Abstract
Expression of the carbohydrate antigens sialyl Lewis x (sLex) and a (sLea) was evaluated in feline mammary gland tumours (FMGT). Immunohistochemical analysis of tissues from 21 FMGT patients and 11 healthy cats revealed significantly higher sLex and sLea antigen expression in adenocarcinoma tissues compared with that of normal mammary tissues (P <0.01). Serum concentration of sLex was evaluated using an enzyme-linked immunosorbent assay and was significantly higher in the 11 FMGT patients (4.71 ± 10.1 U/ml) than the 22 patients with other disease (2.69 ± 1.59 U/ml) (P = 0.03) and the 22 healthy cats (3.71 ± 1.10 U/ml), although the latter difference was not significant. Although the number of cases examined in this study was small, our findings suggest that aberrant expression of sLe antigens may be induced by tumourigenesis in FMGT and that sLe antigens are potential prognostic tumour markers for FMGT.
Introduction
Feline mammary gland tumours (FMGT) are the third most common type of tumour found in cats following haematopoietic neoplasms and skin tumours.1–4 Approximately 90% of FMGT are histologically malignant, and show rapid progression and metastasis at an early stage of disease.1–4 Usually classified as adenocarcinomas, FMGT often invade locally and easily metastasise to regional lymph nodes, the lung and pleura.1–4 Conventional treatments, such as surgery, chemotherapy and radiation therapy, may not confer adequate benefits in advanced cases. 5 Several prognostic indicators have been reported, including tumour size, mitotic count, clinical stage and surgical procedure.4–7 Several molecular markers, such as human epidermal growth factor receptor-2, cyclooxygenase-2 and vascular endothelial growth factor, have been reported to be associated with clinical outcome in immunohistochemical analysis.8–11
Tumour metastasis is a multistep process that involves detachment of cells from the primary mass, invasion of blood or lymph vessels, interaction with the endothelium, extravasation at distant sites and formation of new tumour foci. 12 Various factors are required for this process, including cell adhesion molecules, matrix metalloproteinases, angiogenic factors and chemokines.12,13 Several adhesion molecules have been identified as playing an important role in the extravasation cascade, 12 and it is now widely believed that adhesion between E-selectin and its ligands, sialyl Lewis x (sLex) and sialyl Lewis a (sLea) initiates a significant step in tumour extravasation. 14 The carbohydrate ligands sLex and sLea are known to adhere to E-selectin, a member of one of the selectin families whose expression is induced on vascular endothelial cells by inflammatory cytokines. 14 These carbohydrate ligands are expressed on monocytes, granulocytes, natural killer cells and activated lymphocytes, and their adhesion to E-selectin is the first step of interaction between the vascular endothelium and leukocytes during the inflammatory process. It has become apparent that cancer cells mimic the behaviour of immune cells to spread throughout the body and form metastatic foci.13–15 By exposing sLe antigens on their cell surface, disseminated circulating cancer cells may extravasate from the bloodstream into the peripheral tissues, in whose vessels selectins are highly expressed (see Figure 2 in Kannagi et al 15 ). Malignant transformation is frequently associated with this drastic alteration in the surface expression of carbohydrate determinants.16,17
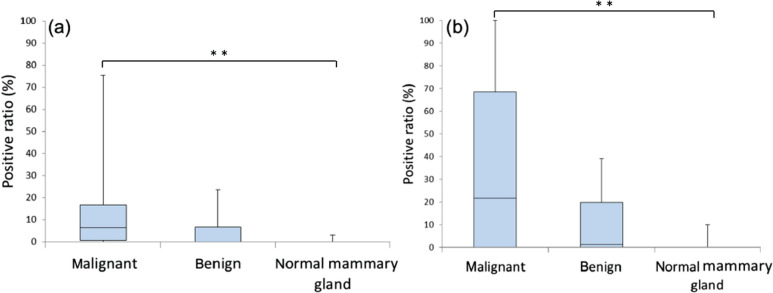
Figure 2.
