Abstract
Feline injection site sarcomas affect 1–10 cats per every 10,000 vaccinated and are associated with high mortality. Radical resection may be curative, but is often associated with prolonged recovery, disfigurement and loss of function when tumors occur at currently recommended injection sites. The objective of this study was to assess alternatives to currently recommended vaccination sites in terms of preference by oncology practitioners, ease of injection and serological responses. Surgical, radiation and medical oncology practitioners were surveyed regarding their preference for vaccination sites based on the ease of tumor resection. A six-point Likert scale was used to measure each cat’s behavioral reaction to vaccination when injected subcutaneously in the distal hind limb or the distal tail. Serum collected before and 1–2 months after vaccination was tested for antibody titers against feline panleukopenia virus (FPV) and rabies virus (RV). The preferred sites for vaccination by 94 oncology practitioners were below the stifle (41%) and the tail (30%). There were no significant differences in the cats’ behavioral reaction to vaccination below the stifle (n = 31) and in the distal tail (n = 29). Of the cats seronegative for FPV at the time of vaccination, 100% developed protective antibody titers (≥40) against FPV 1–2 months following vaccination. For cats seronegative for RV, all but one cat (tail vaccine) developed acceptable antibody titers (≥0.5 IU/ml) against RV. Tail vaccination was well tolerated and elicited similar serological responses to vaccination in the distal limbs.
Introduction
Vaccines have been associated with life-threatening sarcomas in 1–10 of every 10,000 cats vaccinated. 1 Although the proportion of affected cats is low, vaccination is such a common procedure that several thousand new cases occur each year in the USA. Feline injection-site sarcoma (FISS) is poorly controlled with conservative surgery, chemotherapy and radiation therapy, and most cats die of local recurrence or distant metastasis. Recently, radical excision by surgical specialists incorporating surgical margins of at least 5 cm laterally and resection of two muscle planes deep resulted in long-term survival of a majority of cats. 2
Historically, cats were commonly vaccinated in the interscapular region owing to ease of administration. Following the recognition that vaccination was associated with malignant tumor development and that wide and deep surgical excision was difficult to achieve at that site, international guidelines were developed that recommended vaccination in sites more amenable to radical excision. Currently, the American Association of Feline Practitioners recommends that vaccines containing feline panleukopenia virus (FPV), feline herpesvirus-1 and feline calicivirus antigens be injected subcutaneously below the elbow joint of the right forelimb, and vaccines containing feline leukemia virus (FeLV) or feline immunodeficiency virus (FIV) antigen or rabies virus (RV) antigen be injected below the stifle joint on the left and right hind limb, respectively. 3 The World Small Animal Veterinary Association recommends that vaccines be injected in the lateral thorax or abdomen. 4
Radical surgical excision of tumors forming at traditionally recommended vaccination sites is often disfiguring (eg, in the case of limb amputation), painful and expensive. 2 Many cat owners elect not to pursue treatment with curative intent because of its cost and invasiveness. The purpose of this pilot study was to assess alternatives to currently recommended vaccination sites in terms of preference by oncologists, ease of injection and serological responses.
Materials and methods
Oncology survey
An online survey (using SurveyMonkey) was distributed via the listservs of the specialty colleges and special interest groups for medical oncology, surgical oncology and radiation oncology to solicit opinions regarding the optimal vaccination sites in cats, as defined by the site in which surgical treatment of FISS would have the best outcome. The survey could be completed anonymously, but respondents were also permitted to provide their email address if they wished to receive the survey results. The survey consisted of two questions and a space to provide written responses. The first question asked:
For each anatomic site below, indicate your level of agreement with the following statement: ‘This is an excellent site to vaccinate a cat.’ Consider only the issue of potential surgical treatment of injection-site sarcoma, not other issues such as type of vaccine or ease of administration.
The answer options included a five-point Likert scale ranging from ‘strongly disagree’ to ‘strongly agree’. Eleven potential vaccination sites were listed: inter-scapular, lateral scapula, between the shoulder and the elbow, below the elbow, lateral thorax, lumbar region, lateral abdomen, ventral abdomen, between the hip and the stifle, below the stifle and the tail. The second question asked respondents:
Considering only surgical treatment of injection-site sarcomas, what are your top three recommended sites for vaccination in cats?
Respondents were asked to select their top-, second- and third-ranked vaccination sites from the same anatomic site list. To weight the responses, first choices were awarded three points, second choices two points and third choices one point. Respondents were also asked to describe their primary area of practice with the options of radiation, surgical and/or medical oncology.
Cats
A total of 60 adult cats presented for spaying or neutering in a community cat trap–neuter–return program (Operation Catnip, Gainesville, FL, USA) were enrolled in the study. Cats were selected if they were tame (not feral), outwardly healthy, had a full-length tail and presented by a caregiver who believed they could return the cat for follow-up evaluation approximately 1–2 months after the initial visit. Each cat was examined, photographed, weighed and had the length of its tail measured. The study protocol was approved by the Institutional Animal Care and Use Committee at the University of Florida.
