Abstract
Clinical summary:
Dental treatment was carried out in an 8.5-year-old castrated male domestic shorthair cat found to have tooth resorption. Right mandibular, and right and left maxillary nerve blocks were administered using a 1 ml syringe attached to a 25 G x 5/8 inch needle and an intraoral technique. The following day the cat displayed blepharospasm of the right eye. The ocular signs progressed and 5 days later an ophthalmologist confirmed a blind, glaucomatous right eye. It was suspected that the eye had suffered a penetrating injury during dental surgery. Enucleation of the right eye was performed and gross and histopathological examination revealed a penetrating wound consistent with a needle tract injury.
Practical relevance:
Complications arising from veterinary dental regional anaesthesia appear to be rare; however, it may be that they are under-reported. This case report highlights the risks involved and reviews the safest and most efficacious regional anaesthesia technique for the feline maxilla.
An elegant and contemporary approach to pain management in small animal dentistry involves the use of multimodal and preemptive analgesia.1 –5 Peripheral neural blockade describes the use of local anaesthetic agents to selectively block specific nerves, thus desensitising certain regions of the body or head (regional anaesthesia or nerve block).3,4 This may form part of a multimodal approach while simultaneously contributing to pre-emptive analgesia. Easily identifiable landmarks are often used as a guide to direct needle placement. Various blocks are described in the veterinary literature for both dogs and cats.3 –9
Human dentists administer thousands of local anaesthetic injections daily, this being the most common form of perioperative pain control. 10 Misjudging the anatomy of the area concerned may not only result in inadequate analgesia, but more serious local or systemic complications. These incidents appear to be relatively rare, but even experienced human dental practitioners are urged to take time to review the anatomy involved.10–12 In human medicine the research tends to focus on nerve injury associated with regional nerve block, but the overall risk associated with regional anaesthesia remains poorly defined. 13
While complications associated with regional anaesthesia in dogs and cats are described, these appear to be extrapolated from human research into systemic toxicity.14,15 To the authors’ knowledge there have been no reports in the literature of local complications and only one report of a severe, systemic complication arising from a dental nerve block in the cat. 16 It may be that complications arising from veterinary dental regional anaesthesia are genuinely rare. Alternatively, it may be that they are under-reported.
This report describes globe penetration in a cat following maxillary nerve block during dental surgery. The aim is to highlight this risk and to review the safest and most efficacious regional anaesthesia technique for the feline maxilla.
Case history
An 8.5-year-old castrated male domestic short hair cat was presented to a first opinion practice for routine annual vaccination. During the clinical examination, tooth resorption was observed on the buccal aspect of the permanent right maxillary third premolar tooth (107). No other clinical abnormalities were noted on oral or general physical examination. A recommendation was made for dental assessment and treatment under general anaesthesia.
A week later, the cat was admitted to the practice for dental treatment. Pre-anaesthetic blood tests (albumin, alkaline phosphatase, alanine aminotransferase, urea, creatinine, globulin, glucose, total protein, sodium, potassium and chloride) were within normal limits. An intravenous catheter was placed in the left cephalic vein in a sterile manner and Hartmann’s solution administered intravenously at maintenance rate (2 ml/kg/h) rising to 4 ml/kg/h during anaesthesia. The cat was premedicated with a combination of acepromazine (0.03 mg/kg) and buprenorphine (20 µg/kg) given by intramuscular injection. Induction of anaesthesia was achieved using propofol (6 mg/kg) intravenously to effect over 40 s. The trachea was intubated with a size 4.0 mm endotracheal tube of suitable length. This was inflated until the escape of gas was prevented with the application of moderate positive pressure. Anaesthesia was maintained using isoflurane mixed with oxygen delivered via a mini-Lack circuit. Non-invasive blood pressure, oxygen saturation, body temperature, heart rate, respiratory rate and end-tidal CO2 were monitored continuously throughout the procedure and recorded every 5 mins. Body temperature was supported with the use of a circulating warm air blanket.
