Abstract
Practical relevance:
Periodontal disease is commonly encountered in feline practice. Gingivitis, followed by inflammation of the rest of the periodontal tissues, can lead to chronic oral infection, bacteraemia, pain and ultimately tooth loss. Given adequate plaque control and thorough, consistent dental home care, gingivitis is a reversible and controllable condition. Periodontitis, however, is an essentially irreversible and progressive condition. Treatment aims to control tissue inflammation, returning the gingiva to clinical health and preventing destruction of the periodontium in other parts of the mouth.
Clinical challenges:
Diagnosis must be established using a combination of oral examination under anaesthesia and dental radiography. Periodontitis leads to tooth attachment loss, and given the short length of most cat teeth, probing depths of 1 mm or more should alert the clinician to the presence of periodontitis. The decision of whether to extract or preserve affected teeth needs careful consideration. In practice, as periodontitis is often associated with type 1 tooth resorption, extraction is often required, but the slender and delicate nature of feline tooth roots, compounded by the destructive nature of tooth resorption, can frustrate extraction attempts. As highlighted in this article, iatrogenic damage to teeth is also a real risk if periodontal therapy procedures (including scaling and polishing) are not performed carefully. The challenges of providing home care in the cat are additionally discussed.
Evidence base:
The authors have drawn upon, wherever possible, an evidence base relating strictly to the feline patient. Where there is a lack of published research, evidence from canine and human studies is assessed.
Periodontal disease is a collection of diseases characterised by plaque-induced inflammation of the periodontal tissues that together make up the periodontium. In health, the periodontium securely maintains the teeth in the jaws. The classification of human periodontal diseases is wide, including not only gingival diseases, but necrotising diseases, periodontal abscess, and chronic as well as aggressive forms of periodontitis. 1 The two main feline periodontal diseases that require clear understanding are gingivitis and periodontitis. However, juvenile forms of these conditions exist, and the practitioner must be able to differentiate these from other inflammatory conditions in the mouth, such as chronic gingivostomatitis.
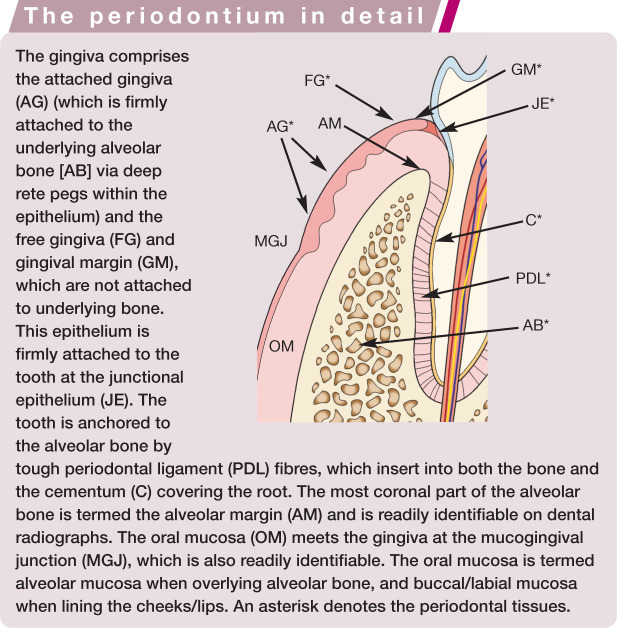
The healthy periodontium
The periodontium is comprised of cementum (covering the tooth root surface), periodontal ligament (securely anchored in the cementum and alveolar bone), alveolar bone (compact bone that lines the walls of the alveoli and is seen radiographically as the lamina dura) and the gingiva (Figures 1 and 2). The gingiva is the covering of the alveolar bone that protects the other periodontal structures, and is separated from the alveolar mucosa by the mucogingival junction. 2 The gingiva, divided into free and attached parts, is perforated by the teeth protruding through it into the oral cavity. Surrounding each tooth is, therefore, a collar of free gingiva. The gingiva then reflects back on itself becoming sulcular epithelium and then junctional epithelium, which attaches to the tooth itself via hemidesmosomes. Thus a potential space, the gingival sulcus, is formed. The sulcus is bathed in gingival crevicular fluid, which flows continuously outwards from the base of the sulcus.
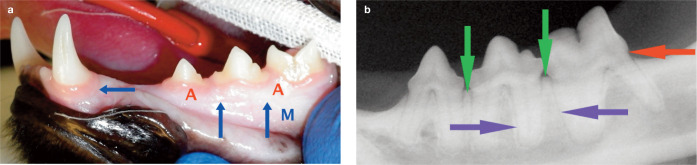
Figure 1.
Periodontal tissues in clinical health. (a) Attached gingiva is denoted (A), the alveolar mucosa (M) and the mucogingival junction is indicated by the blue arrows. There are minimal deposits on the tooth surfaces, and no bleeding was evident on periodontal probing. (b) Radiographically, the alveolar bone margin (green arrow) is within 1 mm of the cemento-enamel junction (red arrow). The periodontal ligament (blue arrows) is visible as a radiolucent line of consistent width surrounding the tooth roots
Figure 2.
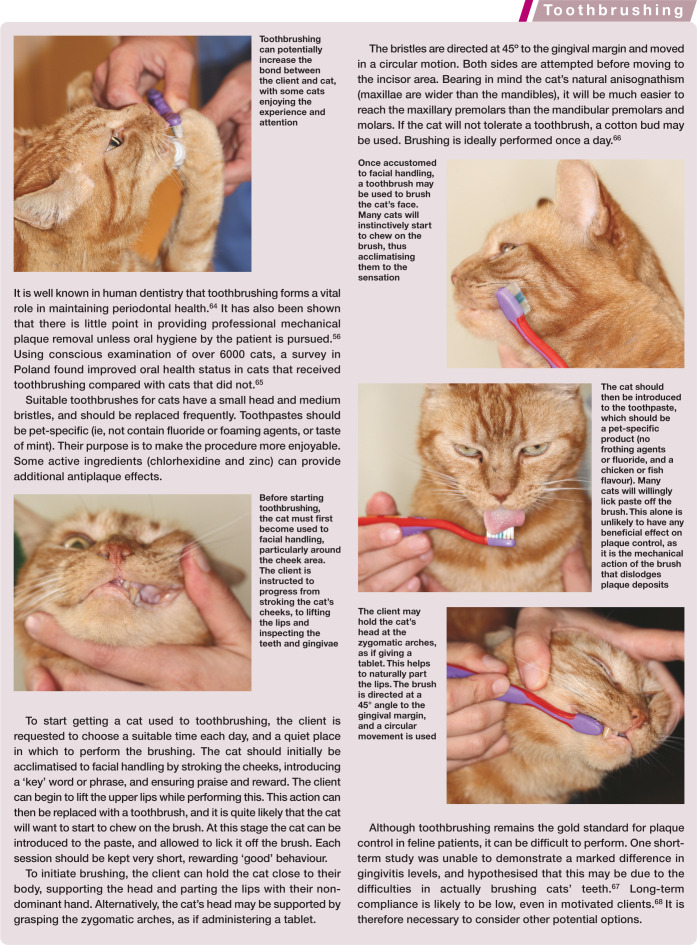
Basic tooth anatomy. The crown of the tooth is covered in enamel (E), and the roots are covered in cementum (C). Dentine (D) comprises the majority of the hard tissue of the tooth. Within the tooth is the pulp (P). This is a soft tissue composed of arteries, veins and nerves, which enter through the apex of the root (A) (these are branches from the maxillary and mandibular artery, vein and nerves [*]), as well as lymphatics, fibroblasts, odontoblasts and undifferentiated mesenchymal cells. Within the crown the pulp space is termed the pulp chamber and within the root the root canal. The periodontal ligament (PDL) anchors the tooth within the alvelous by attaching to the cementum and alveolar bone (AB). The furcation (F) is where the roots divide and, in health, this area is filled with alveolar bone
Nowhere else in the body is the epithelium broached in such a manner, and so this junctional epithelium forms the principal seal between the oral environment and the underlying tissues. The anatomy of this junction must be fully understood in order to comprehend the pathogenesis of periodontal disease. 2

The attached gingiva is firmly adherent to the underlying alveolar bone, while the free gingiva is not attached to underlying bone. The oral epithelium of the attached gingiva is keratinised and the lamina propria beneath contains many coarse collagen bundles firmly attaching the gingiva to the underlying periosteum. 3 (This firm attachment can make mucogingivoperiosteal flap creation during open extractions quite difficult if inappropriate or blunt instruments are used.) The alveolar mucosa, by contrast, is non-keratinised epithelium, with a loose lamina propria containing many elastic fibres. (This elasticity enables closure of any flaps created during extraction attempts as long as the underlying inelastic periosteum is incised.)
The healthy gingival sulcus in the cat has a probing depth of less than 0.5 mm (1 mm for the canine tooth) and should not bleed when probed. The measurement is taken from the free gingival margin to the most apical part that the probe reaches.

Plaque
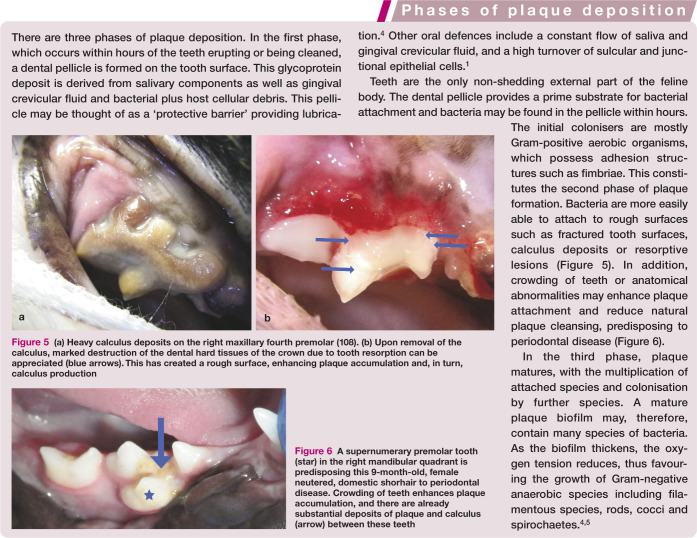
Periodontal disease represents the host’s inflammatory response to plaque bacteria. The bacteria are part of a biofilm, where a protective community of bacteria form, attach to a surface (the tooth) and multiply. Plaque is a soft deposit, and is opaque white or grey in colour (Figures 3 and 4). Unless abundant, it is not readily seen with the naked eye, but becomes visible when a plaque disclosing solution is used. Sweeping a periodontal probe across the tooth at the gingival margin may demonstrate plaque accumulation.
Figure 3.
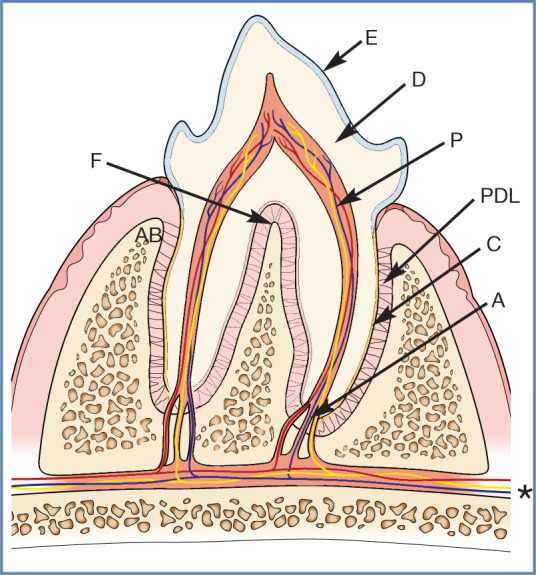
Plaque is an opaque, soft deposit that is adherent to teeth and other oral tissues. In this image, a probe has been swept along the gingival margin, collecting plaque. Note also the heavy calculus deposits on the maxillary third premolar (107), mandibular fourth premolar and first molar (408, 409), and the missing third premolar (407)
Figure 4.
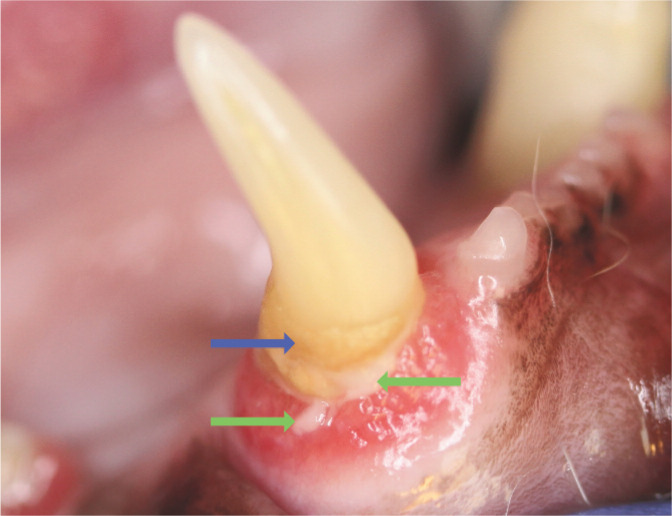
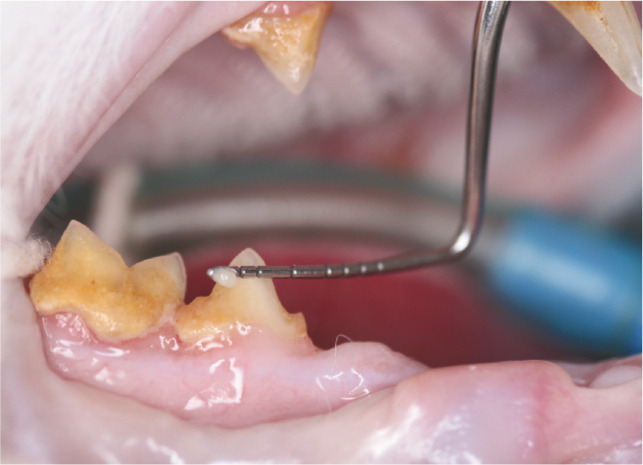
Abundant plaque is visible on this tooth (green arrows) and calculus is developing as a result (blue arrow). Gingival recession and alveolar bone loss have exposed the root surface; cementum is rougher than enamel, predisposing to plaque attachment
Plaque deposition occurs in three phases (see box). Plaque is initially supragingival (at or above the gingival margin) but soon approaches the gingival margin, before becoming subgingival (within the sulcus).
