Abstract
Sleeping and resting respiratory rates are commonly measured variables in patients with cardiac disease. However, little information is available on these variables in healthy client-owned cats or cats with subclinical heart disease (SHD). Therefore, we examined and characterized the sleeping respiratory rate (SRR) and resting respiratory rate (RRR) in 59 echocardiographically normal (EN) and 28 apparently healthy (AH) cats, and 54 SHD cats acquired by the cat owners in the home environment on eight to 10 separate occasions. The within-cat mean sleeping respiratory rate (SRRmean) in EN cats, AH cats and SHD cats with mild or moderate left atrial (LA) enlargement (as defined by quantiles of the ratio of the LA to the aorta [LA:AO]) was consistently <30 breaths/min; median SRRmean approximated 21 breaths/min. The SRRmean of SHD cats with severe LA enlargement sometimes exceeded 30 breaths/min, and was higher than SRRmean of other SHD cats (P <0.05). The within-cat mean resting respiratory rate was consistently higher than SRRmean (P <0.05). Age and geographic location, but not bodyweight, affected SRRmean in EN and AH cats. Within-cat SRR and within-cat RRR did not vary markedly from day-to-day, as evidenced by a low within-cat coefficient of variation. Data acquisition was considered easy or non-problematic by most participants. Our data provide useful guidelines for SRR and RRR, obtained in the home environment, in healthy cats and cats with SHD, and might prove useful in managing cats with clinical heart disease. Cats with SRRmean >30 breaths/min and cats with multiple SRR measurements >30 breaths/min likely warrant additional evaluation.
Introduction
Left-sided congestive heart failure (CHF) is a syndrome characterized in cats by development of pulmonary congestion and edema, or pleural effusion secondary to severe left heart disease. 1 Clinical signs of CHF in cats include dyspnea and/or varying degrees of tachypnea. These can be subtle and difficult to detect early in the course of developing CHF, but can progress to marked signs, often with apparent rapidity. 1 Diagnosis of CHF often relies on results from several tests, and traditionally requires the demonstration of severe cardiac disease, radiographic evidence of pulmonary interstitial or alveolar opacity or pleural effusion, and consistent clinical signs. However, radiographic presentation of pulmonary edema in cats is much less consistent than in dogs, making interpretation difficult in many cases, especially when complicated by the presence of pleural effusion. 2 Furthermore, attempts at an echocardiographic or radiographic diagnosis of heart disease in cats with severe dyspnea can further compromise an already critically unstable patient.
Over the last two decades, serum biomarker changes have been proposed as adjunct diagnostic tests for identification of CHF in cats; however, these studies have examined diagnostic accuracy and validity of the assays, rather than demonstrating their clinical utility.3 –9 In dogs, resting respiratory rate (RRR) proved the most sensitive and specific single diagnostic test for identifying CHF as a cause of clinical signs in dogs with heart disease and was an independent variable in predicting CHF in multivariable regression analysis. 10 A further study demonstrated that resolution of the CHF resulted in a reduction in RRR into pre-CHF ranges. 11 Thus, respiratory rate has the potential to be a sensitive, albeit unspecific, indicator of developing or recurring CHF in cats. Continued, frequent monitoring of this easily measured clinical variable could allow more timely therapeutic intervention or modulation in cats with subclinical heart disease but severe cardiac remodeling [eg, those with marked left atrial (LA) enlargement] or known prior history of CHF. Many veterinary cardiologists currently recommend that owners monitor respiratory rates in subclinical canine and feline cardiac patients at home to help determine either the onset of CHF, or in clinical patients to assess effectiveness of CHF therapy. Anecdotally, veterinary cardiologists have suggested that a sleeping respiratory rate (SRR) <30 breaths/min likely excludes CHF as a cause of clinical signs; however, to the best of our knowledge, no published data exist confirming this threshold value in cats. Prior studies of cats in a laboratory environment documented resting (awake) respiratory rates of 22–23 breaths/min. 12 We recently documented that SRR in healthy dogs was, indeed, <30 breaths/min, with no dog in that study having an average SRR >24 breaths/min. 13 Additionally, we found that dogs with subclinical left-sided heart disease of varying severity also had SRR <30 breaths/min. 14 Whether healthy cats and cats with subclinical left-sided heart disease that could result in CHF have similar SRRs remains unknown.
Therefore, we characterized and compared at-home measurements of SRR in healthy adult cats and cats with echocardiographic diagnosis of subclinical heart disease [mostly hypertrophic cardiomyopathy (HCM)] that could result in left-sided CHF. Additionally, we compared SRR and RRR in a subset of healthy cats and all cats with subclinical heart disease.