Positive ratios of (a) sLex and (b) sLea expression from adenocarcinoma, benign and normal mammary gland tissues. The value is depicted as data plot and mean (**P <0.01)
Aberrant cell surface glycosylation is a common phenotypic change that occurs during carcinogenesis, 16 and sLex and sLea determinants are well-known cancer-associated glycans.16,17 These antigens are tetrasaccharides derived from type 2 (β-Gal-(1-4)-GlcNAc) and type 1 (β-Gal-(1-3)-GlcNAc) disaccharide sequences with fucose and sialic acid substitutions, respectively. 18 In humans, expression of sLex and sLea is reported to be markedly increased in carcinoma cells.15,18 The sLex determinant was found to be strongly involved in the adhesion of breast, ovarian and pulmonary cancer cells.14,19 In breast carcinoma, the presence of sLex also correlates with poor prognosis 20 and serum sLex antigen levels are increased in patients with advanced breast cancers. 21 In contrast, sLea antigen levels are high in cancers of the digestive organs.22,23 There are some reports of sLe antigens expression in mammary gland tumours of small animals; however, information regarding sLe antigens is still limited.24–26
The purpose of this study was to evaluate whether or not sLe antigen expression correlates with histopathological features of malignancy and clinical outcomes of FMGTs. To achieve this goal, sLex and sLea tissue expression, and serum sLex levels were examined in spontaneous FMGT patients to evaluate the relationship between sLe antigen expression and the clinicopathological features of FMGT.
Materials and methods
This study was approved by the Ethics Committee of Animal Use and Care Committee of the Graduate School of Agricultural and Life Sciences, The University of Tokyo.
Tissue samples
FMGT tissues were obtained from 21 patients who underwent unilateral mastectomy at the University of Tokyo Veterinary Medical Center between April 2006 and November 2010. Normal mammary gland tissues were obtained from 11 healthy cats. Age, breed, body weight, history of spaying, histopathological diagnosis, tumour size and site, regional lymph node involvement and distant metastasis by thoracic X-ray findings at time of referral to our hospital were obtained from medical records. In our hospital, regional lymph node involvement was examined by palpation. If palpable, we performed cytology to confirm nodal status. Clinical outcome was confirmed by a telephone interview with the client or referring practitioner. Abdominal ultrasound or post-mortem examinations were not performed. Tumour size was calculated based on the maximum diameter of the largest mass from the patient. Distant metastasis was confirmed by thoracic radiography in all cases. The World Health Organization clinical stage was used for the classification of FMGT staging, 4 and survival time was defined as the time from surgery to death.
Serum samples
Serum samples were collected from 55 cats between January 2009 and October 2010, of which 11 had FMGT, 22 had been referred to the Veterinary Medical Center with other diseases and the remaining 22 were healthy cats. Other diseases included tumours other than FMGT, inflammatory diseases, bone fracture, etc. After blood sampling, serum was separated and stored at –20°C until analysis. The medical records of the patients with other diseases were also reviewed.
Antibodies
Mouse monoclonal anti-human sLex (clone CSLEX1, dilution rate 1:500) was obtained from BD Biosciences and mouse monoclonal anti-human sLea (clone 1H4, dilution rate 1: 100) was obtained from Seikagaku.
Immunohistochemistry
Expression and localisation of sLex and sLea on the tissue samples were analysed by immunohistochemistry. Tissues were fixed in 10% neutral-buffered formalin for 2–3 days and embedded in paraffin. A series of 2–4 μm-thick sections were cut for immunohistology and histological diagnosis by light microscopy. Formalin-fixed paraffin-embedded sections were deparaffinised in xylene and rehydrated through a series of graded ethanols followed by distilled water. Antigen retrieval was performed for sLea by autoclaving slides for 10 mins at 121°C in citrate buffer (pH 6.0); examination of sLex did not require this process. Immunohistochemical procedures were performed using a DAKO ENVISION+kit/HRP (DAB) (DAKO Japan). Sections were washed in phosphate-buffered saline and the endogenous peroxidase activity was abolished by treatment with 0.3% hydrogen peroxide in Tris-buffered saline containing 0.1% Tween 20 (TBS-T). The sections were then incubated with 5% normal goat serum in TBS-T for 1 h, followed by the primary antibody (sLex and sLea at a dilution of 1:1000) for 2 h at 37°C. Sections of canine normal oesophagus and canine mammary gland adenocarcinoma were used as positive controls for sLex and sLea respectively. Sections that stained only the secondary antibody were evaluated as negative controls. After washing with TBS-T, samples were treated with the polymer solution containing the horseradish peroxidase (HRP)-conjugated antibody against mouse IgG at 37°C for 30 mins. Samples were then washed again and visualised with the DAB/hydrogen peroxide solution (DAKO). For light microscopy, sections were stained with haematoxylin and eosin. For the evaluation of immunohistochemistry, five photos were taken randomly at 200× magnification for each slide. The total cell area and the sLex-positive or sLea-positive cell area were measured using ImageJ software. Briefly, we distinguished the positive epithelial cells and drew the positive cell area manually. Also, the tumoural cell area was drawn manually. The positive ratio (positive cell area/total cell area) was then calculated, and the mean of five positive ratios was used as the positive ratio for each slide.