Over 700 species of bacteria have been implicated in human periodontal disease, but much less is known about the composition of feline plaque.5,6 The oral cavity contains many commensal species that may be considered part of a healthy oral micro-biome. However, some species of bacteria are more commonly found in disease states, the so-called periodontopathogens. 5 One study of 32 cats found that black pigmented Bacteroides and Peptostreptococcus anaerobius were more prevalent in diseased sites. 7 Pasteurella multocida was found commonly in the mouths of cats but was not associated with periodontal disease severity. 7 Porphyromonas gulae and Tannerella forsythia have also been implicated as significant periodontopathogens.8,9 The concept that only certain plaque is pathogenic due to its particular bacterial composition is known as the specific-plaque hypothesis. 4
Figure 6.
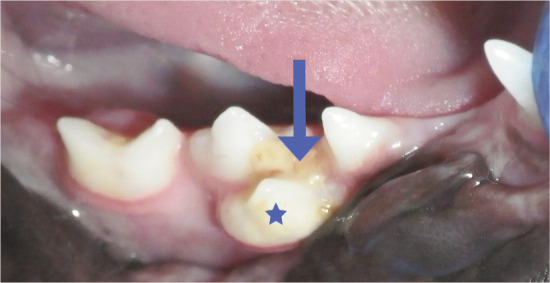
A supernumerary premolar tooth (star) in the right mandibular quadrant is predisposing this 9-month-old, female neutered, domestic shorhair to periodontal disease. Crowding of teeth enhances plaque accumulation, and there are already substantial deposits of plaque and calculus (arrow) between these teeth
Calculus
Calculus (tartar) is a hard dental deposit formed by the mineralisation of plaque (Figures 4, 5 and 7). it is composed of inorganic and organic parts, and is always covered by a layer of plaque. 10 The component minerals come both from saliva and gingival crevicular fluid. Mineralisation may start within hours of plaque accumulation, and the process may be complete within 2 weeks.
Figure 5.
(a) Heavy calculus deposits on the right maxillary fourth premolar (108). (b) Upon removal of the calculus, marked destruction of the dental hard tissues of the crown due to tooth resorption can be appreciated (blue arrows). This has created a rough surface, enhancing plaque accumulation and, in turn, calculus production
Figure 7.
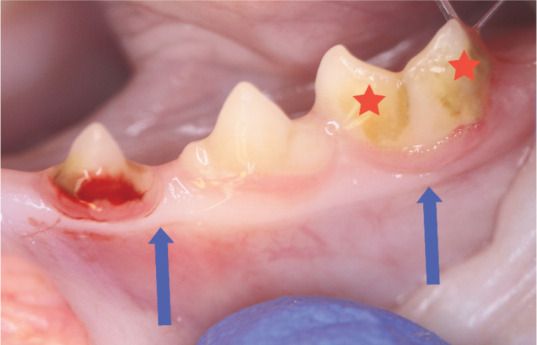
Mild calculus deposits on the third premolar (307) and first molar (309) (stars). Note the marginal gingivitis associated with 307, which has shown bleeding on probing. The margin is swollen and oedematous. The mucogingival junction is shown (blue arrows). No bone loss was seen radiographically
Calculus is not a primary cause of periodontal disease but, rather, a predisposing factor. It provides a rough, plaqueretentive surface, favouring the accumulation of pathogenic plaque bacteria. Abundant calculus may be associated with little gingival inflammation, and vice versa. Therefore, a judgement on the severity of periodontal disease should not be made based on the volume of calculus deposits.
Calculus may be supragingival or subgingival. Supragingival calculus is visible in the oral cavity; subgingival calculus is found beneath the gingival margin and, therefore, is not immediately obvious on clinical examination. All calculus deposits, however, must be thoroughly removed during treatment because of their ability to accumulate plaque. 10
Gingivitis
Gingivitis (inflammation of the gingiva) may occur within 2–3 weeks if plaque biofilm is not removed (Figures 8–10). 11 Periodontopathogens may synthesise products that are damaging to the host cells, in particular the junctional epithelial cells that form the barrier at the gingival attachment to the tooth. This allows bacteria and their products access to the connective tissue layer beneath the epithelium. Initially this triggers vasodilation and an increased blood flow to the area, with diapedesis of leukocytes (especially polymorphonuclear neutrophils) alongside an increase in gingival crevicular fluid flow.5,12 Clinically this may manifest as erythema, loss of the ‘knife-like’ edge to the gingival margin and bleeding on probing. Bleeding on probing may actually be detected before any colour changes (see Figure 7) and thus is of great value in detecting early gingivitis. 13
Figure 8.
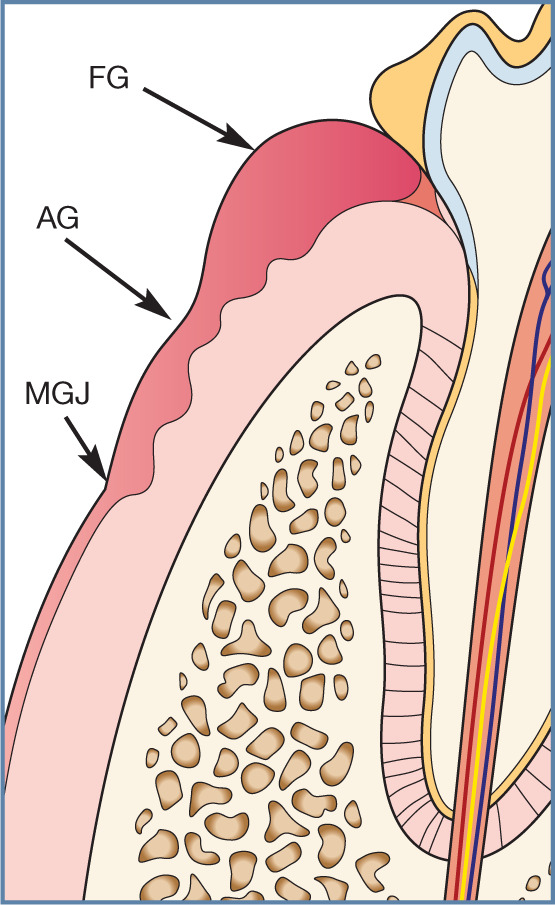
Gingivitis. The free gingival margin (FG) is swollen, oedematous and inflamed. This inflammation may extend to the attached gingiva (AG) but does not typically cross the mucogingival junction (MGJ). The level at which the junctional epithelium is attached to the tooth is not altered, and there is no destruction of bone or periodontal ligament tissues
Figure 9.
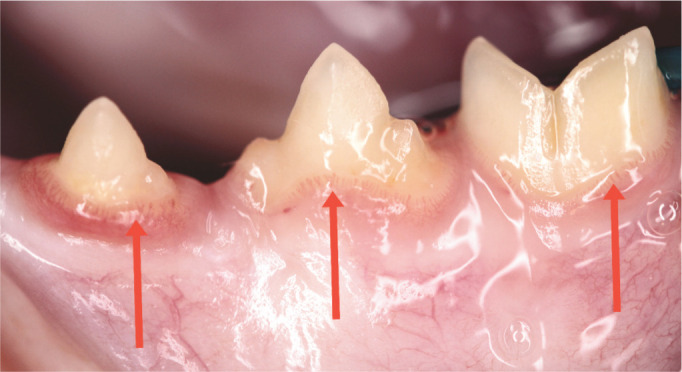
Early gingivitis. The gingival margin in the left mandibular premolars and molar (307–309) is oedematous with marginal blood vessels prominent
Figure 10.
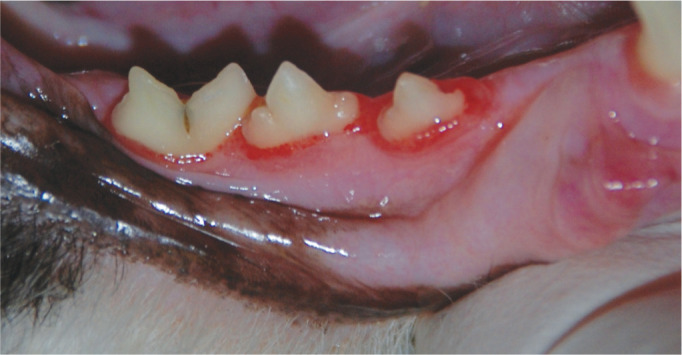
Marginal gingivitis is seen affecting 408 and 409. In 407, the inflammation is extending onto the attached gingiva. The inflammation, however, has not crossed the mucogingival junction
The subsequent host inflammatory response dictates whether this condition resolves, reaches a state of equilibrium or evolves to a state of chronic inflammation. 1 So, although specific periodontopathogens are necessary to initiate the disease process, it is ultimately the host’s response to these bacteria that determines the course of the disease. It is possible for the host and the biofilm to exist in a symbiotic relationship; if the disease never progresses to periodontitis, this symbiosis is maintained. 1
Periodontitis
Gingivitis is essentially a reversible condition as long as plaque is mechanically removed from the tooth surface. The disease, however, may progress to periodontitis affecting the attachment apparatus (cementum, alveolar bone and periodontal ligament). Initially the soft tissue attachment to the tooth is lost, followed by loss of alveolar bone (Figures 11 –16). Ultimately, so much attachment may be lost that the tooth is shed from the mouth (see Table 1).
Figure 11.
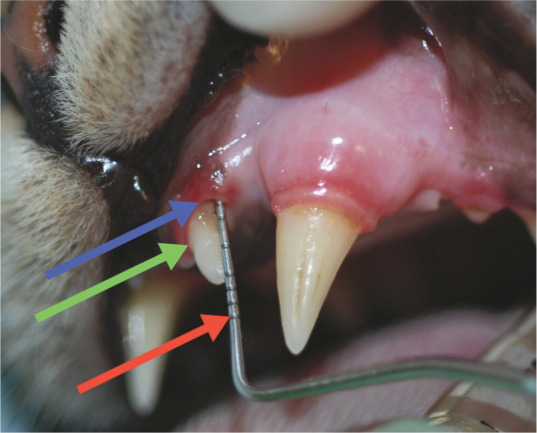
Periodontitis. Attachment loss may be demonstrated in several ways. Here, there is gingival recession affecting the left maxillary third incisor (203): the distance between the blue arrow (gingival margin) and green arrow (cemento-enamel junction) is 2 mm. In addition, the probe is inserted into a pocket of 2 mm depth. Thus there is overall clinical attachment loss of 4 mm for this tooth. A Williams 14 W periodontal probe is being used, which has markings at 1, 2, 3, 5, 7, 8, 9 and 10 mm (the red arrow indicates the 10 mm marking)
Figure 12.
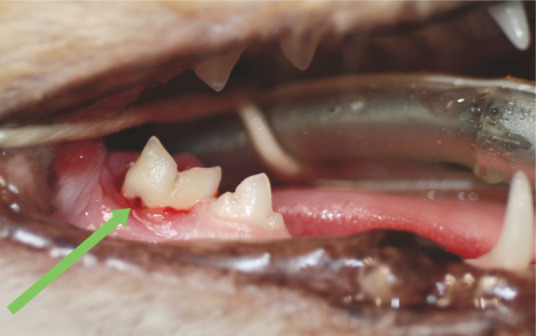
Furcation exposure. Here gingival recession and alveolar bone loss in the furcation area of the right mandibular first molar (409) has revealed the furcation (green arrow). Note this lies beneath the distal cusp of the crown
Figure 13.
Two examples of grade 3 furcation exposure. The periodontal probe passes all the way through the furcation, demonstrating marked periodontal attachment loss. (a) Note that the furcation in this tooth (409) is positioned beneath the distal cusp, unlike other two-rooted teeth, where the furcation is under the main cusp of the crown. (b) The probe passes easily between the mesial roots and distal root of the left maxillary fourth premolar (208)
Figure 14.
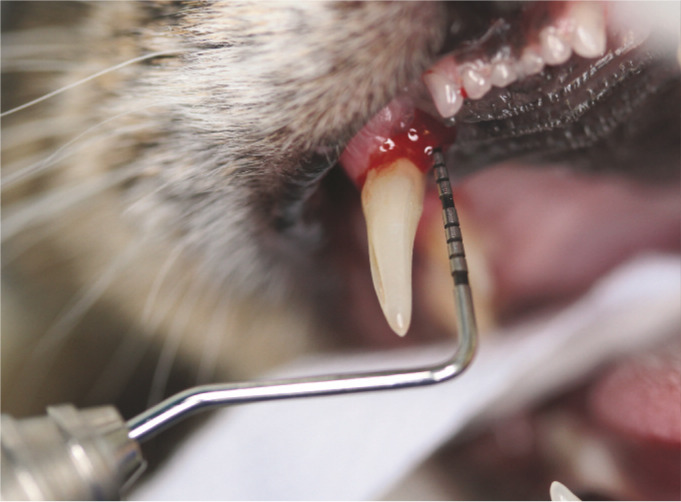
Deep periodontal pocket (6 mm) on the palatal aspect of the right maxillary canine (104). It is vital to probe the entire circumference of the tooth, otherwise focal pockets may be missed. Note the bleeding induced by probing. A UNC-15 probe (markings every mm for 15 mm) is being used
Figure 15.