Oral examination and intraoral dental radiography confirmed the presence of tooth resorption in several teeth. A treatment plan was formulated that included the provision of regional anaesthesia. Right mandibular, and right and left maxillary nerve blocks were administered using a 1 ml syringe attached to a 25 G x 5/8 inch needle, providing a total dose of 0.75 mg/kg bupivacaine. The maxillary nerve blocks were performed using an intraoral technique, advancing the needle dorsally caudal to the molar tooth, directing the needle tip towards the maxillary foramen in the pterygopalatine fossa. To assist this, the needle was pre-bent to a 45° angle to direct the tip rostrally towards the foramen. Aspiration was performed before injection of the bupivacaine. Crown amputation was performed on the right maxillary third premolar (107), while the left maxillary third premolar (207) and right mandibular third premolar (407) were extracted in an open manner. The gingiva was sutured with poliglecaprone 25 5–0 (1M) in a simple interrupted pattern.
Recovery from anaesthesia was uneventful. The cat was discharged and the owner instructed to keep the cat indoors overnight. Meloxicam was prescribed to be given orally once daily for postoperative pain control. The next morning the cat displayed blepharospasm of the right eye, and veterinary attention was sought.
Examination demonstrated a small superficial corneal ulcer, subtle corneal oedema, a very cloudy anterior chamber and iritis. The retina could not be visualised. Topical ofloxacin drops (at a frequency of one drop, six times daily) were added to the systemic medication.
Two days later the patient was reviewed and found to be dull and anorexic. Ocular examination revealed ongoing signs of uveitis. The corneal ulcer had healed, thus topical drops of a combined steroid/antibiotic (dexamethasone, neomycin and polymyxin B) were prescribed. The cat’s body temperature was 38.5°C. A blood sample was taken for testing for feline leukaemia virus, feline immunodeficiency virus and coronavirus, and for routine biochemistry and haematology. A toxoplasmosis titre was not assessed at this time. Results were within normal limits apart from a mild eosinophilia (0.96 x 109/1; normal 0.10–0.79 x 109/1). No improvement in the condition of the eye was noted, and 2 days later the opinions of two of the authors (RP and DM) were sought.
Ophthalmic examination and pathology
Ophthalmic examination confirmed a blind right eye. The eye demonstrated blepharospasm, a negative menace response and a negative direct pupillary light response. Marked uveitis and secondary glaucoma were apparent with diffuse corneal oedema, large keratic precipitates over the corneal endothelium, a large clot of organising inflammatory cells and some small organising blood clots in the ventral anterior chamber, an organising inflammatory membrane across the pupil resulting in some distortion in pupil shape, and iritis. It was not possible to visualise any posterior segment structures through the anterior segment opacities. The left eye appeared unremarkable.
Intraocular pressures were 43 and 9 mmHg in the right and left eyes, respectively. Ocular ultrasonography was performed and demonstrated increased echogenicity of the aqueous, thickened iris tissue, some hyperechoic strands (suspected inflammatory membranes) within the vitreal chamber that appeared to be arising from the ventrolateral aspect of the globe, and retinal detachment.
Since the eye problem became evident within 24 h of dental surgery, it was suspected that the cat had suffered a penetrating ocular injury during the dental procedure. A very poor prognosis was given for the right eye and enucleation of the eye was advised. A standard transpalpebral enucleation technique was undertaken under general anaesthesia. The cat made an uneventful recovery from surgery.
The globe was fixed in neutral buffered formalin and submitted for pathological examination. On gross examination, a small (0.5–0.7 mm) focal penetrating injury was identified in the ventral sclera (Figure 1). The globe was then sectioned in half (Figure 2) to include the scleral lesion, routinely processed for histopathology and embedded in paraffin. Sections were cut at 4 µm and stained with haematoxylin and eosin.
Figure 1.
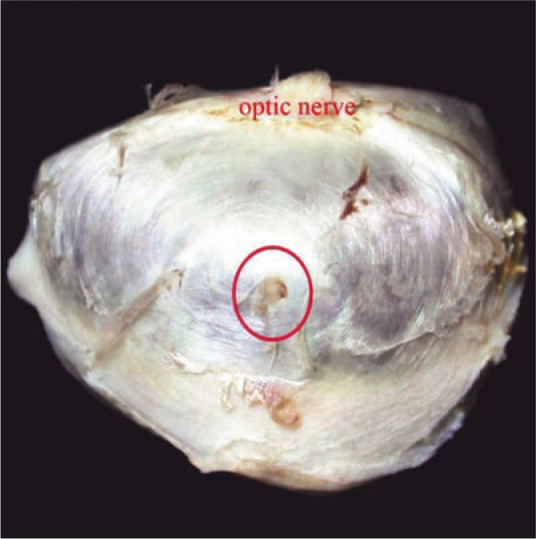
Focal penetrating injury (circled) in the ventral sclera
Figure 2.