Measurement of serum sLex concentration
Serum concentrations of sLex were measured by a sandwich enzyme immunoassay (EIA) using the N-test EIA plate CSLEX kit (Nittobo Medical). Briefly, serum samples were reacted with the first anti-sLex monoclonal antibody (clone: CSLEX1) immobilised in wells of the plate at 37°C for 2 h. Next, the second anti-sLex monoclonal antibody labelled with HRP was added and incubated for 2 h at 37°C. Bound, HRP-labelled antibodies reacted with the substrate tetramethylbenzidine for 10 mins at room temperature, and optical densities were measured at 450 nm with a microplate reader (Model 680 Microplate Reader; Bio-Rad). Concentrations of sLex were expressed as U/ml.
Statistical analysis
Statistical analyses of immunohistochemistry data were carried out with the Kruskal–Wallis H-test and the Mann–Whitney U-test with Bonferroni’s correction. Comparison of variables among groups according to the degree of sLe expression was carried out with the X2 test or Kruskal–Wallis H-test. Statistical analyses of serum sLex concentrations were carried out with the Kruskal–Wallis H-test. Patient survival was analysed using the Kaplan–Meier method and evaluated by the log rank test. Dead cats with lung metastasis and dyspnoea were defined as death. The cats that died from other disease or unknown causes were censored. The cats that survived at the investigation were also censored. All these analyses were performed using Excel and Stat View, and a probability of less than 5% (P <0.05) was considered significant.
Results
Clinicopathological features of patients with FMGT
The clinicopathological features of the 21 FMGT patients are listed in Table 1. Clinical stages were determined based on the maximum size of the tumour in each patient, status of lymph node and distant metastasis at time of referral. All cats were female, and the mean age at surgery was 11.8 years (range 7.8–15.3 years). Of these 21 cats, two were mixed breed, 14 were Japanese domestic cats, and the remaining five were Himalayan, Abyssinian, Persian, Russian Blue and Scottish Fold. After surgery resection, only three cats received chemotherapy, including carboplatin and doxorubicin, vincristine, adriamycin, and cyclophosphamide (VAC) protocol and metronomic chemotherapy (combination of cyclophosphamide and piroxicam), respectively. These cats were excluded from the data of clinical outcome. Ten of these cats developed distant metastasis. Normal cats were all intact female and Japanese domestic cats. The mean age at tissue collection was 3.7 years (range 1.4–8.8 years) and the mean body weight was 4.3 kg (range 2.7–5.3 kg) when they were euthanased for other experiments.
Table 1.