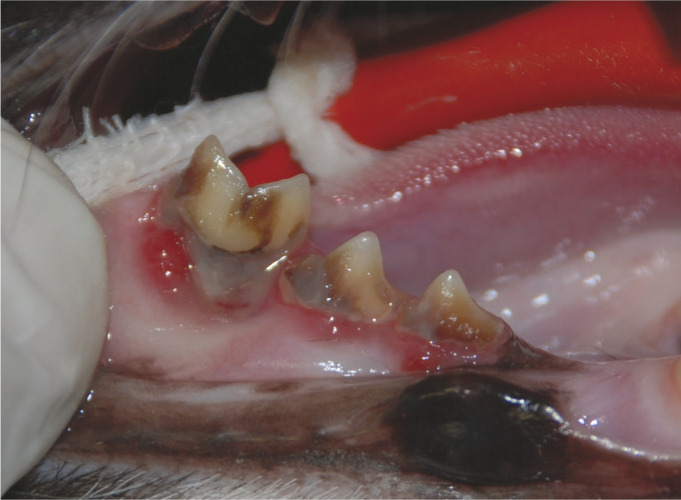
Severe periodontitis. Marked dental deposits and gingivitis are affecting the right mandibular third and fourth premolars (407, 408) and first molar (409). In addition there is gingival recession and alveolar bone loss affecting the mesial root of tooth 409. This tooth is also ‘supererupted’ and mobile
Figure 16.
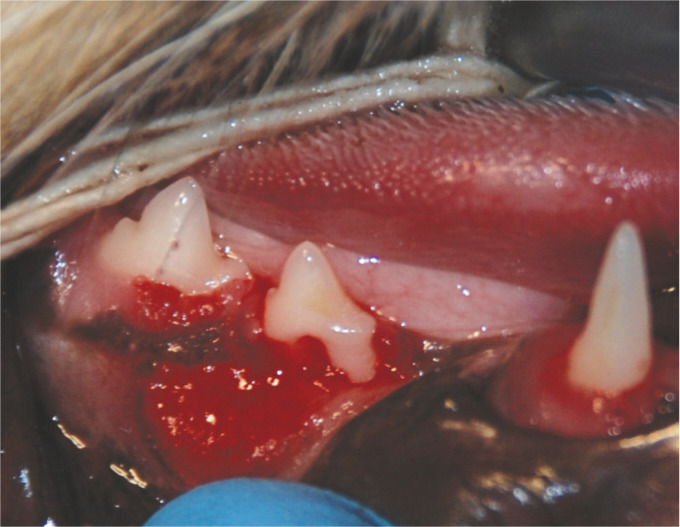
Severe focal periodontitis of the right mandibular third premolar (407). There is loss of gingival tissue as well as alveolar bone, exposing much of the buccal surface of the mesial root of this tooth
Table 1.
Staging of periodontal disease
| Stage | Description | Attachment loss* (%) | Furcation exposure grade |
|---|---|---|---|
| 0 | Healthy | 0 | 0 |
| 1 | Gingivitis | 0 | 0 |
| 2 | Early periodontitis | <25 | F1 |
| 3 | Moderate periodontitis | <50 | F2 |
| 4 | Severe periodontitis | >50 | F3 |
AVDC nomenclature 18
As measured either by probing of clinical attachment level, or radiographic determination of the distance of the alveolar margin from the cemento-enamel junction relative to length of the root
Bacterial components that contribute to this process have been identified and have known virulence factors. Some virulence factors (such as fimbriae) allow the bacteria to colonise and invade host tissues, while other factors actually cause host tissue damage (eg, production of proteolytic enzymes such as collagenase). 14 In part, this may be due to stimulation of the host’s own immune system. For instance bacterial lipopolysaccharide may stimulate release of pro-inflammatory cytokines such as interleukin (IL-1, IL-6), tumor necrosis factor and prostaglandin E2; and host tissue proteinases such as matrix metalloproteinases. 14 The ratio of receptor activator of NF-kB ligand (RANKL) to osteoprotegerin is also increased in disease states and may serve as a biomarker of disease. 15 Together, these mediators are responsible for collagen destruction within the periodontal ligament, and the activation of osteoclasts, which drive bone destruction. During this process the junctional epithelial attachment to the tooth migrates apically. It is this migration that explains the formation of the periodontal pocket, and allows the periodontal probe to enter deeper upon probing (see Figures 11 and 14).
Destruction of periodontal tissues manifests clinically and radiographically in various ways. As well as the formation of periodontal pockets (discussed below), there may be gingival recession, bone loss (horizontal and vertical; see later), exposure of root surfaces (cementum) and tooth mobility. The furcation of multi-rooted teeth may also become obvious visually or tactilely (Figures 12 and 13). The furcation is the site of division of roots in these teeth and, in health, the furcation area is filled with bone (see Figure 2). In some cases, recession may be seen without the formation of a periodontal pocket (gingiva and alveolar bone are lost at the same rate), but there is still evidence of tooth attachment loss.
Periodontitis does not progress along a linear path, but involves periods of quiescence interspersed with bursts of aggressive destruction. 16 Ultimately, the teeth may become mobile and are shed from the body. Periodontitis is not spontaneous but, as mentioned, is a consequence of untreated gingivitis; in other words, gingivitis always precedes periodontitis. 17 Furthermore, periodontitis may be localised or generalised. Therefore, unless a full oral examination is completed under general anaesthesia, incorporating dental radiography, areas of periodontitis may be missed.
Periodontal pocket formation
The periodontal pocket is a pathologically deepened gingival sulcus. 16 A ‘pseudopocket’, by contrast, may be formed if there is increased height of the gingival margin with no alteration in junctional epithelial attachment. This is seen with gingival enlargement (eg, gingival hyperplasia, see later) and is significant due to its plaque-retaining nature.
A true periodontal pocket is a sequela to the destruction of periodontal tissues. Suprabony pockets may be formed where the base of the pocket is coronal to the level of alveolar bone, whereas an infrabony pocket has the base of the pocket apical to the level of adjacent bone, with the pocket forming between the root and alveolar bone. This is also known as horizontal and vertical bone loss, respectively (Figures 17 –20). In cats with healthy periodontal tissues, the alveolar margin is expected to be within 1 mm of the cemento-enamel junction (Figure 1b). 19 In horizontal bone loss, full-thickness alveolar wall is lost buccally and/or palatally (lingually) and the ‘new’ bone margin (apical to the original margin) is seen as a horizontal line, usually parallel to the original alveolar margin. Horizontal bone loss often affects more than one adjacent tooth. 19 Vertical bone loss, however, commonly affects one tooth or one root of one tooth (Figure 20).
Figure 17.
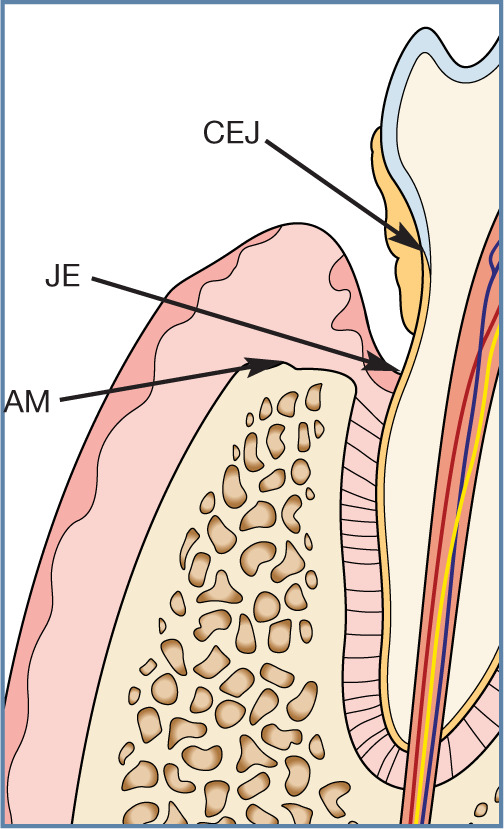
Horizontal bone loss in periodontitis. The alveolar bone and periodontal ligament are destroyed in an apical direction. The alveolar margin (AM) is now >1 mm from the cemento-enamel junction (CEJ). The junctional epithelium attachment of gingiva to the tooth is now also in a more apical direction (JE). Note that this may not necessarily give rise to increased probing depths if the gingiva has also receded
Figure 18.
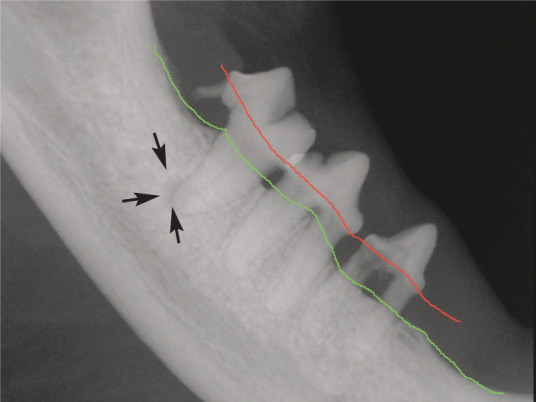
Radiographic appearance of horizontal bone loss in periodontitis. The level of the alveolar bone margin is shown in green. The normal level should be at the cemento-enamel junction (red). Bone is lost in a horizontal manner, and affects multiple teeth in a quadrant; this also soon exposes the furcation (area between roots). Note the periapical lucency around the mesial root of 409 (arrows) due to endodontic infection entering through the resorbing distal root
Figure 19.
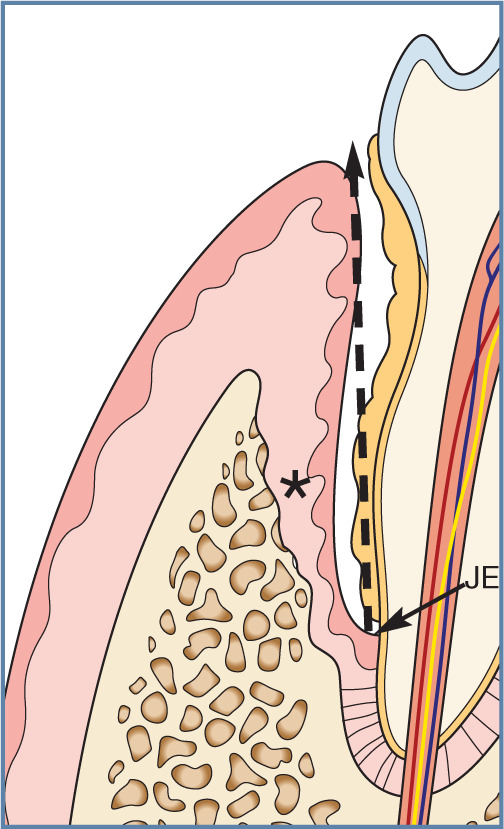
Vertical bone loss in periodontitis. Marked bone (*) and periodontal ligament destruction is seen, and the attachment of the junctional epithelium (JE) to the tooth is at a more apical position. This has resulted in the formation of a deep pocket (broken arrow), which may be measured with a periodontal probe
Figure 20.
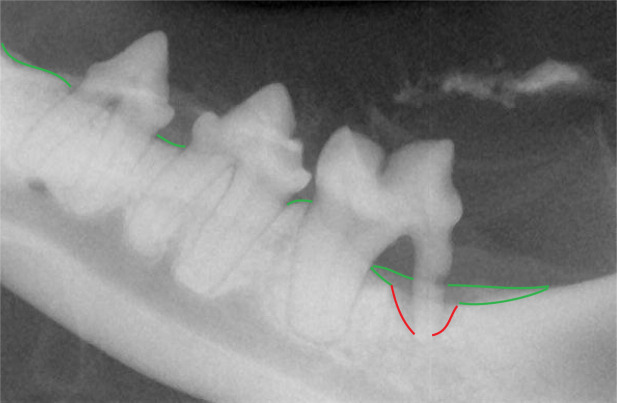
Radiographic appearance of vertical bone loss in periodontitis. Typically one root or one tooth is affected. Here the distal root of the left mandibular first molar (309) is affected (red lines). The alveolar bone margin is shown in green, and it is evident that there is also horizontal bone loss affecting this tooth. Thus, vertical and horizontal bone loss may be seen concurrently. Note also the resorption affecting the distal root
In a retrospective study of the dental records of 147 cats at a university teaching hospital, radiographic patterns of periodontitis were assessed. 19 Twenty-eight percent of cats had normal bone height with no evidence of horizontal or vertical bone loss (ie, no evidence of periodontitis), while 55% showed evidence of generalised horizontal bone loss. Vertical bone loss was seen focally in 33% of cats. In addition, tooth resorption was noted in 67% of cats. Purebred cats in the study were no more likely than mixed-breed cats to have signs of peri-odontitis, but, when present, it was more likely to be moderate or severe. Horizontal bone loss is the most common radiographic pattern of periodontal bone loss in cats, a finding corroborated in a study by Girard et al. 20
Radiographic analysis alone will underestimate the amount of attachment loss as a radiograph is a two-dimensional projection of a three-dimensional structure, and an estimated 30–60% of mineralisation must be lost before it will be seen radiographically;19,21,22 furthermore, attachment loss involves soft as well as hard tissues. The only way of reliably identifying periodontal pockets is with careful probing of the gingival sulcus around each tooth in the mouth, accompanied by dental radiography. In one study, 25% of cats had evidence of periodontitis and bone loss on radiographs that was more severe than clinically apparent. 23
Clinical findings, treatment planning and treatment are all recorded on a dental chart as part of the required medico-legal patient records (see accompanying article on oral examination in Part 1 of this Special Issue series).