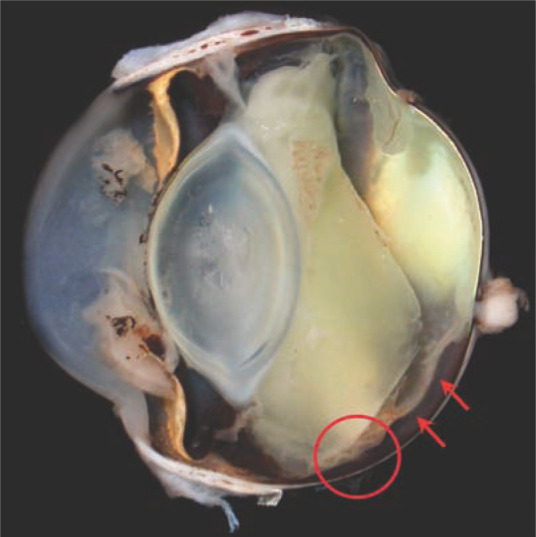
The sectioned globe. The circle demarcates the site of the penetrating scleral injury. Note the associated ventral retinal detachment (arrows) and endophthalmitis
Histopathological examination confirmed the presence of a focal linear penetrating wound disrupting the sclera, choroid and retina (Figure 3); findings included focal pathological detachment of the ventral retina, neutrophilic and lymphoplasmacytic endophthalmitis, mild intraocular haemorrhage and secondary glaucoma. The discrete linear morphology of the penetrating injury was consistent with that expected from a needle tract and did not support scleral rupture secondary to blunt trauma.
Figure 3.
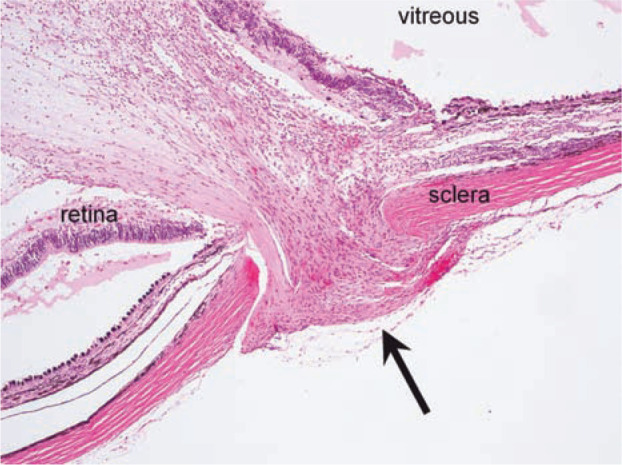
Histopathological examination confirms the presence of a focal well-delineated full-thickness penetrating injury (arrow) that involves the sclera, choroid and retina. Reparative fibroblastic tissue partially fills the defect. The retina is pathologically detached. Haematoxylin and eosin, x 40
Discussion
The contemporary approach to veterinary practice demands careful anticipation and prevention of pain in patients under our care.1 –5,17 This includes the provision of multimodal analgesia, whereby different classes of drugs are used to block the ultimate conscious perception of pain by interrupting the pain pathway at different points, to maximise analgesic provision.1,7,18 The rationale of pre-emptive analgesia is to prevent initial afferent signals reaching the central nervous system (CNS), and thus reduce the risk of altered processing of afferent input that could amplify postoperative pain. 19 Local anaesthetics are the only drugs that produce complete blockade of peripheral nociceptive input, and therefore offer the most effective way of preventing central sensitisation. 20
Peripheral neural blockade describes the use of local anaesthetic agents to desensitise specific nerves using anatomical landmarks and provide analgesia to a region of the body or head, and is the most common form of perioperative pain control used in human dentistry. 10 Veterinary patients may benefit from local anaesthetic techniques under general anaesthesia as decreased perioperative pain may result in better autonomic stability and reduced cardiovascular, respiratory or CNS depression, contributing to a safer anaesthetic, smooth recovery and rapid discharge from the hospital.3 –7,20 Some longer acting drugs such as bupivacaine may also provide analgesia into the postoperative period. 21
The overall incidence of complications associated with human dental anaesthesia appears to be low, with local anaesthetic drugs being relatively safe when used with care.10–12 Placement of the agent is critical in both ensuring efficacy and reducing unwanted side effects.11,12 Complications may arise as a direct result of needle placement, or due to toxic effects of the drug used. 11 Toxicity may result from overdose or inadvertent intravenous administration of the drug, and in veterinary patients has led to CNS disturbances or cardiorespiratory arrest.22,23 Safety precautions therefore include aspirating before injection to ensure the drug is not administered intravenously, and calculating the maximal dose permissible to avoid overdosing the patient.3,6,7,20 Local complications in humans have reportedly included needle breakage, trismus (inability to open the jaw), prolonged anaesthesia or paraesthesia, paralysis of motor nerves and interference with special senses such as vision.11,12,24–26
The reporting of procedure-related complications is common in both human and veterinary medicine. However, there is a lack of acceptable and reliable reporting criteria in relation to regional anaesthesia. A systematic review in 2009 of human regional anaesthesia peer-reviewed literature found both an inconsistency and lack of outcome reporting in prospective studies, meaning that comparing results for safety-related issues may be difficult. 13 There are reports of complications arising from regional anaesthesia in the dog and cat, but these are primarily associated with epidural administration of drugs.22,23 There are very few reported complications arising from the use of dental nerve blocks in dogs and cats, but severe cardiovascular depression has been reported after a mandibular nerve block in a cat using bupivacaine. 16 Lingual trauma has been reported in the horse following inferior alveolar nerve block, 27 and this complication is anecdotally reported in cats and dogs.