Clinicopathological features of 21 feline mammary gland tumour (FMGT) patients
| Adenocarcinoma (n = 19) |
Adenoma (n = 2) |
||
|---|---|---|---|
| Age (years) | Mean ± SD | 11.6 ± 2.0 | 13.3 ± 1.3 |
| Ovarian status | Intact | 7 | 1 |
| Spayed | 12 | 1 | |
| Weight (kg) | Mean ± SD | 4.0 ± 0.9 | 2.8 ± 0.4 |
| Clinical stage | I | 6 | 2 |
| II | 5 | 0 | |
| III | 6 | 0 | |
| IV | 2 | 0 | |
| Tumour size (cm) | Mean ± SD | 2.7 ± 1.6 | 0.7 ± 0.2 |
| <2 | 6 | 2 | |
| 2–3 | 7 | 0 | |
| >3 | 6 | 0 | |
| Regional lymph node | Positive | 3 | 0 |
| involvement* | Negative | 12 | 2 |
| Distant metastasis* | Positive | 9 | 1** |
| Negative | 6 | 1 | |
| Outcome* | Alive | 4 | 0 |
| Dead (FMGT) | 8 | 1** | |
| Dead (unknown cause) | 3 | 0 | |
| Dead (other disease) | 0 | 1 | |
| Lost to follow- up | 1 | 0 |
At the end of study period (excluding the three cats that received chemotherapy)
This cat died owing to the recurrence and metastasis of adenocarcinoma
Expression of sLex and sLea in FMGT tissues
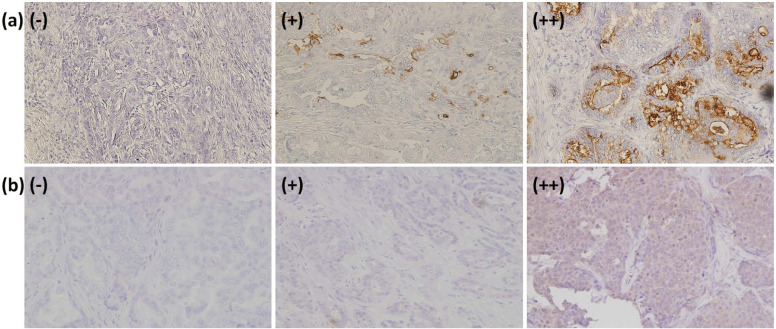
We obtained 53 FMGT tissue specimens from 21 patients. Histological diagnoses were as follows: 43 adenocarcinomas, five adenomas, three mammary hyperplasias, and two metastatic tissue specimens of the pleura and peritoneum. Typical immunohistochemical findings of sLe antigens on FMGT tissues are depicted in Figure 1. sLex expression was expressed on the cell membrane and in the cytoplasm, while sLea was expressed diffusely throughout the cell.
Figure 1.
Immunohistochemical analysis of (a) sLex and (b) sLea on tissues of feline mammary gland tumour FMGT patients. - = low (expression level under 4.9%); + = moderate (expression level between 0.5% and 29.9%); ++ = high (expression level >30%)
Expression levels were defined as follows: low expression (-) was defined as <4.9% of the positive rate; moderate expression (+) was defined as between 5% and 29.9%; and high expression (++) was defined as >30%. The expression levels of sLex and sLea on the tissues of the FMGT patients are summarised according to this classification in Table 2. Among the 43 adenocarcinoma tissue specimens, 35 (81.3%) showed low (n = 17, 39.5%) -to-moderate (n = 18, 41.9%) sLex antigen expression levels, while eight (18.6%) showed high expression (Table 2). Similarly, sLea epression levels on adenocarcinomas were low-to-moderate in 22 (51.1%) tissues, but high in 21 (48.8%) tissues (Table 2). Among the five adenoma tissues examined, only one demonstrated positive staining (+) of sLex, while the remaining four adenoma, all three hyperplasia and 11 normal mammary gland tissue specimens showed low sLex and sLea expression. In the two metastatic tissue specimens, sLex and sLea expression was low in one, whereas the other showed high expression of both antigens.
Table 2.
sLex and sLea expression on different histological tissue types
| Number | (-) | (+) | (++) | |
|---|---|---|---|---|
| sLex | ||||
| Adenocarcinoma | 43 | 17 (39.5%) | 18 (41.9%) | 8 (18.6%) |
| Adenoma | 5 | 4 (80%) | 1 (20%) | 0 |
| Hyperplasia | 3 | 2 (66.7%) | 1 (33.3%) | 0 |
| Normal mammary gland | 11 | 11 (100%) | 0 | 0 |
| Metastasis lesion | 2 | 1 (50%) | 0 | 1 (50%) |
| sLea | ||||
| Adenocarcinoma | 43 | 15 (34.9%) | 7 (16.3%) | 21 (48.8%) |
| Adenoma | 5 | 2 (40%) | 2 (40%) | 1 (20%) |
| Hyperplasia | 3 | 3 (100%) | 0 | 0 |
| Normal mammary gland | 11 | 10 (90.9%) | 1 (9.1%) | 0 |
| Metastasis lesion | 2 | 1 (50%) | 0 | 1 (50%) |
The mean values of the positive ratios of sLex and sLea expression in malignant (adenocarcinoma), benign (adenoma and hyperplasia) and normal mammary tissues are shown in Figure 2. Using the Mann–Whitney U-test with Bonferroni’s correction, significant differences were observed in sLe antigen expression between the adenocarcinoma and normal mammary gland tissues (P <0.01). In contrast, no statistically significant differences were observed between the adenocarcinoma and benign tissues, although the benign tissues tended to show lower expressions than adenocarcinoma for both sLe antigens.