Association of periodontitis with other dental/oral diseases
Tooth resorption
Resorption of cementum and dentine is often seen adjacent to areas of periodontal inflammation (type 1 tooth resorption – see accompanying article in this Special Issue series, pages 37–43). 27 In the radiographic study by Lommer and Verstraete, 19 cats with tooth resorption were more likely to have severe focal vertical bone loss and less likely to have normal alveolar bone height than cats without tooth resorption. In a case-control study of 534 teeth with tooth resorption, periodontitis was seen in 72% of teeth with type 1 (external inflammatory) resorption, but only 15.6% of teeth with type 2 (replacement) resorption. These were statistically significant differences, suggesting that an inflammatory trigger drives type 1 resorption. 28 A correlation between type 1 resorption and periodontal disease was also found in the study by Girard et al 20 (Figure 21).
Figure 21.
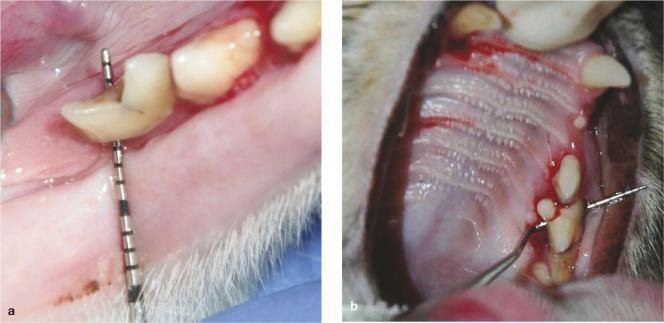
(a,b) Periodontitis may be associated with type 1 (external inflammatory) tooth resorption, as in this 2.5-year-old Siamese cat suffering from an aggressive form of periodontitis. Note the horizontal bone loss, furcation exposure and type 1 resorption in all mandibular premolars and molars. Complete extraction of these teeth is indicated
Feline chronic gingivostomatitis
In a retrospective case-control study of 101 cats with feline chronic gingivostomatitis (FCGS), it was found that all cats with FCGS had periodontitis, with 77% showing semi-generalised or generalised alveolar bone loss, which was a significantly higher proportion than in cats without FCGS (P <0.001). 29 Furthermore, the cats with FCGS had a significantly (P <0.001) higher prevalence of external inflammatory root resorption (type 1) than cats without FCGS. This is of clinical significance because type 1 resorption predisposes to root fracture, and root remnants may be a predisposing cause of refractory stomatitis after premolar–molar/full mouth extractions in the cat. 30 Plaque is known to be a cause of periodontal disease in the cat, and is likely to be a contributory factor in FCGS.
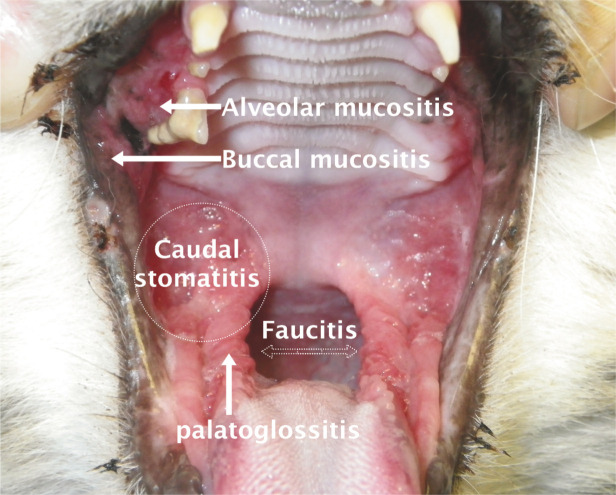
However, the clinician must be cautious in diagnosing FCGS. Severe gingivitis in a patient, with calicivirus isolation using PCR technology, does not automatically provide a diagnosis of FCGS. Many cases of juvenile gingivitis may be mistaken for FCGS. The inflammation in FCGS, by definition, extends beyond the mucogingival junction to encompass the alveolar mucosa and other soft tissues including the lingual mucosa, glossopalatine folds, caudal oral mucosa and occasionally the fauces (Figure 22). If inflammation is confined to gingival tissues, by definition a diagnosis of FCGS cannot be made. The administration of antiviral and immunomodulatory treatments to young cats suffering from intense gingivitis has no scientific basis.
Figure 22.
Correct terminology for inflammation seen in feline chronic gingivostomatis (FCGS). The caudal area of the mouth (commonly and incorrectly referred to as the fauces) does not have a specific anatomical term; inflammation here should be referred to as caudal stomatitis or mucositis. The fauces are actually the lateral walls of the oropharynx, housing the tonsils. Inflammation here is termed faucitis but would usually only be identified under anaesthesia. Inflammation may extend beyond the mucogingival junction to involve the alveolar mucosa (alveolar mucositis) and eventually cheek mucosa (buccal mucositis). The palatoglossal folds may also be affected in this frustrating condition. Caudal stomatitis must be identified in order to diagnose FCGS
Buccal bone expansion
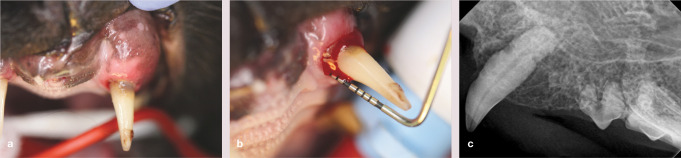
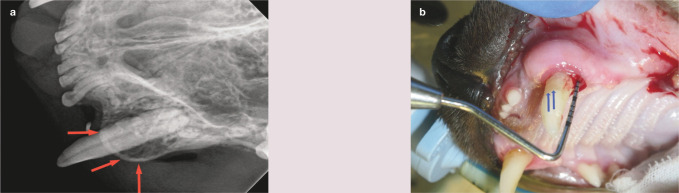
The normal width of the buccal alveolar bone at the canine teeth is <2 mm. 19 Expansion of the buccal alveolar bone overlying the maxillary canine teeth, creating a bulbous appearance, may be seen and has been postulated to be due to chronic alveolar osteitis (Figures 23 and 24).31–33 Expansion of the buccal alveolar bone overlying the canine teeth was found in 53% of cats in the radiographic study by Lommer and Verstraete, 19 but further research is warranted to determine the exact pathogenesis of this phenomenon.
Figure 23.
(a) Marked buccal bone expansion and periodontitis affecting the left maxillary canine (204). (b) Deep periodontal probing (7 mm) is evident on the palatal aspect. (c) The radiograph shows the expansion, loss of the periodontal ligament space due to vertical bone loss, and also external resorption of the root
Figure 24.
(a) Buccal bone expansion (red arrows) and periodontitis affecting the left maxillary canine (204). (b) There is a probing depth of 6 mm on the distal aspect of this tooth. Note that the tooth is also supererupted; the cemento-enamel junction is indicated by the blue arrows
Supereruption/extrusion of canine teeth
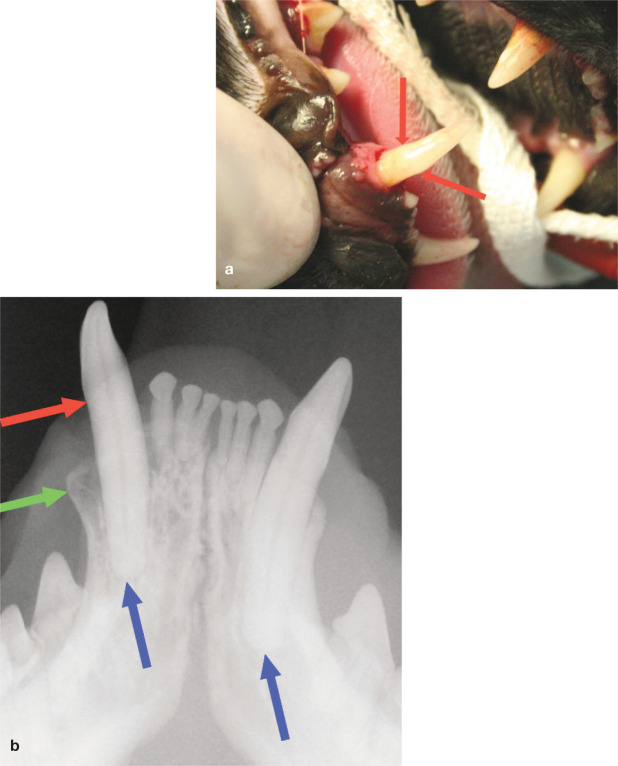
it has been suggested that abnormal extrusion (supereruption) of the canine teeth may also be associated with periodontal disease. 32 This is where the crown of one or more canine teeth appears to be longer compared with the contralateral canine (Figure 25). The alveolar margin would normally be within 1 mm of the cemento-enamel junction; in this condition the distance is visibly increased, with the cemento-enamel junction some distance from the gingiva. 19 A positive correlation has also been found between this condition and tooth resorption. 34 In the same study, hypercementosis was found in all extruded teeth examined histologically, and may play a role in the pathogenesis.
Figure 25.
Supereruption of the canine tooth. (a) The right mandibular canine (404) is abnormally extruded from the mouth. The cemento-enamel junction (red arrows) can be seen in a coronally displaced position, as also shown on the radiograph (b). Buccal bone expansion can additionally be seen in the radiograph (green arrow). Note the relative position of the canine root apices (blue arrows)
The authors’ own clinical findings, and the opinion of other authors, is that abnormal extrusion is often accompanied by alveolar bone expansion; in some cases external resorption is also seen radiographically. 31
Association of periodontal disease with systemic diseases
The statistical association of periodontal disease with various systemic diseases (such as chronic kidney disease [CKd], cardiovascular disease, chronic respiratory disease and diabetes mellitus), as well as pre-term births and premature death, has been well described in people and, to a lesser extent, dogs.38 –42 However, a definitive cause and effect has yet to be epidemiologically demonstrated despite strong and significant associations. 1 Plausible hypotheses include the release of bacteria from biofilms into the periodontal pocket, which then gain access to the circulation via ulcerations in the pocket epithelium; and/or promotion of systemic inflammation by bacteria, via stimulation of production of pro-inflammatory cytokines and acute phase proteins. 1
Less is known, however, about the systemic implications of periodontal disease in the cat. One study has assessed the correlation between systemic health indices and severity of periodontitis in cats. 43 Immunoglobulin G levels and total globulins were positively correlated with periodontal disease status in 30 cats and showed significant reductions after periodontal therapy. Another study has suggested that periodontal disease may be a risk factor for CKD in the cat, although the study design limited this to a coexistence, rather than cause and effect. 44
It is worth noting that some types of treatment for CKD (in particular, the calcium channel blocker, amlodipine) may contribute to periodontal disease by triggering gingival hyperplasia (Figure 26). 39 Anecdotally, glycaemic control appears improved in diabetic cats after periodontal therapy and may warrant reductions in insulin dosing. One study of a small population of Burmese cats identified dental disease requiring treatment as a risk factor for diabetes. 45 Unfortunately, however, the term ‘dental disease’ is too vague to allow meaningful conclusions, but this area of medical research warrants further attention.
Figure 26.
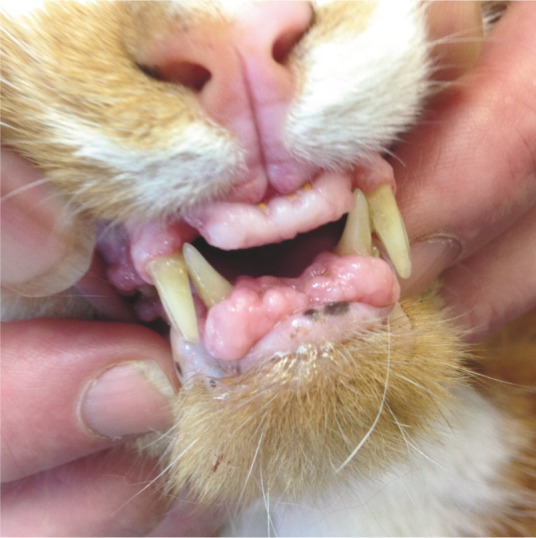
Gingival enlargement due to drug administration. This cat was being treated with the calcium channel blocker amlodipine for systemic hypertension associated with chronic kidney disease. Courtesy of Chirag Patel
Juvenile periodontal disease
Juvenile gingivitis may be seen in young cats around the time of adult tooth eruption (Figure 27), and is associated with marked gingival inflammation. This should not be misdiagnosed as FCGS – a thorough oral examination will not show any inflammation extending onto the alveolar mucosa (unless CUPS lesions are present) or caudal oral area. Calicivirus testing is not warranted in these cats as the virus has no connection with periodontal disease.
Juvenile hyperplastic gingivitis may also be seen in some young cats under 9 months of age (Figures 28 and 29). The gingiva may become very inflamed and enlarged, particularly over the maxillary fourth premolar teeth, creating a pseudopocket. Treatment involves gingivectomy and professional periodontal therapy, followed by thorough dental home care potentially for 2 years, at which point the condition may settle. Again, this should not be mistaken for FCGS, a condition that has an unclear and complex aetiology. There is no evidence to support the use of an antiviral or immunomodulatory approach in cats with juvenile periodontal disease.
Juvenile periodontitis may be seen as a rapidly progressive condition in young Siamese, Maine Coon and domestic shorthair cats, initially involving severe and aggressive periodontal inflammation (Figure 30). 46 The treatment of choice in these cases is extraction of affected teeth (Figure 21), gingivectomy where required, and thereafter regular professional therapy combined with thorough dental home care.
Figure 27.