Vaccination acceptance score
All cats received a modified live virus feline viral rhinotracheitis–calicivirus–panleukopenia virus (FVRCP) vaccine (Fel-O-Guard Plus 3; Boehringer Ingelheim) and an inactivated RV vaccine (Rabvac 3 TF; Fort Dodge) subcutaneously using a 3 ml syringe with a 23 gauge needle. The package inserts for these vaccines do not proscribe an anatomic location for injection when administered via the subcutaneous route. Every other cat was assigned to receive vaccines at a traditional site (the lateral hind limb below the stifle) or in the alternative site (dorsum of the distal third of the tail). In cats assigned to the traditional hind limb group (n = 31), the FVRCP vaccine was administered in the left leg and the RV vaccine was administered in the right leg. In cats assigned to the alternative tail group (n = 29), the RV vaccine was administered 2 cm distally to the FVRCP vaccine (Figure 1). The ‘acceptance score’ for vaccination was assigned by the person administering the vaccines (JKL) after observing the behavioral reaction each cat displayed during vaccination and recording it on a six-point scale: no physical reaction (1), vocalization or limb/tail moved away (2), whole body moved away (3), escape behavior (4), aggression (5) and vaccination not possible (6). Each vaccination event was scored separately so each cat had two scores. A score of 1–2 was considered to indicate that the cat ‘accepted’ the vaccination procedure. After scoring, an identification microchip (HomeAgain Anti-migration Microchip; Intervet) was implanted subcutaneously in the inter-scapular region to facilitate follow-up. Following vaccination, cats were anesthetized and prepared for sterilization surgery according to the clinic’s protocols as previously described. 5
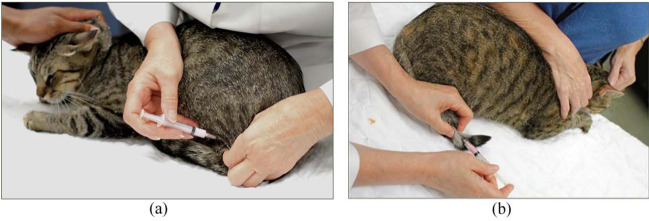
Figure 1.
Cats were vaccinated in the hind limbs below the stifle (a) or in the distal third of the tail (b) while gently restrained
Post-vaccination assessment
Caregivers were instructed to return cats in 1–2 months for follow-up evaluation. A physical examination was performed by a veterinarian blinded to the injection site. The hind limbs and tail were palpated; any anomalies were measured with calipers and recorded. Booster FVRCP vaccines were administered in the left hind leg following blood collection for serology.
Serology
Antibody responses against two highly fatal infections — RV and FPV — were evaluated by measuring antibody titers before and after vaccination in a subset of cats whose caregivers agreed to return them for retesting. Immediately prior to vaccination, 4 ml of blood was collected by jugular or medial saphenous venepuncture into serum separator tubes, allowed to clot for 30 mins and then centrifuged for 20 mins. A second sample was collected at the time of the post-vaccination assessment. Laboratory personnel testing the samples were blinded to the vaccination site. Serum from both samples was tested for FeLV and heartworm antigen, and FIV antibody at the time of collection by an enzyme-linked immunosorbent assay (Snap Feline Triple test; Idexx Laboratories). The remaining serum was stored at −20ºC pending analysis. Antiviral antibody titers in the paired serum samples were determined via hemagglutination inhibition (antibodies against FPV) (Animal Health Diagnostic Center, College of Veterinary Medicine, Cornell University) and virus neutralization via the rapid fluorescent focus inhibition test (antibodies against RV) (Department of Diagnostic Medicine and Pathobiology, College of Veterinary Medicine, Kansas State University). Based on previous laboratory challenge and field studies, the laboratory considers antibody titers of ≥40 to be consistent with protective immunity against FPV. 5 For the purposes of this study, seroconversion from a negative to a protective FPV titer was considered indicative of acceptable response to vaccination. A value of 0.5 IU/ml or greater is considered an acceptable response to vaccination for RV, although no absolute cut-off for protection is established.6,7
Statistical analysis
Survey results underwent descriptive analysis only. Scores for acceptance of vaccination for each vaccine were compared between the leg and tail sites, and the proportions of cats with acceptable responses to vaccination were compared via Fisher’s exact test (Epi Info 7.0.9.34; Center for Disease and Control and Prevention). Mean acceptance scores between the first and second vaccine for each site were compared via the t-test. (VassarStats). Differences were considered significant if P <0.05.