Relationship between expression of sLe antigens and clinicopathological features
The correlation of sLex and sLea expression to clinicopathological features in FMGT was examined, and the results are shown in Table 3. No significant differences were found among groups exhibiting either low (-), moderate (+) or high (++) sLe expression and each of the clinicopathological variables.
Table 3.
Relationship between sLex and sLea expression, and clinicopathological features in feline mammary gland tumour (FMGT) patients
| Clinicopathological features | sLex expression |
P | ||
|---|---|---|---|---|
| (-) | (+) | (++) | ||
| Age (years) | NS | |||
| Mean ± SD | 11.7 ± 2.4 | 11.7 ± 1.4 | 12.2 ± 2.9 | |
| Ovarian status | NS | |||
| Intact | 1 | 6 | 1 | |
| Spayed | 5 | 4 | 4 | |
| Weight (kg) | NS | |||
| Mean ± SD | 4.4 ± 1.3 | 3.6 ± 0.9 | 3.8 ± 0.1 | |
| Clinical stage | NS | |||
| I | 1 | 4 | 3 | |
| II | 2 | 2 | 1 | |
| III | 3 | 3 | 0 | |
| IV | 0 | 1 | 1 | |
| Maximum tumour size (cm) | ||||
| Mean ± SD | 2.6 ± 1.5 | 2.9 ± 1.5 | 1.4 ± 0.5 | NS |
| <2 | 1 | 3 | 4 | NS |
| 2–3 | 3 | 4 | 1 | |
| >3 | 2 | 3 | 0 | |
| Regional lymph node involvement* | NS | |||
| Positive | 1 | 1 | 0 | |
| Negative | 4 | 8 | 4 | |
| Distant metastasis* | NS | |||
| Positive | 2 | 6 | 2 | |
| Negative | 3 | 3 | 2 | |
| Clinicopathological features | sLea expression | P | ||
| (-) | (+) | (++) | ||
| Age (years) | ||||
| Mean ± SD | 12.7 ± 1.6 | 11.4 ± 4.1 | 11.7 ± 1.9 | NS |
| Ovarian status Intact |
1 | 0 | 7 | NS |
| Spayed | 2 | 2 | 9 | |
| Weight (kg) Mean ± SD |
5.5 ± 1.1 | 3.3 ± 0.5 | 3.6 ± 0.7 | NS |
| Clinical stage | NS | |||
| I | 0 | 1 | 7 | |
| II | 2 | 0 | 3 | |
| III | 1 | 0 | 5 | |
| IV | 0 | 1 | 1 | |
| Maximum tumour size (cm) | NS | |||
| Mean ± SD | 3.5 ± 1.3 | 1.8 ± 1.8 | 2.4 ± 1.4 | |
| <2 | 0 | 1 | 7 | NS |
| 2–3 | 2 | 1 | 5 | |
| >3 | 1 | 0 | 4 | |
| Regional lymph node involvement* | NS | |||
| Positive | 0 | 1 | 1 | |
| Negative | 3 | 1 | 12 | |
| Distant metastasis* | NS | |||
| Positive | 2 | 1 | 7 | |
| Negative | 1 | 2 | 6 | |
NS = not significant
At the end of study period (excluding the three cats that received chemotherapy)
Long-term follow-up was performed on 20 patients, and one patient was lost to follow-up. Fourteen cats died owing to FMGT, one cat died from other disease and one cat died from an unknown cause. A follow-up survey could not be performed for in one cat, and four cats are still alive without any metastasis. Among the 14 patients that died of FMGT, 10 showed high sLea expression. According to the results of Kaplan–Meier curves and log rank test, however, there was no significant difference based on the expression level of both sLe antigens (sLex: P = 0.47; sLea: P = 0.19).
sLex concentrations in serum of FMGT patients
The serum sLex concentrations of mammary adenocarcinoma patients, patients with other disease and normal cats are shown in Figure 3. The normal cat population was demographically similar to the normal population used for tissue collection. Mean values (± SD) were 4.71 (± 10.1) (range 0.636–46.733), 2.69 (± 1.59) (range 0.318–6.013) and 3.71 (± 1.10) (range 0–5.37) U/ml, respectively. Significant differences were found in these values between mammary adenocarcinoma patients and those with other diseases (P = 0.03). In contrast, statistically there was no difference between mammary adenocarcinoma patients and normal cats; however, normal cats did exhibit a tendency toward lower sLex concentrations more than mammary adenocarcinoma patients did.