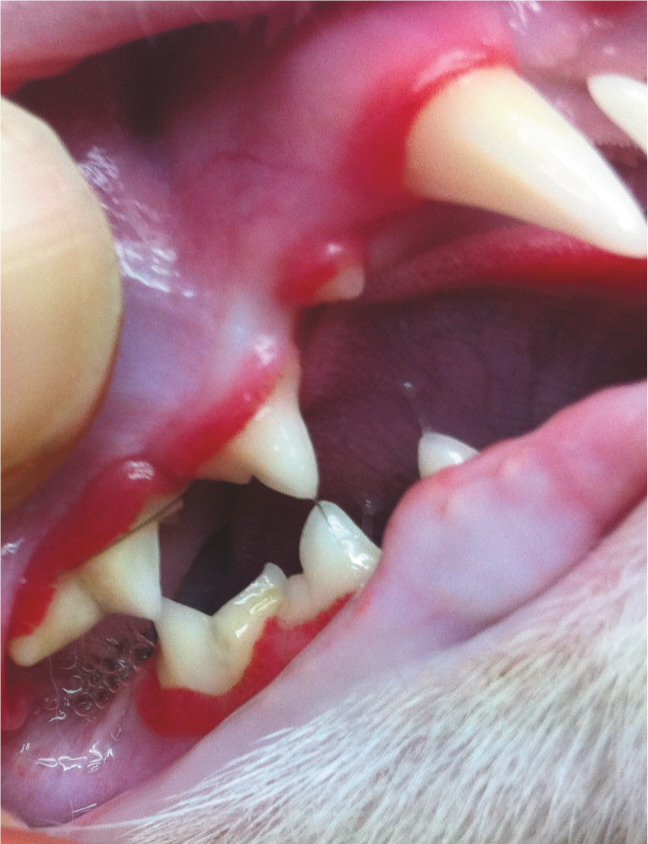
Juvenile gingivitis in a 10-month-old male neutered domestic shorthair. The inflammation is affecting much of the attached gingiva. It should not be mistaken for chronic gingivostomatitis
Figure 28.
Juvenile hyperplastic gingivitis in an 8-month-old male neutered domestic shorthair. The enlarged gingival tissues are creating pseudopockets, which will have deep probing depths. The attachment level is not altered, hence these are not pathological pockets. Treatment includes gingivectomy to remove the enlarged tissue, scaling and polishing of teeth, followed by plaque control at home
Figure 29.
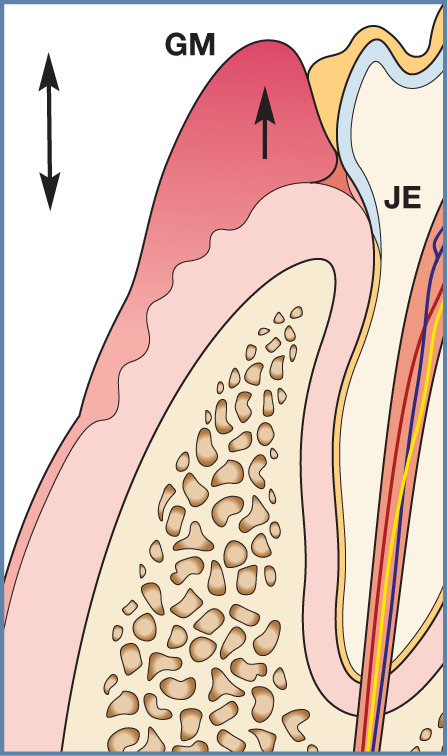
Gingival hyperplasia is a histological and not a clinical diagnosis. The gingiva enlarges (arrow) due to an increase in the number of gingival cells. Clinically, the gingival margin is seen in a more coronal direction, causing increased probing depths (pseudopockets; double-ended arrow). These are not true pathological pockets as the attachment level of the junctional epithelium to the tooth is not altered
Figure 30.
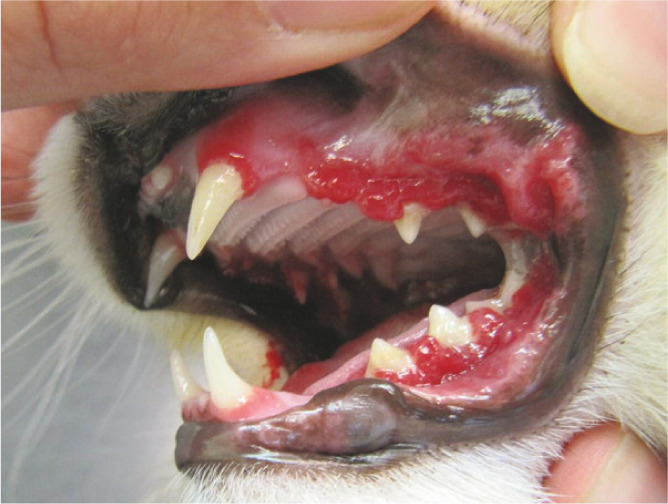
Juvenile periodontitis in a 1-year-old male neutered Maine Coon. Note the severe gingivitis affecting the entire attached gingiva, areas of gingival recession and gingival enlargement. There is also evidence of inflammation in the buccal mucosa of the cheeks (CUPS lesions) and buccal mucositis in the commissures of the lips; this is similar to that seen in dogs and is an inappropriate immunological reaction to plaque present on the teeth. Courtesy of Elise Robertson
Treatment aims and first principles
The aims of treatment are to remove dental plaque and any predisposing factors (such as calculus or overcrowding of teeth) to allow inflamed tissues (gingivitis) to return to normal health and to stop progression of disease and involvement of further sites (periodontitis). Any professional treatment must be followed up by dental home care, otherwise the disease state will relapse and likely progress.
The polymicrobial nature of dental plaque means that antibiotic treatment alone is never a suitable treatment for feline periodontal disease. Microbes within a biofilm do not behave in the same way as planktonic bacteria. Resistance to antimicrobial agents is significantly enhanced in a biofilm environment and the indigenous nature of many bacterial species means that re-infection will occur quickly.4,6 The practice of pulse-dosing of antibiotics has no supporting scientific evidence, and may in fact contribute towards methicillin-resistant Staphylococcus aureus infections in dogs and cats. 47
Prior to anaesthesia it is essential that the veterinary surgeon obtains the signalment of the patient, takes a full history and performs both a conscious oral examination and general physical examination. Periodontal disease becomes more common as patients age, as do concurrent diseases. The all-too-common notion that a cat is ‘too old for a dental’ has no place in modern practice. Periodontal disease, tooth resorption and broken teeth are often over-looked sources of (sometimes significant) pain in this species. 48 Approaching general anaes-thesia in a carefully considered manner can provide all patients with the opportunity to have painful dental problems addressed, regardless of age. A practical overview of peri-operative anaesthetic care of the feline dental patient is provided in an accompanying article in this Special issue series (pages 23–36).
Once anaesthetised, a full and comprehensive dental and oral evaluation must be performed, and findings thoroughly recorded. Further discussion on oral examination and dental radiography in the cat can be found in Part 1 of this Special issue series (November 2014, pages 873–886 and 887–899, respectively).
Rinsing the mouth with an oral 0.12% chlorhexidine gluconate or acetate solution at this stage can help to reduce bacteraemia for the patient, and the resultant bacteria-laden aerosol in the dental room, increasing operator safety. To further increase operator safety, the donning of personal protective equipment, including gloves, goggles, face mask and protective clothing, is strongly advised. 49
Scaling of teeth
Scaling of teeth removes calculus deposits and may be performed using hand instruments alone or a combination of sonic or ultrasonic and hand instruments. 50 Hand scaling is as effective as ultrasonic scaling, although more time-consuming and fatiguing. 51
All supra- and subgingival deposits must be thoroughly identified and removed during treatment – removal of only supragingival calculus is purely a cosmetic exercise.
Hand instruments
Hand instruments have sharp working edges, and are manipulated over the tooth surface to mechanically break the bond between the tooth and calculus deposits. 50
Hand scalers have a sharp-pointed tip and a sharp working edge (Figures 31 and 32). Various designs are available, including Jacquette and sickle styles.
Curettes are blunt-tipped instruments with a sharp working edge, and are used to debride root surfaces (Figures 32 and 33). Various designs are available in human dentistry, including a universal design that may be used on any tooth, and Gracey curettes, which are area-specific within the mouth and identified with specific numbers. 51 The latter have an offset blade with one cutting edge (lower edge is cutting, upper edge is non-cutting); each cutting edge is also curved to adapt to the root surface. The instrument is usually double ended, and each end will be a mirror image of the opposite end. Note that, due to the small size of feline teeth, many curettes are completely inappropriate. Instruments with smaller blade lengths and widths are useful (eg, Micro Mini Five and Mini Five; Hu-Friedy).
Figure 31.
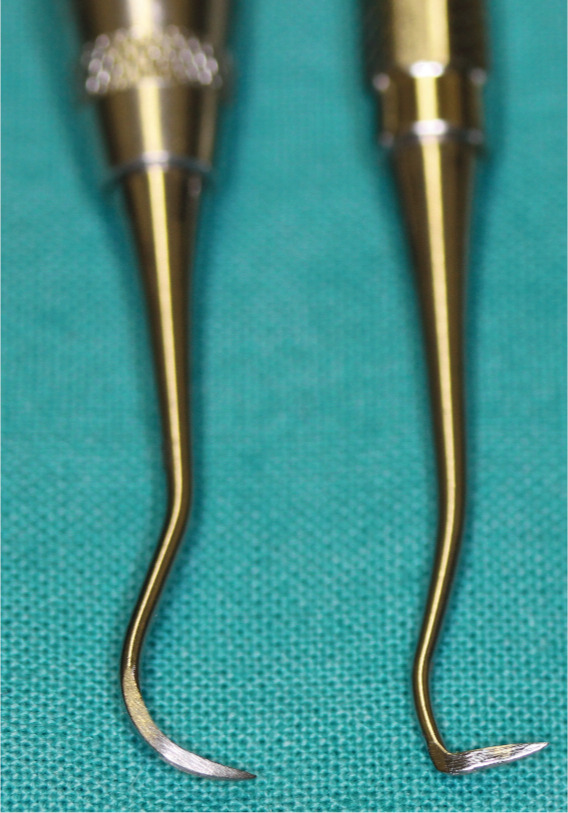
Hand scalers: sickle scaler (left) and Jacquette scaler (right). These are used in a pull motion to remove supragingival calculus deposits. Note that the sharp tip and back could damage epithelial tissue if inserted beneath the gingival margin
Figure 32.
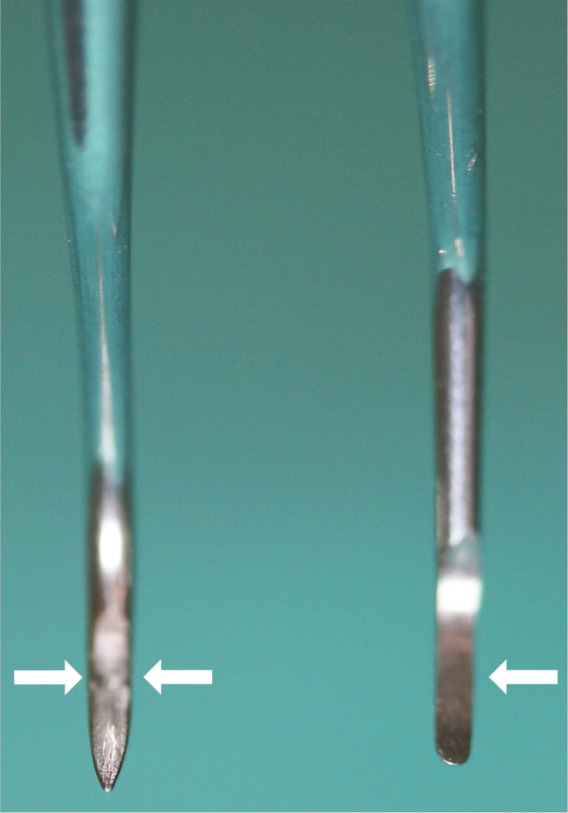
Image comparing the pointed tip (‘toe’) of the scaler (left) and rounded toe of the curette (right). The Gracey curette has one offset cutting edge (the lower edge, arrow), while the scaler has two cutting edges (arrows)
Figure 33.
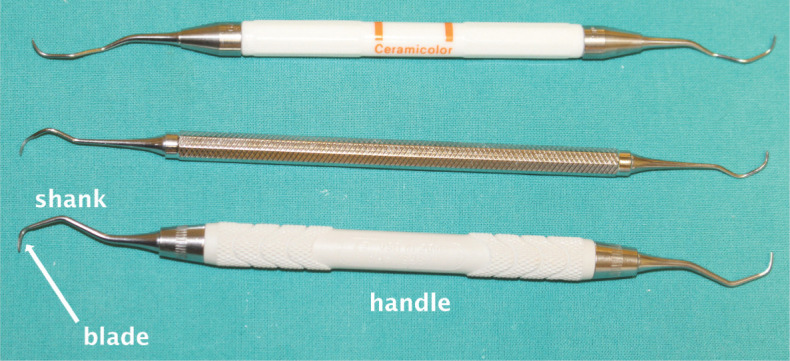
Gracey curettes for supra- and subgingival calculus removal. These instruments are numbered for use in the human mouth, with lower numbers being used on anterior teeth and higher numbers on posterior teeth. The blade has one cutting edge, but a rounded tip safe for subgingival use. A shank that is offset from the handle is seen in posterior instruments to enable access to the teeth
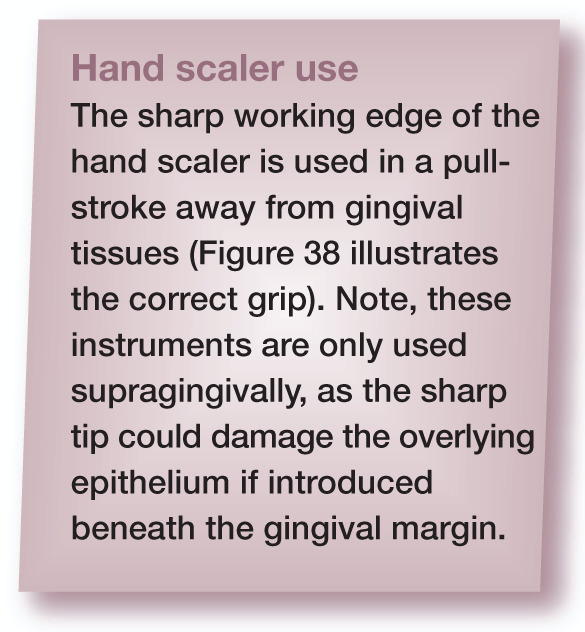

Figure 34.