Regional anaesthesia is also used during human ophthalmic surgery and complications including globe perforation or penetration are reported. 28 Similar regional anaesthetic techniques are used in cattle, horses, dogs and cats for ocular surgery.29,30 The blocks may also be associated with globe perforation and penetration due to the blind placement of the needle. Cadaveric studies in horses suggest that ultrasound guidance may improve the safety and efficacy of the retrobulbar nerve block. 31
Orbital penetration by dental elevators has been reported during tooth extraction in dogs and cats. 32 This is a particular risk during extraction of caudal maxillary molar teeth due to the proximity of the tooth roots to the ventral orbit.33,34
Anatomy
There are three primary divisions of the sensory part of the trigeminal nerve: ophthalmic, maxillary and mandibular. The maxillary nerve supplies sensation to the cheeks, nose, soft and hard palates, upper teeth and gingivae. 35 It leaves the cranial vault via the round foramen, coursing ventral to the orbit in the pterygopalatine fossa, before entering the infraorbital canal via the maxillary foramen. Within the infraorbital canal, branches of the infraorbital nerve supply all teeth before exiting the canal at the infraorbital foramen where it becomes purely sensory to the skin of the nose and upper lip. 35 The infraorbital canal is much shorter in the cat than the dog and may only be a few millimetres in length.4,34,35 The cat possesses large, prominent eyes with an incomplete orbit. The orbital ligament connects the zygomatic and frontal bones in the dorsotemporal region. 36 Important soft tissue structures in the ventral orbit include the maxillary and ophthalmic arteries, orbital veins and venous plexus, and pterygopalatine nerve.30,33,36 The tooth roots of the maxillary fourth premolar and first molar in the cat lie very close to the ventral orbit.32,33
Maxillary nerve block
Using local anaesthetic agent to block the maxillary nerve before it enters the infraorbital canal should desensitise the ipsilateral upper teeth and lip, nose, maxilla, incisive bone, and hard and soft palates.4,6 Several techniques have been described to perform the maxillary nerve block in the dog and cat, but unfortunately a distinction is often not made between the canine and feline patient.4 –9 Extrapolating from a technique used in dogs may not be appropriate due to anatomical differences between the species. Furthermore, some publications are inaccurate and misleading.9,37
The percutaneous approach inserts the needle just below the ventral border of the zygomatic arch, at the junction of the maxilla parallel to the hard palate, and directs it towards the pterygopalatine fossa and maxillary foramen.3,6,38 There are two intraoral approaches described in cats. The first directs the needle dorsally via the notch palpable at the hard and soft palate juncture caudal to the molar tooth. 4 Disadvantages of this technique concern the blind placement of a needle in the retrobulbar space.4,7 The second intraoral approach is via the infraorbital canal and is described as the deep infraorbital block. 6 Some authors suggest this approach should be chosen in the cat as the infraorbital canal is so short it allows advancement of the needle to the maxillary nerve via the infraorbital foramen.4,7
Another intraoral approach is described in dogs using an intravenous catheter to place local anaesthetic at the level of the lateral canthus of the eye via the infraorbital foramen, similar to the deep infraorbital block. 39 Use of a catheter in this study was hypothesised to reduce the risk of iatrogenic needle damage to infraorbital nerves and blood vessels, and the distribution of dye in this study supported this hypothesis. Further studies would be required in a clinical setting to confirm these findings. What is unknown, however, with this procedure is the effect of pressure on the infraorbital nerve after injecting a volume of liquid into an enclosed space.