Figure 3.
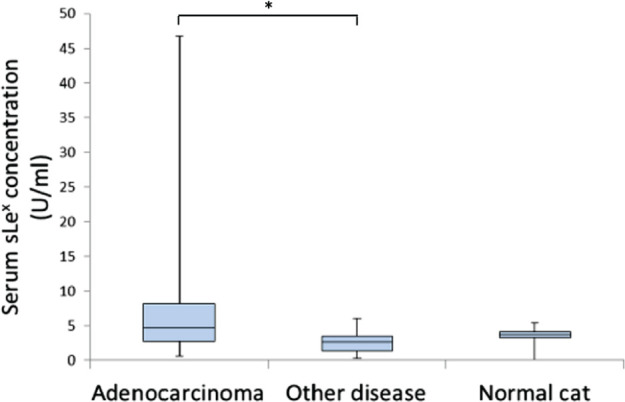
Serum sLex expression in feline mammary gland tumour patients and others. The value is depicted as data plot and mean (*P <0.05)
Discussion
In this study, we evaluated the expressions of sLe antigens in FMGT tissue samples and normal mammary gland tissues from healthy cats. Adenocarcinomas showed significantly higher sLe antigen expression than normal mammary gland tissues did. Most adenocarcinoma tissues also showed higher sLex expression than adenoma tissues, but the difference was not significant. The carbohydrate antigen, sLex, was considered to be expressed with malignant alteration, which is in agreement with previous reports.18,24,26,27 Similar to sLex, sLea showed significantly higher expression levels in adenocarcinoma than in normal tissues. Although the difference was not significant, adenocarcinoma tissues also showed higher sLea expression than adenoma tissues. This result is similar to that of previous reports on human breast cancers,18,27 but differs from previous reports on canine mammary gland tumours (MGT). 25 In this study the mean age of normal cats was younger than that of MGT patients; this difference was considered a limitation of the study. Although sLea expression on MGT is still controversial, aberrant expression of sLe antigens is thought to be induced by malignant changes in the mammary gland cells, which show no sLe antigens expressed under normal conditions. Taken together, results from this study suggest that sLe antigens may be a marker of tumourigenesis in FMGT.
In vitro studies have shown that sLe antigens, which are expressed on tumour cells, play a critical role in adhesion to vascular endothelial cells.14,28,29 This adhesion potential may relate to metastatic potential, which affects patient prognosis. Several clinical studies have been conducted to determine the relationship between tumour malignancy and the degree of sLe antigen expression in several tumours.20,30,31 The results from these studies imply that these carbohydrate antigens play an important role in metastasis. In contrast to these findings, however, our study did not reveal any relationship between sLe antigen expression and clinicopathological features, including distant metastasis. In this study we performed cytological examinations to check the lymph node involvement in only cases with palpable lymphadenopathy. However, lymph node involvement is not always concurrent with swelling of nodes. 5 Furthermore, we only evaluated distant metastasis by thoracic X-ray findings, and no post-mortem examination was performed. These factors may affect the results and were thought to be limitations of this study. In fact, no significant differences in survival were observed based on sLe antigen expression (Kaplan–Meier curves are not shown). Additionally, given the small number of patients included in this study, further studies with a large patient population will be necessary to clarify whether the sLe antigens have significant roles in tumour cell adhesion and metastatic potential or not.
In this study, serum sLex concentrations were measured to evaluate their potential as a tumour marker. Serum tumour markers can be helpful, non-invasive tools to diagnose tumours and provide information on the progression and recurrence of tumours.21,32–34 Serum sLex concentrations were significantly different between mammary adenocarcinoma patients and patients with other diseases. In addition, serum sLex concentrations tended to be higher in mammary adenocarcinoma patients than normal cats, although the difference was not significant. These results suggest that serum sLex levels could serve as a negative predictive serum tumour marker for FMGT. However, studies with large populations are required to determine the usefulness and the cut-off value of the serum sLex concentration as a tumour marker of feline MGT. Some mammary adenocarcinoma patients showed extremely elevated levels of sLex and a considerably poor prognosis with carcinomatous pleuritis. However, a significant correlation between sLex expression level and prognosis was unclear in the longitudinal assessment of these cats. It is important to note that the number of FMGT patients in this study was small and continuous evaluation of serum sLex concentrations was not performed. Thus, further research is needed to determine the usefulness of sLex as a serum FMGT marker.