(a) The curette is introduced into the periodontal pocket with the face flattened against the tooth surface. The instrument is then rotated (arrow) to ensure the terminal shank is parallel with the long axis of the tooth, engaging the cutting edge with the tooth surface. (b and c) The instrument is pulled in the direction of the arrow. Note the two different styles of curette with straight (b) and offset (c) shanks. In both cases the terminal shank is parallel to the tooth axis
Ultrasonic scalers
Ultrasonic scalers work in a similar way to hand scalers, but the tip is electromechanically driven to oscillate, and this movement dislodges the calculus from the tooth surface. The frequency of oscillation is normally between 25 and 42 kHz. The amplitude of tip vibration may be altered using the power setting on the scaler.
Frictional heat is generated by all scalers (some more than others) during tip vibration, and must be dissipated with a fine spray of water coolant (Figure 35) to avoid thermal damage to the tooth (pulpitis and possibly necrosis) and surrounding soft tissues. Increasing the power setting will increase the heat generated. 52 It is essential that this flow of water reaches the scaler tip. Furthermore, the tip must be designed for use under the gingival margin, otherwise the coolant flow may be obstructed. The water flow confers additional bactericidal benefits – the ultrasonic waves formed cause a particular type of fluid flow (acoustic microstreaming) and production and implosion of gas bubbles (cavitation) that disrupt the biofilm. 50
Figure 35.

An adequate water flow should always be ensured before using an ultrasonic scaler on a tooth. The fine halo mist dissipates heat generated during instrument use
Ultrasonic units in dentistry are available in two basic types – magnetostrictive and piezoelectric (Figure 36).
Figure 36.
Ultrasonic scalers. (a) Magnetostrictive metal stack insert, (b) magnetostrictive ferrite rod (showing rod on the left and inserted into the hand piece on the right) and (c) piezoelectric scaler. Note the power setting on the piezoelectric scaler
Magnetostrictive scalers include the stack type and ferrite rod. The stack type are strips of laminated nickel inserted into the hand piece that shorten and lengthen when alternating current is applied. This causes the attached tip to vibrate at a similar frequency. The tip moves in an ellipsoid, figure-of-eight pattern, meaning there are phases when the scaler does not touch the tooth surface. The rod type (42 kHz) tends to produce an elliptical pattern, with fewer ‘dead spots’ during its cycle. Cavitation occurs throughout the vibration cycle.
-
Piezoelectric scalers incorporate piezoelectric crystals in the hand piece that vibrate due to the alternating current, in turn causing the tip to vibrate. Unlike the magnetostrictive scalers, the tip vibrates in a back-and-forth (linear) movement, so can potentially provide increased scaling efficiency.
For optimal and safe scaling using an ultrasonic device, the clinician needs to bear in mind the following:
The highest movement (and therefore efficiency) is at the end of the tip, so the terminal 1–2 mm should be placed in contact with the tooth. The tip will wear down with time and become less efficient, and so should be replaced periodically. Wear guides are available from manufacturers (Figure 37).
Pressing hard on the scaler tip to dislodge tenacious calculus deposits will dampen the vibration, making the process less efficient and also more injurious to the tooth, 50 partly as a result of the increased frictional heat generated. 52 Scalers should be held using the modified pen grip (Figure 38), and a feather-light touch applied.
The tip should be orientated parallel to the tooth surface at all times (Figure 39). Directing it perpendicular to the tooth surface is akin to placing a pneumatic drill on the tooth.
Figure 37.

Manufacturers supply a wear guide for ultrasonic tips, which should be checked regularly and changed when necessary
Figure 38.
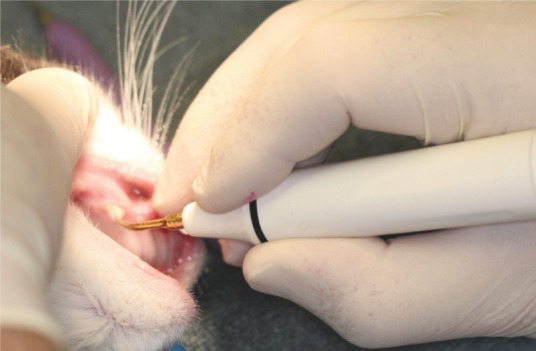
The scaler hand piece should be held using the modified pen grip. The middle finger is extended down towards the tip of the instrument, and the fourth and fifth fingers used as a ‘rest’ to stabilise the working position. This is also the correct grip for hand scalers and curettes
Figure 39.
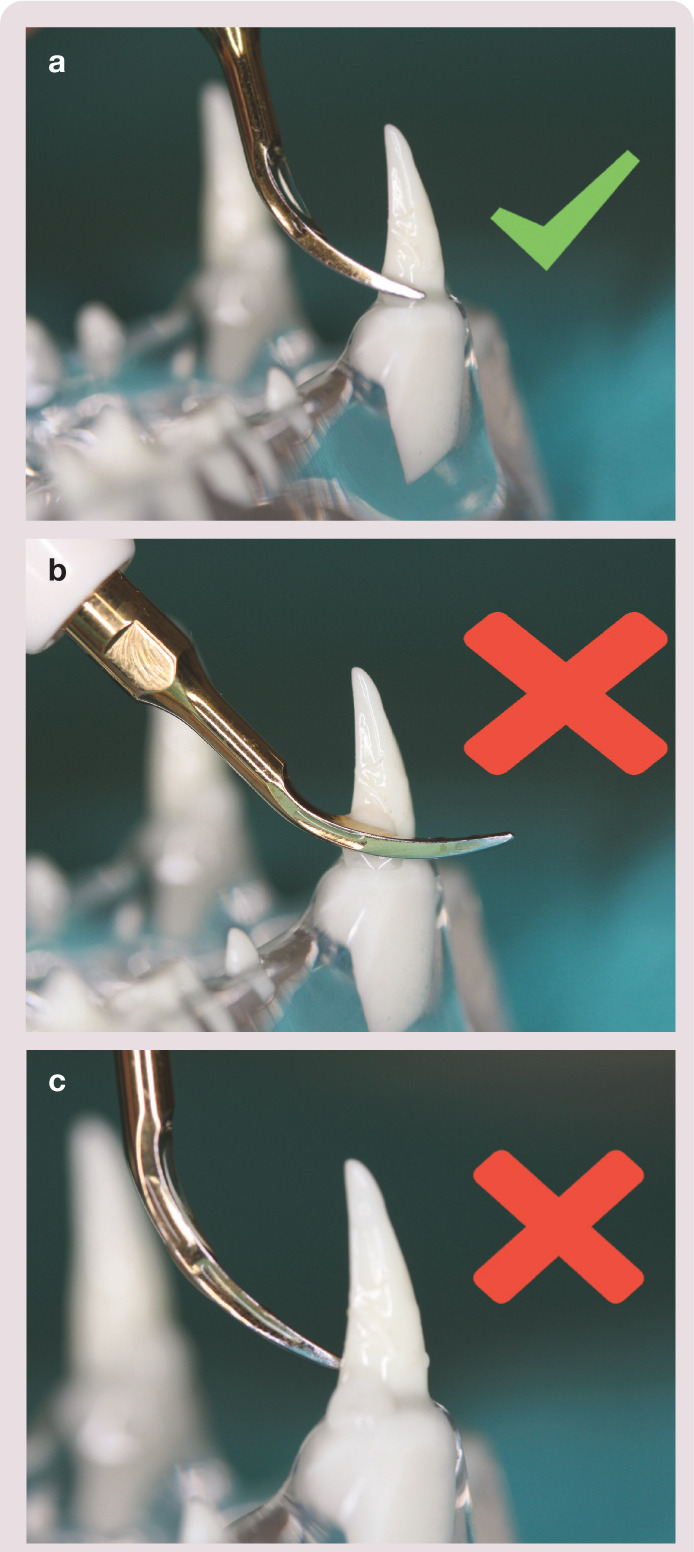
Correct (a) and incorrect (b,c) use of an ultrasonic scaler tip on a tooth
Different types of tip are available for gross supra-gingival calculus deposits, mild-to-moderate supra-gingival deposits, and sub-gingival deposits. Tips designed for subgingival use tend to be long and slender, and should always be used with the power setting on low. Scaler tips are also available that mimic hand curettes for subgingival debridement (Figure 40).
Figure 40.
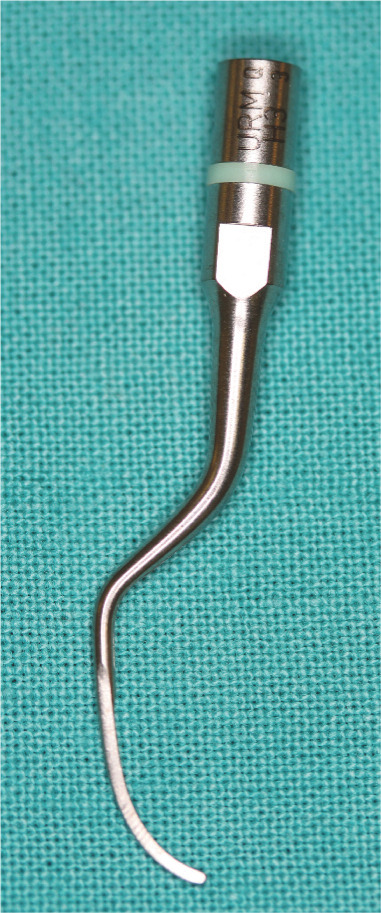
Ultrasonic piezoelectric curette tip, designed to be used subgingivally like a hand curette to debride a periodontal pocket. This instrument is used in a ‘push stroke’, unlike the ‘pull stroke’ of the hand curette.The power setting must be set to low

Polishing of teeth
Polishing removes any residual plaque dental deposits, and in human dentistry is also important in removing stain. it is performed using a soft rubber cup attached to a low-speed hand piece with medium-to-fine prophylaxis paste (Figures 41 and 42).
Figure 41.
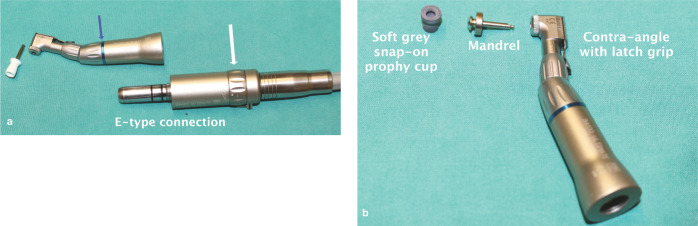
(a,b) Polishing equipment. A contra-angle hand piece is attached to a low-speed motor via an E-type attachment. The blue band (blue arrow in [a]) on the hand piece denotes a 1:1 ratio; that is, the output speed of the hand piece is the same as the motor. The rotating speed can be adjusted by altering the speed ring on the motor (white arrow in [a]). A latch-grip standard white prophy cup is shown in image (a) and a preferable soft grey cup in image (b). This snaps onto a mandrel that has a latch-grip attachment to the hand piece. Screw-in cups are also available
Figure 42.
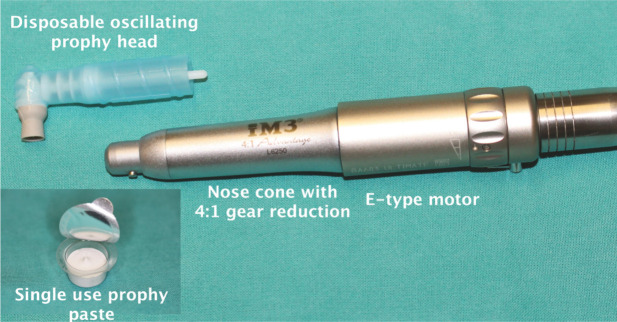
A preferable arrangement for polishing feline teeth is the disposable prophy head, which attaches to a nose cone (also attached to the E-type motor). This nose cone has a 4:1 reducing ability, meaning the rotating speed of the cup is 4 x slower than the motor. The grey cup is soft and, instead of rotating through 360°, it oscillates back and forth. (inset) A single-use volume of fine prophylaxis paste
The function of polishing has been somewhat misunderstood over the years, and has been a subject of controversy in the human dental field since the 1970s. Dogma states that ‘polishing must be performed after scaling to smooth out scratches in the tooth surface and delay plaque re-colonisation’, but there is no solid evidence to support this. Regular ‘routine scaling and polishing’ removes tooth substance, and over a number of years of a human’s life may potentially remove excessive amounts. A move towards ‘selective polishing’ was taken by the American Dental Hygienists’ Association in the 1990s, which is to polish teeth in order to remove stain (ie, a cosmetic, rather than therapeutic exercise).