Materials and methods
Data
Data collection occurred in two phases. We recruited pet owners (most commonly clinicians and veterinary students) with echocardiographically normal (EN) cats or apparently healthy (AH) cats to collect SRR from their cats in their home environment by emailing class lists at several veterinary schools, emailing the membership of the Veterinary Information Network (www.vin.com), and asking colleagues, acquaintances, clients and friends to provide data on their cats. Data collection began in December 2009 and ended in February 2013. Participants were recruited from various regions of the USA, Sweden, Italy and Israel. Thus, the study was composed of a convenience sample, rather than a random sampling of cats.
We invited veterinary cardiologists to provide data on client-owned cats with subclinical left-sided heart disease (SHD) that they examined in the course of their clinical work, using a standardized collection form. Clients were invited to participate in data collection and were asked to collect SRR and RRR data on their cats in their home environment after the echocardiographic diagnosis of SHD. We defined subclinical heart disease as ‘without evidence of CHF or syncope’. We defined the absence of CHF as not requiring diuretic treatment. Thus, all SHD cats in the study were not receiving diuretics at the time of data acquisition or for several months after. The decision not to treat any of the cats with diuretics was made by the participating cardiologists who examined the SHD cats prior to data collection.
This study was purely observational, voluntary and non-invasive, and, as such, required no institutional approval. In EN cats, the echocardiographic evaluation was performed by cardiologists either immediately prior to SRR or RRR data collection, or at some point after the acquisition of SRR data, with the assumption that a cat that was EN after SRR data collection had to have been EN during SRR data collection. EN cats were examined either as part of HCM screening in client-owned cats, or as part of teaching in cases where veterinarians’ or veterinary students’ cats were observed. In SHD cats, the echocardiographic evaluation was performed immediately prior to SRR or RRR data collection as part of a cardiac evaluation.
We excluded cats with a history of CHF, those receiving diuretics, those with other severe systemic diseases (eg, asthma and pulmonary diseases) and age <6 months. The majority of AH cats did not undergo individual physical examinations, echocardiographic evaluation or any other diagnostic tests prior to inclusion, but required an owner history of general and cardiovascular health, and a physical examination by their regular veterinarian within the previous 12 months. Additionally, owners were contacted 1–2 years after SRR data collection and surveyed about development of any clinical signs of respiratory or heart disease during that period. Cats with evidence of cardiac disease or respiratory disease were excluded from the study or considered SHD cats if confirmed by echocardiographic evaluation.
All cats with subclinical cardiac disease underwent a comprehensive echocardiographic evaluation by a veterinary cardiologist to characterize the disease present and to ascertain the disease severity. The reason for the cardiac evaluations of the SHD cats was not determined for any cat. Cardiologists were asked to provide linear measurements of the LA and aorta from the right-parasternal short-axis two-dimensional projection, 15 to determine if systolic anterior motion of the mitral valve was present, and, if present, to measure the left ventricular outflow tract systolic peak velocity. Additionally, the cardiologists performing the examination were asked to detail any medications (cardiac or non-cardiac) the cats were receiving at the time of the examination.
Client participants collected 10–12 SRR measurements from their cats in their home environment during ‘deep sleep’ by counting breaths for 1 min, with instructions to avoid measuring SRR when cats were in ‘active motor sleep’ (eg, paddling, twitching). No more than two measurements per day, separated by at least 30 mins, were allowed (therefore, the most rapid data acquisition would take 5 days), and there was no upper limit to the time over which collection could occur. During periods of data collection, cats were to be kept in a ‘thermoneutral’ environment, meaning that extremes of heat and cold should be avoided, but temperature ranges that would be acceptable were not specified, and participants did not record ambient room temperatures at the time of collection. Measurements of RRR were performed in a similar fashion, except that cats were not expected to be sleeping. Participants were advised not to measure RRR if the cat was obviously purring.
Participants recorded date of birth (approximated if not known), gender and reproductive status, bodyweight, body condition score, breed and respiratory rates. Participants with EN and AH cats also recorded ease of data acquisition (rating acquisition as ‘easy’, ‘generally non-problematic’, ‘somewhat difficult’ and ‘hard’).
Statistical method
We stratified the cats as either ‘EN’, ‘AH’ or ‘SHD’, and analyzed these groups separately. We first calculated the within-cat mean respiratory rate (SRRmean, RRRmean) and SD (SRRsd, RRRsd), as well as the maximum and minimum within-cat SRR and RRR for each cat’s set of 8–10 SRR and RRR measurements. These within-cat variables were then examined by box-and-whisker plots to describe the data distribution for the SRR and RRR means, SD, maxima, minima and coefficient of variation (CV) for the entire study sample. We examined these variables for normality using a Wilk–Shapiro test.
We compared SRR and RRR between EN, AH and SHD cats with Kruskal–Wallis tests. Because EN and AH cats did not differ, we merged these two cohorts for further analysis of the effects of age, bodyweight and geographic location on SRR.