Conclusions
This study demonstrated that adenocarcinoma tissues from FMGT patients exhibited higher sLe antigen expression than benign and normal mammary tissues did. These results suggest that sLe antigens may be expressed following malignant transformation of FMGT, similar to human breast cancers and canine MGT. In addition, serum levels of sLex in patients with adenocarcinoma seemed to be higher than in those with benign FMGT and in normal cats. Although the relationship between their expression and FMGT patient prognosis remains to be elucidated, sLe antigen expression potentially correlates with malignancy and prognosis of FMGT, and may be a useful marker for FMGT. Further studies are needed to clarify the biological role of sLe antigens in FMGT and to confirm their usefulness in clinical practice.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors do not have any potential conflicts of interest to declare.
Accepted: 12 August 2013
References
- 1. Dorn CR, Taylor DO, Frye FL, Hibbard HH. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. I. Methodology and description of cases. J Natl Cancer Inst 1968; 40: 295–305. [PubMed] [Google Scholar]
- 2. Dorn CR, Taylor DO, Schneider R, et al. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. II. Cancer morbidity in dogs and cats from Alameda Country. J Natl Cancer Inst 1968; 40: 307–318. [PubMed] [Google Scholar]
- 3. Hayes HM, Milne KL, Mandell CP. Epidemiological features of feline mammary carcinoma. Vet Rec 1981; 108: 476–479. [DOI] [PubMed] [Google Scholar]
- 4. Lana SE, Rutteman GR, Withrow SJ. Tumors of the mammary gland. In: Withrow SJ, Vail DM. (eds). Small animal clinical oncology. 4th edn. Toronto: Saunders Elsevier, 2001, pp 628–636. [Google Scholar]
- 5. Giménez F, Hecht S, Craig LE, Legendre AM. Early detection, aggressive therapy: optimizing the management of feline mammary masses. J Feline Med Surg 2010; 12: 214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ito T, Kadosawa T, Mochizuki M, et al. Prognosis of malignant mammary tumor in 53 cats. J Vet Med Sci 1996; 58: 723–726. [DOI] [PubMed] [Google Scholar]
- 7. Weijer K, Hart AA. Prognostic factors in feline mammary carcinoma. J Natl Cancer Inst 1983; 70: 709–716. [PubMed] [Google Scholar]
- 8. De Maria R, Olivero M, Iussich S, et al. Spontaneous feline mammary carcinoma is a model of HER2 overexpressing poor prognosis human breast cancer. Cancer Res 2005; 65: 907–912. [PubMed] [Google Scholar]
- 9. Millanta F, Lazzeri G, Vannozzi I, et al. Correlation of vascular endothelial growth factor expression to overall survival in feline invasive mammary carcinomas. Vet Pathol 2002; 39: 690–696. [DOI] [PubMed] [Google Scholar]
- 10. Islam MS, Matsumoto M, Hidaka R, et al. Expression of NOS and VEGF in feline mammary tumours and their correlation with angiogenesis. Vet J 2012; 192: 338–344. [DOI] [PubMed] [Google Scholar]
- 11. Millanta F, Citi S, Della Santa D, et al. COX-2 expression in canine and feline invasive mammary carcinomas: correlation with clinicopathological features and prognostic molecular markers. Breast Cancer Res Treat 2006; 98: 115–120. [DOI] [PubMed] [Google Scholar]
- 12. Chambers FA, Groom CA, MacDonald CI. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2002; 2: 563–572. [DOI] [PubMed] [Google Scholar]
- 13. Madsen CD, Sahai E. Cancer dissemination – lessons from leukocytes. Dev Cell 2010; 19: 13–26. [DOI] [PubMed] [Google Scholar]
- 14. Takada A, Ohmori K, Yoneda T, et al. Contribution of carbohydrate antigens sialyl Lewis A and sialyl Lewis X to adhesion of human cancer cells to vascular endothelium. Cancer Res 1993; 53: 354–361. [PubMed] [Google Scholar]