The infraorbital nerve block has been shown to be effective in abolishing reflex-evoked muscle action potentials from stimulated maxillary fourth premolar teeth in cats where the needle was advanced 0.5 cm into the canal. 40 However, the infraorbital canal in the cat is described as being only a few millimetres long (<4 mm); thus use of a long needle placed into this canal, or advancement further than the lateral canthus of the eye, risks penetrating the globe (Figure 4).3,4 Globe penetration can be avoided by not advancing a needle further than 2–3 mm into the canal, and directing the needle ventrally not dorsally towards the globe. 41 Blocking the infraorbital nerve as it exits the foramen seems logical in terms of safety, but it will not provide any dental analgesia as the nerve at this location is purely sensory to the nose and upper lip. 34 Digital compression over the infraorbital foramen for 30 s after injection may help the anaesthetic agent to reach the target area, although there is a lack of evidence to support this practice. 8
Figure 4.
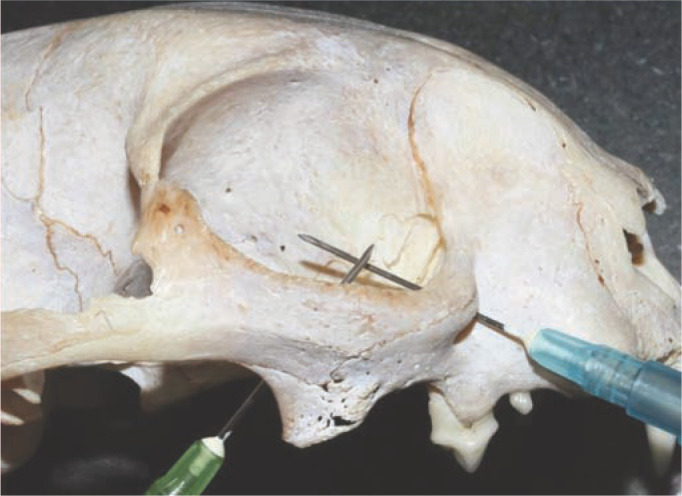
Needle placement in the retrobulbar space or into the infraorbital canal may risk globe penetration
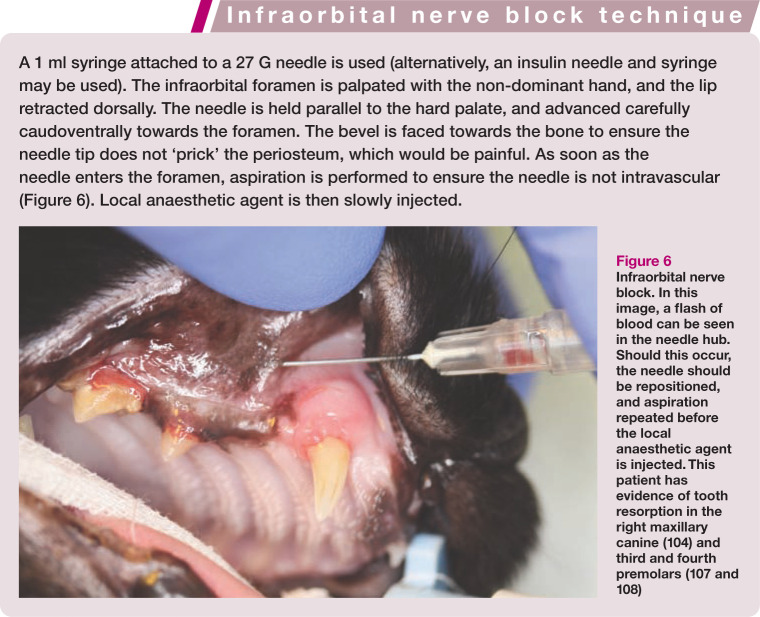
The infraorbital canal is located dorsal to the maxillary third premolar tooth (Figure 5) and can be palpated easily through the oral mucosa. The procedure for infraorbital nerve block is described in the box on page 71, with placement of the needle illustrated in Figure 6.