We then examined the effect of disease severity (as defined by LA size) and use of medications on SRR in SHD cats by two-way analysis of variance. SRR was normally distributed (Kolomogorov–Smirnov test) and error variances showed homogeneity (Levene’s test). We created quantiles of LA enlargement based on LA:aorta (AO) as follows: no enlargement — LA:AO ≤1.4; mild enlargement — LA:AO >1.4 and <1.7; moderate enlargement — LA:AO ≤1.7 and < 2.0; severe enlargement — LA:AO ≥2.0. This four-level factor was then examined together with the use of medications (two-level factor) and included an interaction term (LA size*medications) in the model. Finally, we looked for an association between LA size and use of medications with a Mann-Whitney U-test.
To examine the potential effect of age, location of investigator and bodyweight on SRR in EN and AH cats, we constructed a general linear model using the procMIXED procedure in SAS using age, location, bodyweight, and the interaction of age and location as independent variables in the model. The assumption that the residuals were normally distributed in the model was assessed by visually evaluating the distribution plot of the studentized residuals. Multiple pairwise comparisons were performed on the effects of location with Tukey’s honestly significant difference to preserve comparison-wise error.
To examine the possibility of significant increases or decreases in SRR over the collection period for each participant, we examined the intra-cat coefficients (slopes) of the regressed data. A slope not different from zero would suggest that there was no systematic increase or decrease in SRR over the collection period. We examined within-cat slopes for each level of disease severity by a one-sample t-test to determine if the average slope differed from zero.
Finally, we compared SRR with RRR in all cats with SHD, and the subset of EN and AH cats where both SRR and RRR were acquired with a Wilcoxon signed-ranks test.
We set a comparison-wise error rate at α = 0.05 for most comparisons. For minor comparisons (comparisons of slopes and within-cat CVs), we set the comparison-wise error rate at α = 0.015. All analyses were performed using statistical software PAST v2.14 16 or SAS.
Results
Demographic characteristics for all cats are presented in Table 1. The data collection periods for individual cats ranged from five to 299 days, with a median collection period of 26 days for EN cats, 14 days for AH cats and 21 days for SHD cats.
Table 1.
Subject characteristics of 59 echocardiographically normal (EN) cats, 28 apparently healthy (AH) cats and 54 cats with subclinical heart disease (SHD)
| EN (n = 59) median (range) | AH (n = 28) median (range) | SHD (n = 54) median (range) | |
|---|---|---|---|
| Weight (kg) | 4.5 (2.7–9.1) | 4.7 (3–8.0) | 5.4 (2.3–9.0) |
| Age (months) | 40 (5–181) | 57 (9–172) | 83 (12–184) |
| Gender | |||
| Female intact | 11 | 1 | 4 |
| Female spayed | 22 | 13 | 14 |
| Male intact | 7 | 1 | 4 |
| Male neutered | 19 | 13 | 32 |
| Breed | |||
| DSH | 25 | 26 | 33 |
| Persian | 1 | 8 | |
| Birman | 9 | ||
| DLH | 4 | 2 | |
| British Shorthair | 2 | ||
| Maine Coon | 11 | 2 | |
| Siamese | 1 | 1 | |
| Burmese | 1 | 1 | |
| Russian Blue | 1 | ||
| Sphynx | 2 | ||
| Ocicat | 1 | ||
| Bengal | 2 | 1 | |
| Cornish Rex | 3 | 1 | |
| Scottish Fold | 1 | ||
| Siberian | 1 | ||
| Devon Rex | 1 |
DSH = domestic shorthair; DLH = domestic longhair
EN cats
We obtained data for 59 EN cats. Date of birth for three cats was not available. Four participants provided fewer than 10 data points for their cats; however, their data were included in the analysis. The minimum period between data collection and reporting of the ongoing health status was 1 year (median 2 years).
AH cats
We obtained data on 28 AH cats. No AH cats had been identified with cardiac disease or signs of respiratory/cardiac disease during the intervening period, and all had been examined at least once after the data collection. The minimum period between data collection and reporting of the ongoing health status was 1 year (median 2 years).
Cats with SHD
Fifteen cardiologists provided data on 56 SHD cats; however, we subsequently excluded two cats from the analysis because they were diagnosed with CHF within 1 week of data collection and were considered to potentially have early signs of CHF at the time of data collection. Both of these cats had severe LA enlargement and high SRR (30 and 40 breaths/min). Date of birth for two cats was not available. Three participants provided fewer than 10 data points for their cats; however, their data were included in the analysis. One of these cats died suddenly (cause was undetermined) during the data collection period.
The range of cardiac diseases and severities identified in the SHD cats, along with medications administered to these cats are listed in Table 2. Thirty-two cats were receiving cardiac medications at the time of evaluation; one cat was receiving an antibiotic for a dermatological condition.
Table 2.