- 15. Kannagi R, Izawa M, Koike T, et al. Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci 2004; 95: 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo (glyco) lipid metabolism. Cancer Res 1996; 56: 5309–5318. [PubMed] [Google Scholar]
- 17. Yusa A, Miyazaki K, Kimura N, et al. Epigenetic silencing of the sulfate transporter gene DTDST induces sialyl Lewisx expression and accelerates proliferation of colon cancer cells. Cancer Res 2010; 70: 4064–4073. [DOI] [PubMed] [Google Scholar]
- 18. Cazet A, Julien S, Bobowski M, et al. Consequences of the expression of sialylated antigens in breast cancer. Carbohydr Res 2010; 345: 1377–1383. [DOI] [PubMed] [Google Scholar]
- 19. Saldova R, Royle L, Radcliffe CM, et al. Ovarian cancer is associated with changes in glycosylation in both acute-phase proteins and IgG. Glycobiology 2007; 17: 1344–1356. [DOI] [PubMed] [Google Scholar]
- 20. Nakagoe T, Fukushima K, Itoyanagi N, et al. Expression of ABH/Lewis-related antigens as prognostic factors in patients with breast cancer. J Cancer Res Clin Oncol 2002; 128: 257–264. [DOI] [PubMed] [Google Scholar]
- 21. Abd Hamid UM, Royle L, Saldova R, et al. A strategy to reveal potential glycan markers from serum glycoproteins associated with breast cancer progression. Glycobiology 2008; 18: 1105–1118. [DOI] [PubMed] [Google Scholar]
- 22. Kannagi R. Carbohydrate antigen sialyl Lewis a – its pathophysiological significance and induction mechanism in cancer progression. Chang Gung Med J 2007; 30: 189–209. [PubMed] [Google Scholar]
- 23. Matsui T, Kojima H, Suzuki H, et al. Sialyl Lewis a expression as a predictor of the prognosis of colon carcinoma patients in a prospective randomized clinical trial. Jpn J Clin Oncol 2004; 34: 588–593. [DOI] [PubMed] [Google Scholar]
- 24. Nakagawa T, Uyama R, Ohashi E, et al. The expression of sialyl Lewis x in canine and feline mammary gland tumors. J Vet Med Sci 2002; 64: 949–952. [DOI] [PubMed] [Google Scholar]
- 25. Nowak M, Madej J, Dziegiel P, et al. Tumor-associated carbohydrate antigens: sialyl Lewis a, T/Tn antigens in canine mammary tumors. Vet Pathol 2009; 46: 222–226. [DOI] [PubMed] [Google Scholar]
- 26. Pinho SS, Matos AJ, Lopes C, et al. Sialyl Lewis x expression in canine malignant mammary tumours correlation with clinicopathological features and E-Cadherin expression. BMC Cancer 2007; 7: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Renkonen J, Paavonen T, Renkonen R. Endothelial and epithelial expression of sialyl lewisx and sialyl lewisa in lesions of breast carcinoma. Int J Cancer 1997; 74: 296–300. [DOI] [PubMed] [Google Scholar]
- 28. Nakagawa T, Endo Y, Watanabe M, et al. Adhesional function of canine mammary gland tumor cells expressing sialyl Lewis x. J Vet Med Sci 2009; 71: 1225–1228. [DOI] [PubMed] [Google Scholar]
- 29. Ugorski M, Laskowska A. Sialyl Lewis(a): a tumor-associated carbohydrate antigen involved in adhesion and metastatic potential of cancer cells. Acta Biochim Pol 2002; 49: 303–311. [PubMed] [Google Scholar]
- 30. Matsui T, Kojima H, Suzuki H, et al. Sialyl Lewis a expression as a predictor of the prognosis of colon carcinoma patients in a prospective randomized clinical trial. Jpn J Clin Oncol 2004; 34: 588–593. [DOI] [PubMed] [Google Scholar]
- 31. Saito K, Fujii Y, Kawakami S, et al. Increased expression of sialyl-Lewis a correlates with poor survival in upper urinary tract urothelial cancer patients. Anticancer Res 2003; 23: 3441–3446. [PubMed] [Google Scholar]
- 32. Fujita T, Murayama K, Hanamura T, et al. CSLEX (Sialyl Lewis x) is a useful tumor marker for monitoring of breast cancer patients. Jpn J Clin Oncol 2011; 41: 394–399. [DOI] [PubMed] [Google Scholar]
- 33. Hanski C, Hanski ML, