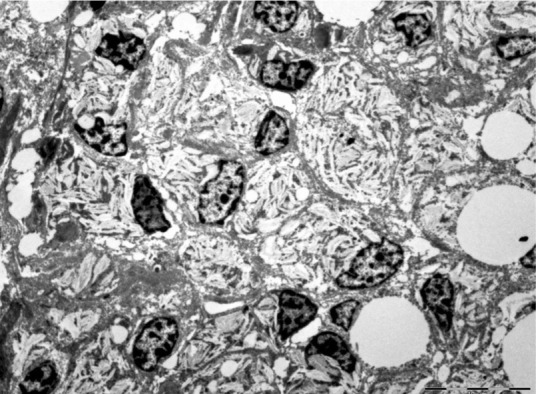
Transmission electron micrograph of feline liver showing groups of reticulohistiocytic cells exhibiting multiple parallel, intracytoplasmic cholesterol crystals, with angular conformations of varying regularity. Bar = 10 μm
The final diagnosis was severe fibrosis and stellate cell lipidosis of the liver associated with metabolic osteopathology and cervical fracture as sequelae to chronic hypervitaminosis A.
Discussion
Hypervitaminosis A has been described in animals, 1 with the long-term effects manifesting primarily in the skeleton as a debilitating metabolic osteopathology (‘deforming cervical spondylosis’).12,13 The cat is particularly susceptible to chronic vitamin A toxicity, most natural cases presenting in adult animals aged between 2 and 9 years with no bias towards gender or breed.4,8,14–16 In the present case, extensive confluent exostoses and ankylosis of the cervicothoracic vertebrae were consistent with chronic hypervitaminosis A.
Differential diagnoses for the radiographic findings in this case included mucopolysaccharidosis (lysosomal storage disease). However, this can be distinguished on the basis of liver histopathology by the presence of round vacuoles in hepatocytes and Kupffer cells, as well as electron microscopy findings of membrane-bound vesicles compatible with the structure of secondary lysosomes or storage bodies made of multilaminar material.17–19
Although the precise pathophysiologic mechanisms of vitamin A toxicity remain unclear, the bone lesions appear to be induced by a direct effect on skeletal tissue. 16 Retinoic acid, a vitamin A metabolite, stimulates the formation and activity of osteoclasts, leading to increased bone resorption and periosteal reaction. 20 Extensive inhibition of collagen synthesis promotes the breakdown of musculotendinous insertions in the periosteum during normal muscle activity, and periosteal trauma is a well-known cause of exostoses.1,6,7
Experimental studies in animals have shown that prolonged and excessive intake of vitamin A causes marked deposition of fat in the reticulohistiocytic cells, especially the liver. 14 In the present case, transmission electron microscopy revealed marked lipid accumulation in hepatic macrophages. Similar macrophage cells were observed in the medullary cords of perihepatic and mesenteric lymph nodes.
Between 50% and 90% of total body vitamin A accumulates in the liver, and 90% of this is rapidly transferred to stellate cells, located in the space of Disse. There it is stored in small cytoplasmic droplets, which are easily visualized by transmission electron microscopy. 21 Ikejiri and Tanikawa 22 demonstrated that ingestion of large amounts of vitamin A resulted in hypertrophy and proliferation of hepatic stellate cells, together with an approximately 50% reduction in the volume of sinusoids and a consequential increase in portal venous resistance and portal hypertension. In the present report, stellate cells showed marked hyperplasia and hypertrophy, with the presence of many intracytoplasmic lipid droplets.
During liver injury, the fine structure of the stellate cells changes considerably. These cells lose their characteristic droplets and activated stellate cells subsequently evolve into myofibroblast-like cells surrounded by newly formed collagen fibrils. 23 Thus, the pathogenesis of vitamin A-induced fibrosis and cirrhosis is most likely mediated by stellate cell activation 10 which, according to Nollevaux et al, 11 may be the net result of retinol toxicity and the release of inflammatory cytokines and fibrogenic products by damaged hepatocytes. Whether the excess of vitamin A per se is the major cause of this phenomenon remains to be elucidated.