Cardiac diseases and medications in 54 cats with subclinical heart disease
| Disease | n |
| Hypertrophic cardiomyopathy | 50 |
| Unclassified cardiomyopathy | 1 |
| Ventricular septal defect | 2 |
| Mitral valve dysplasia | 1 |
| Severity (based on quantiles of LA enlargement) | |
| None (LA:AO ≤1.4) | 22 |
| Mild (LA:AO >1.4, ≤1.7) | 15 |
| Moderate (LA:AO >1.7, <2.0) | 9 |
| Severe (LA:AO ≥2.0) | 8 |
| Medications* | |
| Atenolol | 20 |
| ACE-inhibitor | 13 |
| Clopidogrel | 6 |
| Aspirin | 4 |
LA = left atrium; AO = aorta; ACE = angiotensin-converting enzyme
Medications administered to single patients included pimobendan, diltiazem, taurine and meropenem
SRR measurements
SRR measurements were not normally distributed. Figure 1a shows the box-and-whisker plots for SRR measurements in EN, AH and SHD cats.
Figure 1.

Box-and-whisker plots of mean, maximum and minimum (a) sleeping respiratory rates (SRR) in echocardiographically normal (EN) cats (n = 57), apparently healthy (AH) cats (n = 28) and cats with subclinical heart disease (SHD; n = 54). (b) Resting respiratory rates in EN cats (n = 23) and cats with SHD (n = 50)
Both EN and AH cats had a median within-cat SRRmean of 19 breaths/min (EN range 9–37 breaths/min; AH range 15–31 breaths/min). Within-cat regression coefficients (slopes) for EN and AH cats did not differ from zero (P = 0.4 and P = 0.3, respectively), with mean slopes of −0.04 and −0.08, respectively, indicating that measurements did not change significantly over time.
Three of 57 (5%) EN cats and one AH cat had SRRmean ≥30 breaths/min. Eight of 57 (14%) EN cats and 4/28 (14%) AH cats had individual SRR measurements ≥30 breaths/minute; seven of these eight EN cats and all four of these AH cats had multiple SRR measurements ≥30 breaths/min during the collection period.
Cats with SHD had a median within-cat SRRmean of 21 breaths/min (range 12–41 breaths/min). Within-cat regression coefficients (slopes) did not differ from zero (P = 0.51), with a mean slope of −0.03 for all cats, indicating that measurements did not change significantly over time.
Two of 54 (4%) SHD cats had SRRmean ≥30 breaths/min; 9/54 (17%) had individual SRR measurements ≥30 breaths/min; five of these nine cats had multiple SRR measurements ≥30 breaths/min during the collection period. Table 3 details findings of cats with either severe LA enlargement (n = 6) or cats with SRRmean ≥25 breaths/min (n = 8). One of the cats with SRRmean ≥25 breaths/min and severe LA enlargement was diagnosed with CHF approximately 4 months after obtaining SRR measurements (this cat’s SRRmean was 28 breaths/min at the time of data collection) and two cats remained subclinical for at least 6 months after obtaining SRR measurements (these two cats had SRRmean of 41 and 31 breaths/min, respectively). Outcome data were unavailable for the remaining three cats.
Table 3.
Details and outcomes of cats with either severe left atrial (LA) enlargement or within-cat mean sleeping respiratory rate (SRRmean) ≥25 breaths/min
| Cat | LA size | LA:AO | SRRmean | Outcome |
|---|---|---|---|---|
| 1 | Severe | 2.4 | 41 | No CHF for at least 6 months after data acquisition |
| 2 | Severe | 2.8 | 28 | CHF 4 months after data acquisition |
| 3 | Severe | 3.2 | 22 | ND |
| 4 | Severe | 2.1 | 22 | No CHF for at least 6 months after data acquisition |
| 5 | Severe | 2.3 | 31 | ND |
| 6 | Severe | 2.3 | 25 | ND |
| 7 | Normal | 1.5 | 28 | ND |
| 8 | Normal | 1.2 | 27 | ND |
| 9 | Mild | 1.5 | 28 | ND |
| 10 | Moderate | 1.9 | 25 | ND |
AO = aorta; CHF = congestive heart failure; ND = not determined
The cat that died suddenly in the midst of data collection had a SRRmean of 25 breaths/min from the eight measurements that were obtained, and had no evidence of dyspnea prior to death.
The SRRmean of EN cats did not differ from that of AH cats (P = 1.0) or SHD cats (P = 0.07). The SRRmean of AH cats did not differ from SHD cats (P = 0.25).
RRR measurements
RRR measurements were available for 23 EN cats (Figure 1b). These cats had a median within-cat RRRmean of 25 breaths/min (range 11–38 breaths/min); 4/23 EN cats had a RRRmean ≥30 breaths/min; 7/23 cats had at least 1 RRR measurement ≥30 breaths/min and 19/23 cats had at least 1 RRR measurement ≥25 breaths/min.
RRR measurements were available for 51 SHD cats (Figure 1b). These cats had a median within-cat RRRmean of 27 breaths/min (range 16–46 breaths/min); 18/51 SHD cats had RRRmean ≥30 breaths/min, including 4/6 cats with severe LA enlargement; 33/51 SHD cats had at least 1 RRR measurement ≥30 breaths/min and 27/51 had >1 RRR measurement ≥30 breaths/min. Within-cat regression coefficients (slopes) did not differ from zero (P = 0.04), with a mean slope of −0.28 for all cats, indicating that measurements did not change significantly over time (or, if anything, decreased with time).