Results
Oncology survey
The online survey was completed by 94 veterinarians with a clinical practice in oncology (45 medical, 37 surgical and 12 radiation, with two indicating two areas of practice). The respondents were more likely to agree that the distal limbs and the tail were excellent sites for vaccination than other sites (Figure 2). The preferred sites for vaccination by 94 responding oncology practitioners were below the stifle and the tail (Figure 3). Based on these results, the tail was selected as the alternative to currently recommended sites and was compared with the most popular traditional site, the distal hind limb.
Figure 2.
Responses of 94 oncology practitioners to the question ‘For each anatomic site below, indicate your level of agreement with the following statement: “This is an excellent site to vaccinate a cat.” Consider only the issue of potential surgical treatment of injection-site sarcoma, not other issues such as type of vaccine or ease of administration.’ Values shown are mean scores for each anatomic site
Figure 3.

Responses of 94 oncology practitioners to the question ‘Considering only surgical treatment of injection-site sarcomas, what are your top three recommended sites for vaccination in cats?’. Respondents were asked to select their top-, second- and third-ranked vaccination sites from the same anatomic site options. To weight the responses, first choices were awarded three points, second choices two points and third choices one point
Cats
A total of 24 male (40%) and 36 female (60%) adult cats were enrolled in the study. One female was seropositive for FeLV, one male was seropositive for FIV and one male and one female were positive for heartworm antigen.
Cat acceptance of vaccination
Cats were allowed to sit or lie on the examination table as they preferred. An assistant gently stroked the cat for relaxation and distraction while the vaccinations were administered by a second person. Less handling was required for the tail because it was always easily accessible, whereas the hind limb sometimes had to be repositioned for injection. Subjectively, skin of the hind limb was easier to tent and to inject the 1 ml vaccine volume into than the skin of the tail. Vaccination in the tail required very superficial needle placement and a slow injection technique to avoid increased pressure and leakage of vaccine. Overall, 103 (87%) vaccinations were well tolerated with minimal reaction by the cats (acceptance score of 1–2) (Figure 4). Cats accepted 55 (95%) tail vaccinations and 48 (77%) hind limb vaccinations, with an acceptance score of two or less (P = 0.03), but mean acceptance scores were not significantly different between vaccines administered in the tail (1.6) compared with the hind limb (1.8) (P = 0.24). Although cats were numerically more likely to accept the first injection (55 cats; 92%) with a score of 1–2 than the second (50 cats; 83%); this difference did not reach statistical significance (P = 0.27). The mean acceptance scores for the first vaccine (1.5) were lower than the scores for the second vaccine (1.9) (P = 0.06). Only one cat did not allow a vaccination to be administered in the assigned site (second injection in the tail).
Figure 4.
Behavioral responses of 60 cats to two vaccines administered in the hind limbs or in the tail. Scores of 1–2 were considered to indicate that the cat ‘accepted’ the vaccine
Post-vaccination assessment
Caregivers agreed to return 39 cats for follow-up evaluation, but only 31 (79%) of these cats were actually returned. Cat identity was confirmed by microchip identification. Of the 31 cats returned, 12 (39%) were vaccinated in the hind limb and 19 (61%) were vaccinated in the tail. Of the 62 vaccination sites palpated (two per cat) by a veterinarian who was blinded to the vaccination site, two cats that had been vaccinated in the tail had detectable swelling. In these cats, caliper measurement detected a 1 mm increase in tail diameter at an injection site. None of the injection sites appeared to be painful.
Serological responses to vaccination
Only one of the 31 cats tested had detectable RV antibodies (11.5 IU/ml) prior to vaccination, while all other cats had titers <0.1 IU/ml. Caregivers returned their cats for follow-up serological testing 28–76 days post-vaccination. No additional vaccines were administered between the first and second serological testing dates, even if the cats were late for retesting. At that time, only one cat (3%) did not have an acceptable titer against RV when tested 36 days after vaccination. There was no significant difference in seroconversion between the two vaccination sites. Only 14 cats were seronegative for FPV at the time of vaccination. Of these, six (43%) had been vaccinated in the hind limb and eight (57%) had been vaccinated in the tail. All seronegative cats developed acceptable antibody titers to FPV following vaccination.
Discussion
It has been well-established that best outcomes in treatment of FISS occur when aggressive surgical excision of masses is possible. Veterinarians whose area of practice includes the treatment of FISS selected a traditional vaccination site (the distal hind limb) and a novel site (the distal tail) as their preferred vaccination sites due to the option of obtaining wide surgical margins with amputation. One respondent enthusiastically declared ‘Tail, tail, tail! I’ve been vaccinating my cats in their tails for years and, while I understand the initial hesitancy for practitioners to consider vaccinating in the tail of cats, it is definitely worthwhile from a surgical treatment and potential for cure perspective’.