The detection in stellate cells of desmin has been employed as the ‘gold standard’ in identifying stellate cells in rodent liver, 24 although the marker is only weakly expressed in hepatic stellate cells of dogs 25 and its expression is variable in humans.13,26 In cats with hepatic fibrosis associated with polycystic liver disease, a mild reaction to desmin has been observed in blood vessels in portal areas and myofibroblasts in fibrous septa; by contrast, an intensive reaction has been observed in oval to spindle-shaped cells in perisinusoidal spaces. 27
The protein α-SMA is one of the six actin isoforms expressed in mammalian tissues, and its presence is characteristic of vascular smooth muscle cells 28 and myofibroblasts.29,30 In humans and rats, induction of α-SMA is a reliable marker of stellate cell activation as it is absent from resident cells of the normal or injured liver except for smooth muscle cells surrounding large vessels. 23 However, in the normal canine liver, α-SMA-positive quiescent stellate cells are present throughout the hepatic parenchyma, suggesting an active role in controlling microvascular blood flow. 25 In the normal cat liver, α-SMA reactivity is observed in large vacuole-laden and spindle-shaped perisinusoidal cells distributed randomly around a few bile ducts and blood vessels and under the Glisson’s capsule.27,30 It appears that activation of hepatic stellate cells in cats follows a different mechanism from that in rodents and humans, and suggests that such large vacuole-laden stellate cells might be in activated state. 30
Nollevaux et al 11 demonstrated a close correlation between the severity of perisinusoidal fibrosis and daily retinol intake, while Geubel et al 31 reported 41 cases of liver damage caused by therapeutic administration of vitamin A in humans. Diagnosis in these cases was based on clinical history, liver biopsy, measurement of hepatic vitamin A and the absence of other causes of stellate cell hyperplasia. In the present case, the concentration of hepatic vitamin A was 894.6 µg/g wet tissue, which is higher than that documented in most carnivores (84–280 µg/g wet liver tissue). 32
Hepatocellular dysfunction in hypervitaminosis A can result from diverse types of liver injury, ranging from stellate cell hyperplasia and hypertrophy-induced portal hypertension to varying degrees of fibrosis and, ultimately, cirrhosis. 33 Additionally, vitamin A can activate hepatic macrophages through interferon gamma released by T-lymphocytes. 34 Activated macrophages produce several mediators, including tumor necrosis factor-α (TNF-α), TGF-β1, IL-6 and reactive oxygen species (ROS). Among these, TGF-β1 plays a crucial role in activation of stellate cells and stimulation of the production of extracellular matrix components.23,26 Taken together, multiple cellular processes can contribute to the pathogenesis of liver fibrosis induced by chronic intake of vitamin A.
Conclusions
In the present case, immunostaining for desmin and α-SMA revealed diffuse lipidosis of hepatic stellate cells. The exact mechanism underlying severe liver fibrosis in hypervitaminosis A remains unknown. Therefore, if there is dietary evidence to support increased intake of vitamin A, hypervitaminosis A should be considered in the differential diagnosis of chronic liver disease in cats.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this article.
The authors do not have any potential conflicts of interest to declare.
Date accepted: 9 November 2013
References
- 1. Clark L. Hypervitaminosis A: a review. Aust Vet J 1971; 47: 568–571. [DOI] [PubMed] [Google Scholar]
- 2. Seawright AA, English PB. Deforming cervical spondylosis in the cat. J Pathol Bacteriol 1964; 88: 503–509. [DOI] [PubMed] [Google Scholar]
- 3. Buffington CA. Nutritional diseases and nutritional therapy. In: Sherding RG. (ed). The cat: diseases and clinical management. 2nd ed. New York: Churchill-Livingstone, 1994, pp 161–190. [Google Scholar]
- 4. Morgan JP. Radiographic and myelographic diagnosis of spinal disease. In: August JR. (ed). Consultations in feline internal medicine. 2nd ed. Philadelphia: WB Saunders, 1997, pp 425–428. [Google Scholar]
- 5. Seawright AA, English PB, Gartner RJ. Hypervitaminosis A of the cat. Ad Vet Sci Comp Med 1970; 14: 1–27. [PubMed] [Google Scholar]
- 6. Hough S, Avioli LV, Muir H, Gelderblom D, Jenkins G, Kurasi H, et al. Effects of hypervitaminosis A on the bone and mineral metabolism of the rat. Endocrinology 1988; 122: 2933–2939. [DOI] [PubMed] [Google Scholar]
- 7. Franch J, Pastor J, Franch B, Durall I, Manzanares MC. Back-scattered electron imaging of a non-vertebral case of hypervitaminosis A in a cat. J Feline Med Surg 2000; 2: 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hayes KC. Nutritional problems in cats. taurine deficiency and vitamin A excess. Can Vet J 1982; 23: 2–5. [PMC free article] [PubMed] [Google Scholar]