Comparison of SRR and RRR
Both EN and SHD cats had lower SRRmean than the RRRmean (median: 20 versus 27 breaths/min; P <0.0001). Only one EN and one SHD cat of the 74 cats with both sets of respiratory rate measurements had SRRmean > RRRmean (12 versus 11 breaths/min and 28 versus 25 breaths/min).
Within-cat SRRCV for the combined EN and SHD groups differed from RRRCV (8.9 breaths/min versus 10 breaths/min; P = 0.0008), suggesting that the within-cat variability of SRR was slightly lower than that of RRR.
Effect of cardiac medications and disease severity on SRR
Examination of the SRRmean against LA:AO in SHD cats indicated a non-linear relationship (Figure 2a). We detected an effect of LA size (P <0.001) and cardiac medications (P = 0.003) on SRRmean. Pair-wise comparisons showed that cats with severe LA enlargement had higher SRR than all other SHD cats (Figure 2a, b). There was an interaction between disease severity and medication on SRRmean (P = 0.001). Cats receiving cardiac medications had more severe disease (as determined by LA:AO) than those not receiving medications (P = 0.02).
Figure 2.
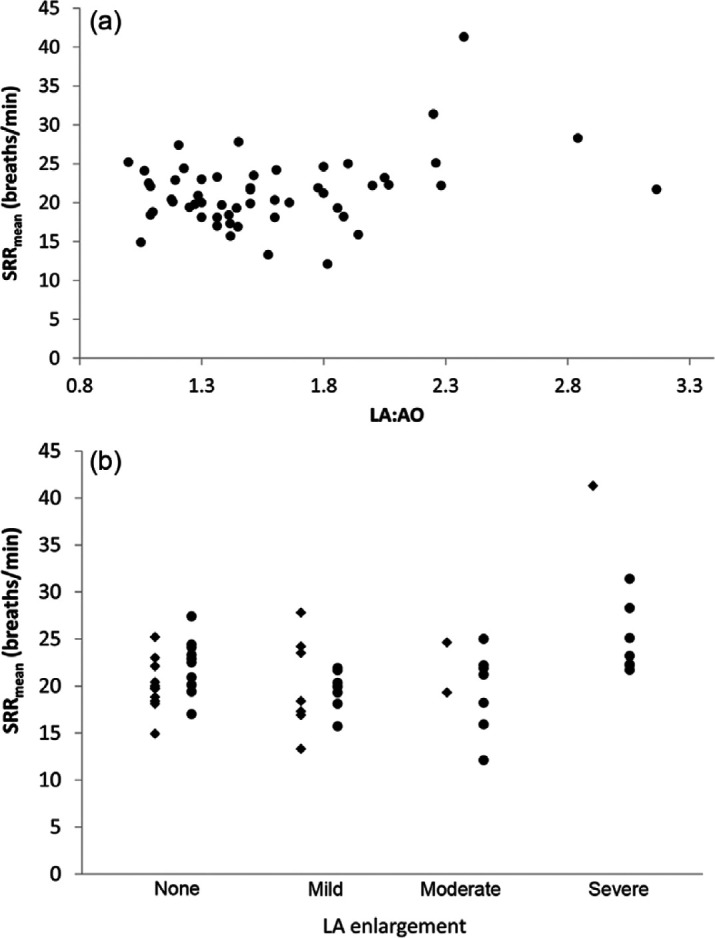
(a) Scatter plot of within-cat mean sleeping respiratory rate (SRRmean) in 54 cats with subclinical heart disease (SHD) against left atrium:aorta (LA:AO). (b) Dot plot SRRmean in cats with different severity of SHD based on left atrial size. Diamonds indicate cats not receiving cardiac medications. Circles indicate cats receiving cardiac medications
Effect of age, bodyweight and geographic location on SRR
SRR in EN and AH cats decreased with increasing age (P = 0.0001) (Figure 3). This decrease was non-linear, and was only apparent for the first 4–5 years. We observed this decrease in cats from all regions where EN and AH data were acquired. Additionally, geographic location appeared to have an independent effect on SRR, with cats in Israel having higher SRR than those in either Sweden or the USA (P = 0.0002 and P = 0.0439, respectively) (Figure 3). There was no interaction between age and location. There was no effect of bodyweight on SRR (P = 0.76).
Figure 3.