Figure 5.
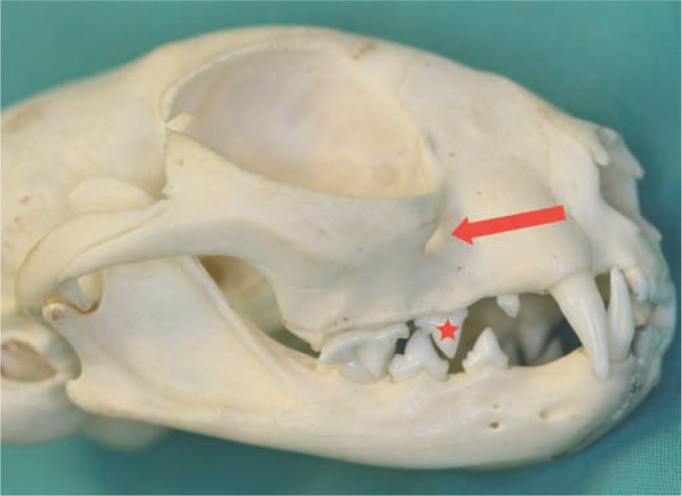
The position of the infraorbital foramen is shown by the arrow. It lies immediately dorsal to the maxillary third premolar tooth (107/207) (asterisk)
Figure 6.
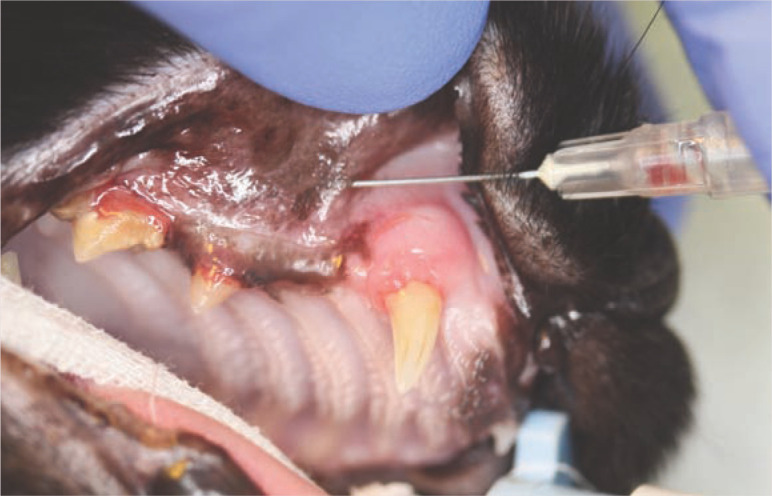
Infraorbital nerve block. In this image, a flash of blood can be seen in the needle hub. Should this occur, the needle should be repositioned, and aspiration repeated before the local anaesthetic agent is injected. This patient has evidence of tooth resorption in the right maxillary canine (104) and third and fourth premolars (107 and 108)
Conclusions
This report describes a catastrophic complication of performing maxillary nerve block in the cat. The provision of balanced and preemptive analgesia will ultimately benefit the patient, so the reader is urged to reflect upon feline-specific anatomy, and continue to provide regional anaesthesia in the most efficacious and safe way. Review of the literature has revealed differences in opinions regarding maxillary regional anaesthesia in the cat. The weakest form of evidence is the consensus statement or opinion. 42 The highest quality published evidence suggests that the infraorbital block is sufficient to desensitise the maxillary fourth premolar (108/208) in the cat. 40 This block would, therefore, be the recommended approach to providing regional anaesthesia to the feline maxilla, as long as the depth and direction of needle placement are carefully gauged.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this case report.
The authors do not have any potential conflicts of interest to declare.