- 9. Bennett D, May C. Joint diseases of dogs and cats. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine. 4th ed. Philadelphia: WB Saunders, 1994, pp 2032–2077. [Google Scholar]
- 10. Babb RR, Kieraldo JH. Cirrhosis due to hypervitaminosis A. Western J Med 1978; 128: 244–246. [PMC free article] [PubMed] [Google Scholar]
- 11. Nollevaux MC, Guiot Y, Horsmans Y, Leclercq I, Rahier J, Geubel AP, et al. Hypervitaminosis A-induced liver fibrosis: stellate cell activation and daily dose consumption. Liver Int 2006; 26: 182–186. [DOI] [PubMed] [Google Scholar]
- 12. Rodahl K. Hypervitaminosis A in the rat. J Nutr 1950; 41: 399–421. [DOI] [PubMed] [Google Scholar]
- 13. Schmitt-Gräff A, Desmoulière A, Gabbiani G. Heterogeneity of myofibroblast phenotypic features: an example of fibroblastic cell plasticity. Virchows Archive 1994; 425: 3–24. [DOI] [PubMed] [Google Scholar]
- 14. Seawright AA, English PB. Hypervitaminosis A and deforming cervical spondylosis of the cat. J Comp Pathol 1967; 77: 29–39. [DOI] [PubMed] [Google Scholar]
- 15. O’Donnell JA, Hayes KC. Nutrition and nutritional disorders. In: Holzworth J. (ed). Diseases of the cat – medicine and surgery. Philadelphia: WB Saunders, 1987, pp 27–28. [Google Scholar]
- 16. Braund KG. Clinical neurology in small animals – localization, diagnosis and treatment. International Veterinary Information Service, Ithaca, NY, 2002. [Google Scholar]
- 17. Haskins ME, Aguirre GD, Jezyk PF, Patterson DF. The pathology of the feline model of mucopolysaccharidosis VI. Am J Pathol 1980; 101: 657–674. [PMC free article] [PubMed] [Google Scholar]
- 18. Yogalingam G, Hopwood JJ, Crawley A, Anson DS. Mild feline mucopolysaccharidosis type V1. J Biol Chem 1998; 273: 13421–13429. [DOI] [PubMed] [Google Scholar]
- 19. Schultheiss PC, Gardner SA, Owens JM, Wenger DA, Thrall MA. Mucopolysaccharidosis VII in a cat. Vet Pathol 2000; 37: 502–505. [DOI] [PubMed] [Google Scholar]
- 20. Scheven BAA, Hamilton NJ. Retinoic acid and 1,25-dihydroxyvitamin D3 stimulate osteoclast formation by different mechanisms. Bone 1990; 11: 53–59. [DOI] [PubMed] [Google Scholar]
- 21. Levine PH, Delgado Y, Theise ND, West AB. Stellate-cell lipidosis in liver biopsy specimens. Recognition and significance. Am J Clin Pathol 2003; 119: 254–258. [DOI] [PubMed] [Google Scholar]
- 22. Ikejiri N, Tanikawa K. Effects of vitamin A and estrogen on the sinusoidal cells in the rat liver. In: Wisse E, Knook DL. (eds). Kupffer cells and other liver sinusoidal cells. Amsterdam: Elsevier/North Holland Biomedical Press, 1977, pp 83–92. [Google Scholar]
- 23. Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 2008; 88: 125–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yokoi Y, Namihisa T, Kuroda H, Komatsu I, Miyazaki A, Watanabe S, Usui K. Immunocytochemical detection of desmin in fat-storing cells (Ito cells). Hepatology 1984; 4: 709–714. [DOI] [PubMed] [Google Scholar]
- 25. Ijzer J, Roskams T, Molenbeek RF, Ultee T, Penning LC, Rothuizen J, et al. Morphological characterisation of portal myofibroblasts and hepatic stellate cells in the normal dog liver. Comp Hepatol 2006; 5: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Friedman SL, Rockey DC, McGuire RF, Maher JJ, Boyles JK, Yamasaki G. Isolated hepatic lipocytes and Kupffer cells from normal human liver: morphological and functional characteristics in primary culture. Hepatology 1992; 15: 234–243. [DOI] [PubMed] [Google Scholar]
- 27. Aleksić-Kovačevič S, Kukolj V, Kureljušič B, Marinković D, Knežević D. Role of hepatic stellate cells (HSCs) in the development of hepatic fibrosis in cats with polycystic kidney disease (PKD). Acta Vet (Beograd) 2010; 60: 391–400. [Google Scholar]
- 28. Seawright AA, English PB, Gartner RJW. Hypervitaminosis A and hyperostosis of the cat. Nature 1965; 206: 1171–1172. [DOI] [PubMed] [Google Scholar]
- 29. Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res 1999; 250: 273–283. [DOI] [PubMed] [Google Scholar]
- 30. Uetsuka K, Nishikawa S, Yasoshima A, Nakayama H, Doi K. Histopathological characteristics of Ito cells and Kupffer cells in the feline liver. J Vet Med Sci 2006; 68: 235–242. [DOI] [PubMed] [Google Scholar]
- 31. Geubel AP, De Galocsy C, Alves N, Rahier J, Dive C. Liver damage caused by therapeutic vitamin A administration: estimate of dose-related toxicity in 41 cases. Gastroenterology 1991; 100: 1701–1709. [DOI] [PubMed] [Google Scholar]
- 32. McCain S, Souza M, Ramsay E, Schumacher J, Hecht S, Thomas W. Diagnosis and surgical treatment of a Chiari I-like malformation in an African lion (Panthera leo). J Zoo Wildlife Med 2008; 39: 421–427. [DOI] [PubMed] [Google Scholar]
- 33. Cheruvattath R, Orrego M, Gautam M, Byrne T, Alam S, Voltchenok M, et al. Vitamin A toxicity: when one a day doesn’t keep the doctor away. Liver Transplant 2006; 12: 1888–1891. [DOI] [PubMed] [Google Scholar]
- 34. Ramanathan VS, Hensley G, French S, Eysselein V, Chung D, Reicher S, Pham B. Hypervitaminosis A inducing intra-hepatic cholestasis – a rare case report. Exp Mol Pathol 2010; 88: 324–325. [DOI] [PubMed] [Google Scholar]