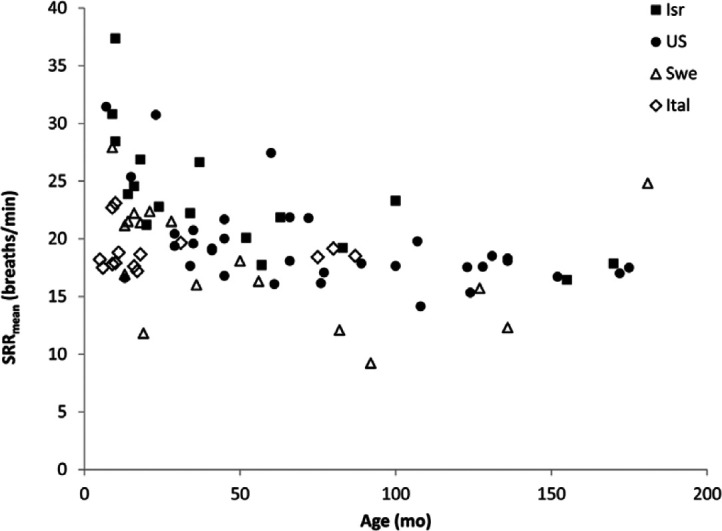
Scatter plot of within-cat mean sleeping respiratory rate (SRRmean) against age in 59 echocardiographically normal (black shapes) and 28 apparently healthy cats (white shapes), separated by geographic location. SRRmean declines with age for the first 4–5 years, and then plateaus. Cats in Israel (Isr) had higher SRRmean than comparably aged cats in Sweden (Swe) or the USA (US). Ital = Italy
Ease of data acquisition
Forty-two of 85 (50%) of participants with EN and AH cats provided feedback on the ease of data acquisition: 19 considered it ‘easy’, 15 considered it ‘generally non-problematic’, three considered it ‘somewhat difficult’ and five considered it ‘hard’.
Discussion
Healthy (EN) cats, AH adult cats and cats with SHD have a SRRmean that infrequently exceeds 30 breaths/min (7% in our study), with a median SRRmean of approximately 20–21 breaths/min and relatively little day-to-day variability. Cats with severe SHD (LA:AO >2.0) have higher SRRmean than cats with mild or moderate SHD, but even in our small sample population of cats with severe SHD, SRRmean infrequently exceeded 30 breaths/min. The SRR and RRR measurements are relatively easily acquired by both trained and untrained observers in most cats. Our study provides guidelines for assessing sleeping respiratory rates in cats collected within the pet’s home environment by the primary care provider (owner), and establishes both variability and maximum thresholds when collected in this manner.
Adult cats without evidence of heart disease (EN and AH groups) had a median SRRmean of approximately 19 breaths/min. However, some cats (14% in our study) occasionally had individual SRR >30 breaths/min. Furthermore, within-cat SRR did not vary much from day-to-day for most cats, as evidenced by the low within-cat coefficient of variation. We found independent effects of age and location of data collection, but no effect of bodyweight, on SRRmean in EN and AH cats, with SRRmean decreasing with age until cats reached approximately 4 years of age, and cats in Sweden having lower SRRmean than cats in either Israel or the USA.
We cannot easily explain the higher SRRmean observed in younger healthy cats. However, it would be reasonable to postulate that these cats are more active and have a higher metabolic rate than older, more sedentary cats, resulting in a higher SRRmean. Similarly, we have no explanatory evidence for the higher SRRmean observed in cats from Israel than those from Sweden or the US. While it is tempting to speculate that Israeli cats live in a warmer environment than those in Sweden or the USA, we asked that participants record SRR and RRR in a ‘thermoneutral’ environment, that is in a setting where ambient temperature (room temperature) was comfortable for the participant. Furthermore, as data collection occurred during all seasons, Swedish cats in winter would likely have been in heated rooms, while those in Israel in summer would likely have been in air-conditioned rooms.
Similar to EN and AH cats, SHD cats had a median SRRmean of approximately 21 breaths/min across the entire spectrum of subclinical disease, which did not differ from that of EN or AH cats. Cats with severe SHD had higher SRRmean than other SHD cats. Cats receiving cardiac medications had a lower SRRmean than SHD cats not receiving cardiac medications; however, this was largely evident only in cats with moderate or severe SHD, where most cats were (and few cats were not) receiving cardiac medications. Therefore, this association should be interpreted cautiously. Furthermore, this observation underscores the possibility that at least some of the severe SHD cats might have had mild CHF that was being controlled with medications at the time of data collection. Conversely, none of the cats required diuretic therapy prior to, during or immediately after the data collection.
Our data provide clinically useful information for clinicians who use SRR as a biomarker for CHF in cats with subclinical heart disease. Our data suggest that most cats have SRRmean <30 breaths/min, similar to our previous findings of SRR in healthy adult dogs and dogs with SHD.13,14 Cats with confirmed SHD and repeated measurements of SRR ≥30 breaths/min warrant further evaluation, as these cats might, in fact, have mild clinical signs of CHF.
We found no effect of bodyweight on SRR. This is similar to our observations of a lack of association between bodyweight and SRR in healthy dogs. 13 While a general allometric effect is observed with respiratory rate and body size, 17 this effect might not hold true within species, but only as a general ‘between-species’ effect.