Date accepted: 17 July 2014
References
- 1. Beckman BW. Pathophysiology and management of surgical and chronic oral pain in dogs and cats. J Vet Dent 2006; 23: 50–60. [DOI] [PubMed] [Google Scholar]
- 2. Colmery B, 3rd. The gold standard of veterinary oral health care. Vet Clin Small Anim 2005; 35: 781–787. [DOI] [PubMed] [Google Scholar]
- 3. Pascoe PJ. Anesthesia and pain management. In: Verstraete FJM, Lommer MJ. (eds). Oral and maxillofacial surgery in dogs and cats. Edinburgh: Saunders Elsevier, 2012, pp 23–42. [Google Scholar]
- 4. Reiter AM. Dental and oral diseases. In: Little SE. (ed). The cat: clinical medicine and management. St Louis, MO: Elsevier Saunders, 2012, 341–342. [Google Scholar]
- 5. Beckman B, Legendre L. Regional nerve blocks for oral surgery in companion animals. Compend Contin Educ Pract Vet 2002; 24: 439–443. [Google Scholar]
- 6. Rochette J. Regional anesthesia and analgesia for oral and dental procedures. Vet Clin North Am Small Anim Pract 2005; 35: 1041–1058. [DOI] [PubMed] [Google Scholar]
- 7. Woodward TM. Pain management and regional anesthesia for the dental patient. Top Companion Anim Med 2008; 23: 106–114. [DOI] [PubMed] [Google Scholar]
- 8. Lantz GC. Regional anesthesia for dentistry and oral surgery. J Vet Dent 2003; 20: 181–186. [PubMed] [Google Scholar]
- 9. O’Morrow C. Advanced dental local nerve block anesthesia. Can Vet J 2010; 51: 1411–1415. [PMC free article] [PubMed] [Google Scholar]
- 10. Daubländer M, Müller R, Lipp MDW. The incidence of complications associated with local anesthesia in dentistry. Anesth Prog 1997; 44: 132–141. [PMC free article] [PubMed] [Google Scholar]
- 11. Crean S-J, Powis A. Neurological complications of local anaesthetics in dentistry. Dent Update 1999; 26: 344–349. [DOI] [PubMed] [Google Scholar]
- 12. Smith MS, Lung KE. Nerve injuries after dental injection: a review of the literature. J Can Dent Assoc 2006; 72: 559–564. [PubMed] [Google Scholar]
- 13. Stojadinovic A, Shockey SM, Croll SM, et al. Quality of reporting of regional anesthesia outcomes in the literature. Pain Med 2009; 10: 1123–1131. [DOI] [PubMed] [Google Scholar]
- 14. Chadwick HS. Toxicity and resuscitation in lidocaine- or bupivacaine-infused cats. Anesthesiology 1985; 63: 385–390. [DOI] [PubMed] [Google Scholar]
- 15. Feldman HS, Arthur GR, Covino BG. Comparative systemic toxicity of convulsant and supraconvulsant doses of intravenous ropivacaine, bupivacaine, and lidocaine in the conscious dog. Anesth Analg 1989; 69: 794–801. [PubMed] [Google Scholar]
- 16. Aprea F, Vettorato E, Corletto F. Severe cardiovascular depression in a cat following a mandibular nerve block with bupivacaine. Vet Anaesth Analg 2011; 38: 614–618. [DOI] [PubMed] [Google Scholar]
- 17. McMillan FD. Comfort as the primary goal in veterinary medical practice. J Am Vet Med Assoc 1998; 212: 1370–1374. [PubMed] [Google Scholar]
- 18. Kehlet H, Dahl J. The value of ‘multimodal’ or ‘balanced analgesia’ in postoperative pain treatment. Anesth Analg 1993; 77: 1048–1056. [DOI] [PubMed] [Google Scholar]
- 19. Krissin I. Preemptive analgesia. Anesthesiology 2000; 93: 1138–1143. [DOI] [PubMed] [Google Scholar]
- 20. Lemke KA, Creighton CA. Analgesia for anesthetised patients. Top Companion Anim Med 2010; 25: 70–82. [DOI] [PubMed] [Google Scholar]
- 21. Chapman PJ. Review: bupivacaine – a long-acting local anaesthetic. Aust Dent J 1987; 32: 288–291. [DOI] [PubMed] [Google Scholar]
- 22. Blass CE, Shires PK. Respiratory paralysis secondary to epidural anesthesia in a dog. J Am Vet Med Assoc 1986; 189: 315–316. [PubMed] [Google Scholar]