The behavioral response of cats was evaluated during vaccination in the two sites most popular with the oncology practitioners. Although the vast majority of cats tolerated vaccination in either site, acceptance was slightly higher in the tail than in the traditional site. Cats are often stressed by visits to veterinary clinics 8 and can become more agitated when restrained in unnatural or uncomfortable positions. This is evidenced by the slight decrease in acceptance following the first vaccine. The high acceptance rate for vaccination was remarkable considering the accompanying stress the cats experienced during transportation in a wire trap to the mass sterilization clinic, and having an examination and venepuncture immediately prior to vaccination. It is possible that cats were more relaxed when allowed to position themselves as they chose during tail vaccination compared with the handler having to position the legs for vaccination. Vaccine acceptance might be even higher in a facility that embraces the calming atmosphere defined by the Cat Friendly Practice program.9,10
Vaccines appeared to be equivalently immunogenic in the tail and in the hind limb. All seronegative cats were effectively immunized against FPV and all but one cat developed acceptable titers against RV. These results are similar to those reported previously following vaccination of cats during trap-neuter-return clinics. 5 The lack of response detected in one cat for RV may be owing to the short interval between vaccination and serological testing (36 days), or may represent the expected failure rate previously reported in cats (2.8%) observed in testing for international travel. 11
Conclusions
This pilot study provided proof-of-concept for vaccination in an anatomic site that can be amputated without severely disfiguring the patient. In addition, tail amputation is a minor surgery that can be performed by a general practitioner on an outpatient basis. This may increase access to curative surgery for many cats afflicted with FISS. It is also possible that vaccination in the distal tail might facilitate early detection of FISS, as the tail is highly visible and pet owners frequently stroke the tail when handling their cats. Early detection is a factor in prevention of metastatic disease in FISS. If tail vaccination is adopted, care should be taken to assure the vaccine is administered in the distal third of the tail and only in cats with long tails. The occurrence of FISS in a more proximal location could be devastating as wide surgical margins would be exceedingly difficult to obtain in the perineal area. Based on the promising findings of this small study, further research is indicated to better characterize the acute and long-term effects of tail vaccination in a larger population of cats of different ages and body sizes, and in cats receiving multiple booster vaccines.
Acknowledgments
We acknowledge Patty Dingman, Sandy MacArthur, Courtney Butts, Kathleen Croy, Jillian Westberg, Charles Hall, Brooke Butler and Lesly Donis for technical assistance.
Footnotes
Funding: This study was supported by grants from Maddie’s Fund, the Merial Veterinary Scholars Program and the Harold H Morris Trust Fund for Research in Diseases of Small Animals.
The authors do not have any potential conflicts of interest to declare.
Accepted: 22 August 2013
References
- 1. Ladlow J. Injection site-associated sarcoma in the cat. J Feline Med Surg 2013; 15: 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Phelps HA, Kuntz CA, Milner RJ, et al. Radical excision with five-centimeter margins for treatment of feline injection-site sarcomas: 91 cases (1998–2002). J Am Vet Med Assoc 2011; 239: 97–106. [DOI] [PubMed] [Google Scholar]
- 3. Scherk M, Ford RB, Gaskell RM, et al. 2013 AAFP Feline Vaccination Advisory Panel Report. J Feline Med Surg 2013; 15: 785–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vaccination Guidelines Group, Day MJ, Horzinek MC, Schultz RD. WSAVA guidelines for the vaccination of dogs and cats. J Small Anim Pract 2010; 51: 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fischer SM, Quest CM, Dubovi EJ, et al. Response of feral cats to vaccination at the time of neutering. J Am Vet Med Assoc 2007; 230: 52–58. [DOI] [PubMed] [Google Scholar]
- 6. Wasniewski M, Cliquet F. Evaluation of ELISA for detection of rabies antibodies in domestic carnivores. J Virol Methods 2012; 179: 166–175. [DOI] [PubMed] [Google Scholar]
- 7. The World Organisation for Animal Health (OIE). Manual of diagnostic tests and vaccines for terrestrial animals 2012: rabies, www.oie.int/en/international-standard-setting/terrestrial-manual/access-online/ (accessed May 11, 2013).
- 8. Belew AM, Barlett T, Brown SA. Evaluation of the white-coat effect in cats. J Vet Intern Med 1999; 13: 134–142. [DOI] [PubMed] [Google Scholar]
- 9. Burns K. Cat friendly practice program takes off: American Association of Feline Practitioners growing with program. J Am Vet Med Assoc 2012; 241: 1264–1265. [PubMed] [Google Scholar]
- 10. Carney HC, Little S, Brownlee-Tomasso D, et al. AAFP and ISFM feline-friendly nursing care guidelines. J Feline Med Surg 2012; 14: 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mansfield KL, Burr PD, Snodgrass DR, et al. Factors affecting the serological response of dogs and cats to rabies vaccination. Vet Rec 2004; 154: 423–426. [DOI] [PubMed] [Google Scholar]