As expected, RRR was slightly higher than SRR in the 23 EN and 51 SHD cats, where paired data were acquired, and was similar to prior observations in healthy research cats. 12 In both groups, median RRRmean was approximately 27 breaths/min and did not differ between EN and SHD cats if cats with severe SHD were excluded from the analysis. Additionally, day-to-day RRR was marginally more variable than SRR, although the difference would likely be considered clinically irrelevant.
Our study did not evaluate the sensitivity and specificity of SRR in the diagnosis of CHF. However, in cats presenting to general practitioners, a high SRR should prompt the clinician to consider further evaluation of both cardiac and non-cardiac causes of tachypnea, such as asthma or other pulmonary disease. Equally important is the fact that a normal SRR does not exclude the presence of heart disease. Finally, while we hypothesize that cats with CHF would have higher SRR than SHD or EN cats, we did not include CHF cats in this study, and therefore cannot conclude that cats with CHF would differ from the cats reported here.
Most participants found data acquisition either ‘easy’ or ‘generally non-problematic’. However, these results should be interpreted cautiously, because the study used a ‘convenience’ sample of data collectors/participants. It is possible that potential participants who found recording SRR impossible or very difficult failed to provide their data to the study coordinators, thereby skewing the results towards participants who could actually acquire the data relatively easily. Nevertheless, our study demonstrates that measuring SRR in cats is achievable by many cat owners.
Our cross-sectional study has several typical limitations. While most of the AH cats recruited to this study did not have complete physical examinations by the investigators, they were considered ‘healthy’ by their owners (mostly veterinary students and veterinarians) with no history of cardiac disease or any other systemic or organ related disease likely to affect the respiratory rate. Furthermore, all cats had undergone a routine physical examination within the 12 months preceding the data collection and a cardiac auscultatory examination at least 12 months after data collection. Additionally, none of the cats had developed clinical signs of respiratory or cardiac disease in the intervening period between data collection and manuscript submission (several cats had died by the time of manuscript submission, but no deaths were related to respiratory or cardiac disease). Finally, the SRR and RRR data from the AH cats did not differ statistically from those of the EN cats. We are reasonably confident that none of these AH cats had severe occult (ie, clinically undetectable) heart disease at the time of data collection — had such disease been present, we would have expected it to manifest clinically during the period between respiratory data collection and follow-up evaluation. We cannot completely exclude the possibility that some AH cats could have had mild occult SHD at the time of data collection. However, our data suggest that EN, AH and SHD cats with mild-to-moderate disease have similar SRRmean. Thus, the distinction between these three groups of cats is clinically irrelevant.
We did not ask participants to record ambient temperatures during the period of their data collection. However, our data collection extended over three continents, multiple climatic zones and multiple seasons within each region, so these numbers can be applied confidently to cats across the globe. While location had an effect on SRRmean (with EN and AH cats in Israel having higher SRRmean than those in other locations), the upper limits of our populations would apply to all cats. We requested that the animals be in a ‘thermoneutral’ environment (ie, not too hot, not too cold) although this was a subjective assessment by the owners. It is possible, however, that, in some instances, higher SRRs were recorded while the animals were hot (eg, a pet sleeping in front of a warm fire, in direct sunlight or in a room with higher ambient temperature).
Many SHD cats were receiving a variety of cardiac medications (mostly beta blockers or angiotensin-converting enzyme inhibitors) at the time of data collection. Cats receiving cardiac medications tended to have more severe disease than those not receiving cardiac medications.
One SHD cat died suddenly during data collection for unknown reasons. While the SRR data obtained from that cat (eight data points) did not differ from other SHD cats, it is possible that this cat died of acute CHF, although the owners did not report any signs consistent with CHF prior to the cat’s death.
Conclusions
Clinicians often use respiratory rates for monitoring cats with substantial heart disease or respiratory disease, and when identifying the onset or recurrence of CHF or the resolution of decompensated CHF with therapy. Additionally, clinicians might have clients measure SRR at home in cats with non-cardiac diseases that present with tachypnea (eg, asthma, stress, heat stroke, pneumonia, etc) to evaluate the effect of therapy. Our data suggest that EN and AH cats without clinical evidence of cardiac disease, and cats with known SHD generally have stable SRRmean <30 breaths/min in the home environment, and slightly higher and more variable RRRmean when measured at home by clients. These data provide clinicians with a strong basis on which to make their assessments of respiratory rates in their feline patients when determining if the SRR is elevated or not. Furthermore, the relatively limited variability in SRR and RRR in cats might allow for clinicians to monitor RR for evidence of worsening disease or, potentially, the onset of CHF, and to use this information to help clients determine appropriate strategies for re-evaluations. Cats with SRRmean >30 breaths/min might warrant additional investigation to determine whether or not decompensated CHF or non-cardiac pathology exists that might account for the tachypnea.