- 23. Swalander DB, Crowe DT, Jr, Hittenmiller DH, et al. Complications associated with the use of indwelling epidural catheters in dogs: 81 cases (1996–1999). J Am Vet Med Assoc 2000; 216: 368–370. [DOI] [PubMed] [Google Scholar]
- 24. Haas DA, Lennon D. A 21 year retrospective study of reports of paraesthesia following local anaesthetic administration. J Can Dent Assoc 1995; 61: 319–330. [PubMed] [Google Scholar]
- 25. Rood JP. Ocular complication of inferior dental nerve block. Brit Dent J 1972; 132: 234. [DOI] [PubMed] [Google Scholar]
- 26. Sambrook PJ, Goss AN. Severe adverse reactions to dental local anaesthetics: prolonged mandibular and lingual nerve anaesthesia. Aust Dent J 2011; 56: 154–159. [DOI] [PubMed] [Google Scholar]
- 27. Caldwell FJ, Easley KJ. Self-inflicted lingual trauma secondary to inferior alveolar nerve block in three horses. Equine Vet Educ 2012; 24: 119–123. [Google Scholar]
- 28. Rubin AP. Complications of local anaesthesia for ophthalmic surgery. Br J Anaesth 1995; 75: 93–96. [DOI] [PubMed] [Google Scholar]
- 29. Edmondson MA. Local and regional anesthesia in cattle. Vet Clin North Am Food Anim Pract 2008; 24: 211–226. [DOI] [PubMed] [Google Scholar]
- 30. Cho J. Surgery of the globe and orbit. Top Companion Anim Med 2008; 23: 23–37. [DOI] [PubMed] [Google Scholar]
- 31. Morath U, Luyet C, Spadavecchia C, et al. Ultrasound-guided retrobulbar nerve block in horses: a cadaveric study. Vet Anaesth Analg 2013; 40: 205–211. [DOI] [PubMed] [Google Scholar]
- 32. Smith MM, Smith EM, La Croix N, et al. Orbital penetration associated with tooth extraction. J Vet Dent 2003; 20: 8–17. [DOI] [PubMed] [Google Scholar]
- 33. Ramsey DT, Marretta SM, Hamor RE, et al. Ophthalmic manifestations and complications of dental disease in dogs and cats. J Am Anim Hosp Assoc 1996; 32: 215–224. [DOI] [PubMed] [Google Scholar]
- 34. Gioso MA, Carvalho VGG. Oral anatomy of the dog and cat in veterinary dentistry practice. Vet Clin North Am Small Anim Pract 2005; 35: 763–780. [DOI] [PubMed] [Google Scholar]
- 35. Hudson LC. Nervous system. In: Hudson LC, Hamilton WP. (eds). Atlas of feline anatomy for veterinarians. Jackson: Teton NewMedia, 2010, pp 194–230. [Google Scholar]
- 36. Hudson LC. Special sensory organs. In: Hudson LC, Hamilton WP. (eds). Atlas of feline anatomy for veterinarians. Jackson: Teton NewMedia, 2010, pp 243–254. [Google Scholar]
- 37. Pang D, Anthony J, Ambros B, et al. Advanced dental local nerve block anesthesia – a comment. Can Vet J 2011; 52: 345. [PMC free article] [PubMed] [Google Scholar]
- 38. Dugdale A. Local anaesthetic techniques for the head: small animals. In: Dugdale A. (ed). Veterinary anaesthesia: principles to practice. Oxford: Wiley-Blackwell, 2010, pp 118–122. [Google Scholar]
- 39. Viscasillas J, Seymour CJ, Brodbelt DC. A cadaver study comparing two approaches for performing maxillary nerve block in dogs. Vet Anaesth Analg 2012; 40: 212–219. [DOI] [PubMed] [Google Scholar]
- 40. Gross ME, Pope ER, Jarboe JM, et al. Regional anesthesia of the infraorbital and inferior alveolar nerves during noninvasive tooth pulp stimulation in halothane-anesthetized cats. Am J Vet Res 2000; 61: 1245–1247. [DOI] [PubMed] [Google Scholar]
- 41. Niemiec BA. Pain management. In: Dental extractions made easier. San diego: Practical Veterinary Publishing, 2012, pp 6–7. [Google Scholar]
- 42. Vandeweerd J, Kirschvink N, Clegg P, et al. Is evidence-based medicine so evident in veterinary research and practice? History, obstacles and perspectives. Vet J 2012; 191: 28–34. [DOI] [PubMed] [Google Scholar]