Considerable debate continues to surround the need for ‘routine scaling and polishing’, particularly a single-visit scale and polish, and the frequency at which this should be performed. A meta-analysis assessing the need for and frequency of routine scaling and polishing was unable to derive any meaningful conclusions on either the frequency of pro-phylactic treatments or the therapeutic effects. 55 Some studies have shown that human patients expect their teeth to be polished, and may feel dissatisfied if this is not performed. 56 So, while the absolute need for polishing in our veterinary patients is debatable (particularly if no oral home care is instigated), if it is performed it must be per-formed safely (see box on the left).
What may subsequently help to delay plaque re-colonisation is the application of a barrier sealant to the teeth once cleaned. A hydrophilic professionally applied liquid sealant has shown short term efficacy in plaque and calculus reduction in the dog. 57 A barrier gel system that is commercially available in some countries has been shown to produce short-term plaque reduction in cats, but no changes in gingivitis or calculus levels. 58 The gel is professionally applied, and then follow-up applications are administered by the client at home.
Use of antibiotics in periodontal disease
Antibiotics may be used either to prevent or treat bacterial infections, and may be delivered locally or systemically.
Antibiotic prophylaxis
Antibiotics for prophylaxis should prevent infection either at the site of surgery or at a distant site in the body.
In human dentistry the prophylactic use of antibiotics has been the subject of fierce debate in recent years. It is known that a transient bacteraemia is produced during procedures such as tooth extraction, scaling and periodontal probing. The bacteraemia has been linked to distant-site infections such as infective endocarditis and atherosclerosis. However, it has also become apparent that similar levels of bacteraemia can occur during chewing and toothbrushing.53,59 The true association between dental procedures and infective endocarditis in humans is probably much less strong than once thought, and a recent systematic review and meta-analysis found no evidence that prophylactic antibiotics reduce the incidence of infective endocarditis. 60
An American Veterinary Dental College position statement on the use of prophylactic antibiotics suggests reserving systemic administration for patients that are either immunocompromised, or suffering from clinically apparent cardiac, renal, hepatic or other systemic diseases. 61 In these patients antibiotic prophylaxis may be accomplished by using a single subcutaneous, intramuscular or intravenous dose of a suitable drug (such as clavulanate–amoxicillin, cefuroxime or ampicillin) at the time of pre-medication.
Antibiotic treatment
it seems rational to include antibiotics in the treatment of periodontal disease as bacteria are the causative agent. However, as discussed earlier, it is rare that a single species is responsible. Rather, a complex biofilm of many species of bacteria is involved, resisting traditional antibiotic doses. Furthermore, the use of systemic antibiotics is not without risks, either of side effects or of contributing to bacterial resistance. 54 The treatment of periodontal disease includes the mechanical removal of plaque, and any predisposing factors such as calculus or tooth crowding. Locally delivered antibiotics including minocycline and doxycycline have been studied in human dentistry. Although studies have shown increases in attachment levels with their use, this may be in the order of <1 mm, so the clinical relevance is debatable.62,63
Should systemic antibiotics be required as an adjunctive treatment in cases of severe or aggressive disease, an appropriate choice should be made that will cover both aerobic and anaerobic species.53,54 Whenever electing to use an antibiotic in a patient, the clinician should give due consideration to the need, benefit and possible risks, and be able to clinically justify the decision.
Home care options for prevention of periodontal disease
Human dentistry is a proactive and preventative discipline. Veterinary dentistry, conversely, still appears to be a largely reactive endeavour, treating disease once it occurs, sometimes at a late stage in the disease process. Clinicians are therefore encouraged to make efforts to prevent periodontal disease before the patient shows clinical signs. At the very least, intervention should occur during the gingivitis stage of disease. This includes educating clients, and starting discussions about periodontal disease and prevention in young kittens.
Plaque will start to re-accumulate within hours of professional treatment, with visible signs of calculus appearing within 2 weeks. It is, therefore, also imperative that any client whose pet receives treatment for periodontal disease under anaesthesia is given clear recommendations about ongoing home care and the potential sequelae of ignoring these; the concept of periodontal disease management, rather than treatment/cure, should also be emphasised. The whole veterinary team should be aware of home care options, and feel confident in discussing them with clients. 49 In choosing the most appropriate home care option for a patient, not only must the veterinary surgeon assess the patient’s behaviour, but there should also be a detailed discussion with the client to determine their expectations, wishes, commitment and physical ability.
Role of diet in periodontal health
It is a common belief that dry foods are protective of periodontal health and wet foods conducive to periodontal disease; and similarly that a raw or natural diet may also be protective against periodontal disease. Research, however, does not necessarily corroborate these tenets. A study in Australia comparing the periodontal health of feral cats eating a natural diet of mammals, birds, reptiles and insects with domestic cats fed a mixture of commercially available canned and dry food found statistically significant lower levels of calculus in the feral cats, but no difference in the prevalence of periodontal disease between the two groups. 69 There were similar findings in a feral population of cats on Marion island, South Africa; that of low calculus levels, but high levels of periodontal disease. 70 In other words, cats fed a natural diet may have teeth that look clean, but periodontal tissues are not necessarily healthy. Also, the Polish survey referred to earlier found a significantly increased risk of poorer oral health in cats fed a home-prepared diet compared with those fed either a dry or wet commercial cat food. 65
Enhanced chewing activity can help to control plaque. increasing both the surface area and thickness of a dry diet with enhanced textural characteristics has been shown to promote plaque reduction. 71 Furthermore, the addition of polyphosphates to such dental-specific diets and treats may help to reduce calculus by chelating salivary calcium. 72 A dental-specific diet may, therefore, help to control plaque and calculus, but the ability to control periodontal disease long-term is not known. Furthermore, it is unknown what the effect is in cases of established periodontitis.
Additional chewing activity can also help to control plaque in cats. The Polish survey demonstrated the benefit of a daily dental treat on the oral health status of cats. 65 Other research has also demonstrated that a daily dental chew may help to reduce accumulation of plaque and calculus as well as reducing gingivitis levels.73,74
Chemical methods of plaque control
Chlorhexidine This agent can penetrate the plaque biofilm and is an effective antimicrobial. 5 It attaches to the pellicle layer on teeth and is available in rinse, spray, gel and toothpaste form. It may be combined with toothbrushing; or, if the client struggles with brushing, it can be applied to the gingival margin using a cotton bud. Chlorhexidine substantivity also means that the antibacterial effect may continue for up to 12 h after application. Some formulations of the rinse, however, have a bitter flavour and may not be well tolerated.
Soluble zinc salts Some oral gels, pastes, and certain dental chews and dental diets contain zinc gluconate or zinc ascorbate. As these salts combine with volatile sulphur compounds they can help to reduce halitosis. In addition, they are antimicrobial. A short-term trial in cats showed that the application of a zinc ascorbate gel resulted in significant reductions in plaque accumulation, gingivitis and periodontal pathogens. 75 When using any antiseptic gel or paste, the client must actively apply the product to the gingival margin. There is no evidence to support the belief that cats may lick these products from their paws to exert a beneficial effect in the mouth.
Water additives A xylitol-containing water additive has been shown to reduce plaque and calculus in a short-term study in cats, although the blinding in the study was not clear. 76 A product containing a mixture of vitamins C and B2, papain and various plant and fruit extracts is available as a water additive, gel and oral spray and claims to reduce plaque accumulation, for which it has the VOHC seal (see box). A recent study has shown that the gel may enhance the effectiveness of tooth brushing in dogs. 77
Food supplements A powdered algal food supplement for cats (and humans) purports to reduce plaque and calculus, although there are no published studies supporting these claims. Several products are available containing RF2 factor, derived from the Chinese rhubarb plant. In vitro studies have demonstrated that the product reduces biofilm formation in a single species Streptococcus mutans colony. 78 This bacterium is commonly associated with caries formation in humans but is not implicated in periodontal disease in cats. Products include a water additive, chews and toothpaste and barrier wax.
Whenever making product recommendations for home care, the clinician should seek out the highest quality evidence to support the recommendations, and not rely on product marketing. If a product claims to be exceptionally good, and we are not using it in our own mouths, we must logically question why!
The future
Therapy in human dentistry is undergoing a shift towards modulating the host’s response to disease, and away from a direct antimicrobial approach. 1 For example, sub-antimicrobial doses of the antibiotic doxycycline appear to exert an anticollagenase effect, helping to reduce periodontal tissue destruction. The anti-inflammatory effect of fatty acids is also being actively researched. A monounsaturated fatty acid complex available in topical and dietary form has been shown to reduce pocket depth in rabbits 79 and cats. 80 Laser technology is looking set to be integrated routinely into human periodontal therapy. Its use can decrease biofilm and endotoxins, increase attachment levels, reduce pocket depth and stimulate healing with enhanced postoperative comfort. 81 While it is obvious that plaque control must remain a part of veterinary periodontal disease management, a single effective treatment or diet remains elusive.
Key Points
Periodontal disease is commonly identified in the cat and is seen in two distinct forms: gingivitis and periodontitis.
Gingivitis is reversible, but left untreated may progress to periodontitis, which is progressive and irreversible.
Juvenile forms of periodontal disease occur and should not be mistaken for chronic gingivostomatitis.
Type 1 tooth resorption is often associated with periodontal inflammation, and affected teeth should be completely extracted. Crown amputation is contraindicated in these cases.
Treatment of periodontal disease revolves around plaque and calculus removal supra- and subgingivally.
Periodontal disease, although plaque induced, may have a multifactorial aetiology.
Thorough effective dental home care is essential if periodontitis is to be prevented.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this article.
The authors do not have any potential conflicts of interest to declare.
Contributor Information
Rachel Perry, Perrydental Vet Ltd, Grove Lodge Veterinary Hospital, Upper Brighton Road, Worthing, West Sussex, BN14 9DL, UK.
Cedric Tutt, Cape Animal Dentistry Service, 78 Rosmead Avenue, Kenilworth, 7708, Cape Town, South Africa.
References
- 1. Dentino A, Lee S, Mailhot J, et al. Principles of periodontology. Periodontol 2000. 2013; 61: 16–53. [DOI] [PubMed] [Google Scholar]
- 2. Fiorellini JP, Kim DM, Uzel NG. Anatomy of the periodontium. In Newman MG, Takei HH, Klokkevold PR, et al. (eds). Carranza’s clinical periodontology. St Louis, MO: Elsevier Saunders, 2012, pp 12–27. [Google Scholar]
- 3. Nanci A. Oral mucosa. In Nanci A. (ed). Ten Cate’s oral histology development, structure and function. St Louis, MO: Mosby Elsevier, 2008, pp 319–367. [Google Scholar]
- 4. Teughels W, Quirynen M, Jakubovics N. Periodontal microbiology. In Newman MG, Takei HH, Klokkevold PR, et al. (eds). Carranza’s clinical periodontology. St Louis, Mo: Elsevier Saunders, 2012, pp 232–270. [Google Scholar]
- 5. Bernimoulin JP. Recent concepts in plaque formation. J Clin Periodontol 2003; 30: 7–9. [DOI] [PubMed] [Google Scholar]
- 6. Palmer RJ. Composition and development of oral bacterial communities. Periodontol 2000. 2014; 64: 20–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mallonee DH, Harvey CE, Venner M, et al. Bacteriology of periodontal disease in the cat. Arch Oral Biol 1988; 33: 677–683. [DOI] [PubMed] [Google Scholar]
- 8. Pérez-Salcedo L, Herrera D, Esteban-Saltiveri D, et al. Isolation and identification of Porphyromonas spp. and other putative pathogens from cats with periodontal disease. J Vet Dent 2013; 30: 208–213. [DOI] [PubMed] [Google Scholar]
- 9. Booij-Vrieling HE, van der Reijden WA, Houwers DJ, et al. Comparison of periodontal pathogens between cats and their owners. Vet Microbiol 2010; 144: 147–152. [DOI] [PubMed] [Google Scholar]
- 10. Jepsen S, deschner J, Braun A, et al. Calculus removal and the prevention of its formation. Periodontol 2000. 2011; 55: 167–188. [DOI] [PubMed] [Google Scholar]
- 11. Löe H, Theilade E, Jensen SB. Experimental gingivitis in man. J Periodontol 1965; 36: 177–187. [DOI] [PubMed] [Google Scholar]
- 12. Fiorellini JP, Kim DM, Uzel NG. Gingival inflammation. In: Newman MG, Takei HH, Klokkevold PR, et al. (eds). Carranza’s clinical periodontology. St Louis, Mo: Elsevier Saunders, 2012, pp 71–75. [Google Scholar]
- 13. Meitner SW, Zander HA, iker HP, et al. Identification of inflamed gingival surfaces. J Clin Periodontol 1979; 6: 93–97. [DOI] [PubMed] [Google Scholar]
- 14. Rossa C, Jr, Kirkwood KL. Molecular biology of the host-microbe interaction in periodontal diseases. In: Newman MG, Takei HH, Klokkevold PR, et al. (eds). Carranza’s clinical periodontology. St Louis, MO: Elsevier Saunders, 2012, pp 285–293. [Google Scholar]
- 15. Belibasakis GN, Bostanci N. The RANK-OPG system in clinical periodontology. J Clin Periodontol 2012; 39: 239–248. [DOI] [PubMed] [Google Scholar]
- 16. Carranza FA, Camargo PM. The periodontal pocket. In: Newman MG, Takei HH, Klokkevold PR, et al. (eds). Carranza’s clinical periodontology. St Louis, MO: Elsevier Saunders, 2012, pp 127–139. [Google Scholar]
- 17. Page RC, Schreuder HE. Pathogenesis of inflammatory periodontal disease. Lab Invest 1976; 33: 235–249. [PubMed] [Google Scholar]
- 18. American Veterinary Dental College. Periodontal disease classification. www.avdc.org/nomencla-ture.html#periostages, May 2012.
- 19. Lommer M, Verstraete FJ. Radiographic patterns of periodontitis in cats: 147 cases (1998–1999). J Am Vet Med Assoc 2001; 218: 230–234. [DOI] [PubMed] [Google Scholar]
- 20. Girard N, Servet E, Biouge V, et al. Periodontal health status in a colony of 109 cats. J Vet Dent 2009; 26: 147–155. [DOI] [PubMed] [Google Scholar]
- 21. Mulligan T, Williams C. Atlas of canine and feline dental radiography. Trenton, NJ: Veterinary Learning Systems, 1998, p 126. [Google Scholar]
- 22. Verstraete FJ. Self assesment colour review of veterinary dentistry. London, Manson Publishing, 1999, p 194. [Google Scholar]
- 23. Verstraete FJ, Kass PH, Terpak CH. Diagnostic value of full-mouth radiography in cats. Am J Vet Res 1998; 59: 692–695. [PubMed] [Google Scholar]
- 24. Lund Em, Armstrong PJ, Kirk CA, et al. Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States. J Am Vet Med Assoc 1999; 214: 1336–1341. [PubMed] [Google Scholar]
- 25. O’Neill DG, Church DB, McGreevy PD, et al. Prevalence of disorders recorded in cats attending primary-care veterinary practices in England. Vet J. Epub ahead of print 7 August 2014. DOI: 10.1016/j.tvjl.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 26. Gengler W, Dubielzig R, Ramer J. Physical examination and radiographic analysis to detect dental and mandibular bone resorption in cats; a study of 81 cases from necropsy. J Vet Dent 1995; 12: 97–100. [PubMed] [Google Scholar]
- 27. Hamp S-E, Lindberg R. Histology of spontaneous periodontitis in dogs. J Periodontal Res 1977; 12: 46–54. [DOI] [PubMed] [Google Scholar]
- 28. DuPont GA, DeBowes LJ. Comparison of periodontitis and root replacement in cat teeth with resorptive lesions. J Vet Dent 2002; 19: 71–75. [DOI] [PubMed] [Google Scholar]
- 29. Farcas N, Lommer MJ, Kass PH, et al. Dental radiographic findings in cats with chronic gingivostomatitis (2002–2012). J Am Vet Med Assoc 2014; 244: 339–345. [DOI] [PubMed] [Google Scholar]
- 30. Hennet P. Chronic gingivo-stomatitis in cats: longterm follow-up of 30 cases treated by dental extractions. J Vet Dent 1997; 14: 15–21. [Google Scholar]
- 31. Lemmons M. Clinical feline dental radiography. Vet Clin North Am Small Anim Pract 2013; 43: 533–554. [DOI] [PubMed] [Google Scholar]
- 32. Bellows J. Oral pathology. In: Feline dentistry: oral assessment, treatment and preventative care. Ames, IA: Wiley-Blackwell, 2010, pp 72, 114, 188–193. [Google Scholar]
- 33. Wiggs RB, Lobprise HB. Domestic feline oral and dental disease. In: Wiggs RB, Lobprise HB. (eds). Veterinary dentistry: principles and practice. Philadelphia: Lippincott-Raven, 1997, p 485. [Google Scholar]
- 34. Lewis JR, Okuda A, Shofer FS, et al. Significant association between tooth extrusion and tooth resorption in domestic cats. J Vet Dent 2008; 25: 86–95. [DOI] [PubMed] [Google Scholar]
- 35. Niemiec B. Periodontal disease. Top Companion Anim Med 2008; 23: 72–80. [DOI] [PubMed] [Google Scholar]
- 36. Demko JL, Cohn LA. Chronic nasal discharge in cats: 75 cases (1993–2004). J Am Vet Med Assoc 2007; 230: 1032–1037. [DOI] [PubMed] [Google Scholar]
- 37. Rosenquist K, Wennerberg J, Schildt EB, et al. Oral status, oral infections and some lifestyle factors as risk factors for oral and oropharyngeal squamous cell carcinoma. A population-based case-control study in southern Sweden. Acta Otolaryngol 2005; 125: 1327–1336. [DOI] [PubMed] [Google Scholar]
- 38. Cullinan MP, Seymour GJ. Periodontal disease and systemic illness: will the evidence ever be enough? Periodontol 2000. 2013; 62: 271–286. [DOI] [PubMed] [Google Scholar]
- 39. Craig RG. Interactions between chronic renal disease and periodontal disease. Oral Dis 2008; 14: 1–7. [DOI] [PubMed] [Google Scholar]
- 40. Chee B, Park B, Bartold PM. Periodontitis and type II diabetes: a two-way relationship. Int J Evid Based Healthc 2013; 11: 317–329. [DOI] [PubMed] [Google Scholar]
- 41. Glickman NT, Glickman NW, Moore GE, et al. Evaluation of the risk of endocarditis and other cardiovascular events on the basis of the severity of periodontal disease in dogs. J Am Vet Med Assoc 2009; 234: 486–494. [DOI] [PubMed] [Google Scholar]
- 42. Glickman LT, Glickman NW, Moore GE, et al. Association between chronic azotemic kidney disease and the severity of periodontal disease in dogs. Prev Vet Med 2011; 99: 193–200. [DOI] [PubMed] [Google Scholar]
- 43. Cave NJ, Bridges JP, Thomas DG. Systemic effects of periodontal disease in cats. Vet Q 2012; 32: 131–144. [DOI] [PubMed] [Google Scholar]
- 44. Greene JP, Lefebvre SL, Wang M, et al. Risk factors associated with the development of chronic kidney disease in cats evaluated at primary care veterinary hospitals. J Am Vet Med Assoc 2014; 244: 320–327. [DOI] [PubMed] [Google Scholar]
- 45. Lederer R, Rand JS, Hughes iP, et al. Chronic or recurring medical problems, dental disease, repeated corticosteroid treatment, and lower physical activity are associated with diabetes in Burmese cats [abstract]. J Vet Intern Med 2003; 17: 433. [Google Scholar]
- 46. Wiggs RB, Lobprise HB. Domestic feline oral and dental disease. In: Wiggs RB, Lobprise HB. (eds). Veterinary dentistry: principles and practice. Philadelphia: Lippincott-Raven, 1997, pp 483–485. [Google Scholar]
- 47. Soares Magalhães RJ, Loeffler A, Lindsay J, et al. Risk factors for methicillin-resistant Staphylococcus aureus (MRSA) infection in dogs and cats: a case-control study. Vet Res 2010; 41: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hellyer P, Rodan I, Brunt J, et al. AAHA/AAFP pain management guidelines for dogs and cats. J Feline Med Surg 2007; 9: 466–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Holmstrom SE, Bellows J, Juriga S, et al. AAHA dental care guidelines for dogs and cats. J Am Anim Hosp Assoc 2013; 49: 75–82. [DOI] [PubMed] [Google Scholar]
- 50. Lea SC, Walmsley Ad. Mechano-physical and biophysical properties of power-driven scalers: driving the future of powered instrument design and evaluation. Periodontol 2000. 2009; 51: 63–78. [DOI] [PubMed] [Google Scholar]
- 51. Wiggs RB, Lobprise HB. Dental equipment. In Wiggs RB, Lobprise HB. (eds). Veterinary dentistry: principles and practice. Philadelphia: Lippincott-Raven, 1997, pp 1–28. [Google Scholar]
- 52. Lea SC, Landini G, Walmsley D. Thermal imaging of ultrasonic scaler tips during tooth instrumentation. J Clin Periodontol 2004; 31: 370–375. [DOI] [PubMed] [Google Scholar]
- 53. Seymour RA, Hogg SD. Antibiotics and chemoprophylaxis. Periodontol 2000. 2008; 46: 80–108. [DOI] [PubMed] [Google Scholar]
- 54. Sarkiala-Kessel EM. Use of antibiotics and antiseptics. In: Verstraete FJM, Lommer MJ. (eds). Oral and maxillofacial surgery in dogs and cats. Edinburgh: Saunders Elsevier, 2012, pp 15–19. [Google Scholar]
- 55. Worthington HV, Clarkson JE, Bryan G, et al. Routine scale and polish for periodontal health in adults. Cochrane Database Syst Rev 2013; 11: CD004625. [DOI] [PubMed] [Google Scholar]
- 56. Needleman I, Suvan J, Moles DR, et al. A systematic review of professional mechanical plaque removal for prevention of periodontal diseases. J Clin Periodontol 2005; 32 Suppl 6: 229–282. [DOI] [PubMed] [Google Scholar]
- 57. Sitzman C. Evaluation of a hydrophilic gingival dental sealant in beagle dogs. J Vet Dent 2013; 30: 150–155. [DOI] [PubMed] [Google Scholar]
- 58. Bellows J, Carithers D, Gross SJ. Efficacy of a barrier gel for reducing the development of plaque, calculus, and gingivitis in cats. J Vet Dent 2012; 29: 89–94. [DOI] [PubMed] [Google Scholar]
- 59. Forner L, Larsen T, Kilian M, et al. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol 2006; 33: 401–407. [DOI] [PubMed] [Google Scholar]
- 60. Glenny A, oliver R, Roberts GJ, et al. Antibiotics for the prophylaxis of bacterial endocarditis in dentistry. Cochrane Database Syst Rev 2013; 10: CD003813. [DOI] [PubMed] [Google Scholar]
- 61. American Veterinary Dental College. AVDC position statements. http://www.avdc.org/statements.html (2005, accessed September 10, 2014).
- 62. Hung H-C, Douglass CW. Meta-analysis of the effect of scaling and root planing, surgical treatment and antibiotic therapies on periodontal probing depth and attachment loss. J Clin Periodontol 2002; 29: 975–986. [DOI] [PubMed] [Google Scholar]
- 63. Matthews D. Local antimicrobials in addition to scaling and root planing provide statistically significant but not clinically important benefit. Evid Based Dent 2013; 14: 87–88. [DOI] [PubMed] [Google Scholar]
- 64. Drisko CL. Periodontal self-care: evidence based support. Periodontol 2000. 2013; 62: 243–255. [DOI] [PubMed] [Google Scholar]
- 65. Buckley C, Colyer A, Skrzywanek M, et al. The impact of home-prepared diets and home oral-hygiene on oral health in cats and dogs. Br J Nutr 2011; 106: S124–S127. [DOI] [PubMed] [Google Scholar]
- 66. Tromp JAH, van Rijn LJ, Jansen J. Experimental gingivitis and frequency of tooth brushing in the beagle dog model. J Clin Periodontol 1986; 13: 190–194. [DOI] [PubMed] [Google Scholar]
- 67. Ingham KE, Gorrel C, Blackburn JM, et al. The effect of toothbrushing on periodontal disease in cats. J Nutr 2002; 132: 1740S–1741S. [DOI] [PubMed] [Google Scholar]
- 68. Miller BR, Harvey CE. Compliance with oral hygiene recommendations following periodontal treatment in client-owned dogs. J Vet Dent 1994; 11: 18–19. [PubMed] [Google Scholar]
- 69. Clarke DE, Cameron A. Relationship between diet, dental calculus and periodontal disease in domestic and feral cats in Australia. Aust Vet J 1998; 76: 690–693. [DOI] [PubMed] [Google Scholar]
- 70. Verstraete FJM, van Aarde RJ, Nieuwoudt BA, et al. The dental pathology of feral cats on Marion Island, part II: periodontitis, external odontoclastic resorption lesions and mandibular thickening. J Comp Pathol 1996; 115: 283–297. [DOI] [PubMed] [Google Scholar]
- 71. Clarke DE, Servet E, Hendriks, et al. Effect of kibble size, shape and additives on plaque in cats. J Vet Dent 2010; 27: 84–89. [Google Scholar]
- 72. Hennet P, Servet E, Soulard Y, et al. Effect of pellet food size and polyphosphates in preventing calculus accumulation in dogs. J Vet Dent 2007; 24: 236–239. [DOI] [PubMed] [Google Scholar]
- 73. Ingham KE, Gorrel C, Bierer TL. Effect of a dental chew on dental substrates and gingivitis in cats. J Vet Dent 2002; 19: 201–204. [DOI] [PubMed] [Google Scholar]
- 74. Gorrel C, Inskeep G, Inskeep T. Benefits of a ‘dental hygiene chew’ on the periodontal health of cats. J Vet Dent 1998; 15: 135–138. [DOI] [PubMed] [Google Scholar]
- 75. Clarke DE. Clinical and microbial effects of oral zinc ascorbate gel in cats. J Vet Dent 2001; 18: 177–183. [DOI] [PubMed] [Google Scholar]
- 76. Clarke DE. Drinking water additive decreases plaque and calculus accumulation in cats. J Vet Dent 2006; 23: 79–82. [DOI] [PubMed] [Google Scholar]
- 77. Milella L, Beckman B, Kane JS. Evaluation of an anti-plaque gel for daily toothbrushing. J Vet Dent 2014; 31: 160–167. [Google Scholar]
- 78. Coenye T, Honraet K, Rigole P, et al. In vitro inhibition of Streptococcus mutans biofilm formation on hydroxyapatite by subinhibitory concentrations of anthraquinones. Antimicrob Agents Chemother 2007; 51: 1541–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hasturk H, Goguet-Surmenian E, Blackwood A, et al. 1-Tetradecanol complex: therapeutic actions in experimental periodontitis. J Periodontol 2009; 80: 1103–1113. [DOI] [PubMed] [Google Scholar]
- 80. Anthony J. Topical tetradecanol complex in the treatment of periodontal disease in cats. Proceedings of the 21st European Congress of Veterinary dentistry; 2012 May, Lisbon. [Google Scholar]
- 81. Low SB, Mott A. Laser technology to manage periodontal disease: a valid concept? J Evid Based Dent Pract 2014; 14 Suppl: 154–159. [DOI] [PubMed] [Google Scholar]