Acknowledgments
This study was presented as an abstract at the 20th ECVIM-CA Congress in Toulouse, France, September 2010. We would like to thank Rodrigo Bicalho for assistance with general linear modeling analysis. We would also like to thank all the veterinary cardiologists who participated in data acquisition for the SHD cats and clients who generously provided data on their pets, as well as the veterinary students and clinicians who provided data for their pets for the EN and AH cats.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
The authors do not have any potential conflicts of interest to declare.
Accepted: 2 September 2013
References
- 1. Kittleson MD. Pathophysiology of heart failure - signs of heart failure. In: Kittleson MD, Kienle RD. (eds). Small animal cardiovascular medicine. Veterinary Information Network, 2010: http://beta.vin.com/Members/proceedings/Proceedings.plx?CID=SACARDIO&PID=11485&O=VIN. Accessed 18 July 2013.
- 2. Rishniw M. Radiography of feline cardiac disease. Vet Clin North Am Small Anim Pract 2000; 30: 395–425. [PubMed] [Google Scholar]
- 3. Connolly DJ, Soares Magalhaes RJ, Fuentes VL, et al. Assessment of the diagnostic accuracy of circulating natriuretic peptide concentrations to distinguish between cats with cardiac and non-cardiac causes of respiratory distress. J Vet Cardiol 2009; 11(Suppl 1): S41–S50. [DOI] [PubMed] [Google Scholar]
- 4. Connolly DJ, Magalhaes RJ, Syme HM, et al. Circulating natriuretic peptides in cats with heart disease. J Vet Intern Med 2008; 22: 96–105. [DOI] [PubMed] [Google Scholar]
- 5. Connolly DJ, Cannata J, Boswood A, et al. Cardiac troponin I in cats with hypertrophic cardiomyopathy. J Feline Med Surg 2003; 5: 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fox PR, Oyama MA, Reynolds C, et al. Utility of plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) to distinguish between congestive heart failure and non-cardiac causes of acute dyspnea in cats. J Vet Cardiol 2009; 11 (Suppl 1): S51–S61. [DOI] [PubMed] [Google Scholar]
- 7. Herndon WE, Rishniw M, Schrope D, et al. Assessment of plasma cardiac troponin I concentration as a means to differentiate cardiac and non-cardiac causes of dyspnea in cats. J Am Vet Med Assoc 2008; 233: 1261–1264. [DOI] [PubMed] [Google Scholar]
- 8. Herndon WE, Kittleson MD, Sanderson K, et al. Cardiac troponin I in feline hypertrophic cardiomyopathy. J Vet Intern Med 2002; 16: 558–564. [DOI] [PubMed] [Google Scholar]
- 9. Zimmering TM, Meneses F, Nolte IJ, Simon D. Measurement of N-terminal proatrial natriuretic peptide in plasma of cats with and without cardiomyopathy. Am J Vet Res 2009; 70: 216–222. [DOI] [PubMed] [Google Scholar]
- 10. Schober KE, Hart TM, Stern JA, et al. Detection of congestive heart failure in dogs by Doppler echocardiography. J Vet Intern Med 2010; 24: 1358–1368. [DOI] [PubMed] [Google Scholar]
- 11. Schober KE, Hart TM, Stern JA, et al. Effects of treatment on respiratory rate, serum natriuretic peptide concentration, and Doppler echocardiographic indices of left ventricular filling pressure in dogs with congestive heart failure secondary to degenerative mitral valve disease and dilated cardiomyopathy. J Am Vet Med Assoc 2011; 239: 468–479. [DOI] [PubMed] [Google Scholar]
- 12. Jennings DB, Szlyk PC. Ventilation and respiratory pattern and timing in resting awake cats. Can J Physiol Pharmacol 1985; 63: 148–154. [DOI] [PubMed] [Google Scholar]
- 13. Rishniw M, Ljungvall I, Porciello F, et al. Sleeping respiratory rates in apparently healthy adult dogs. Res Vet Sci 2012; 93: 965–969. [DOI] [PubMed] [Google Scholar]
- 14. Ohad DG, Rishniw M, Ljungvall I, et al. Sleeping and resting respiratory rates in dogs with subclinical heart disease. J Am Vet Med Assoc 2013; 243: 839–843. [DOI] [PubMed] [Google Scholar]
- 15. Rishniw M, Erb HN. Evaluation of four 2-dimensional echocardiographic methods of assessing left atrial size in dogs. J Vet Intern Med 2000; 14: 429–435. [DOI] [PubMed] [Google Scholar]
- 16. Hammer O, Harper DAT, Ryan PD. PAST: Paleontological Statistics software package for education and data analysis. Palaeontol Electron 2001; 4: http://palaeo-electronica.org/2001_1/past/issue1_01.htm. [Google Scholar]
- 17. Stahl WR. Scaling of respiratory variables in mammals. J Appl Physiol 1967; 22: 453–460. [DOI] [PubMed] [Google Scholar]


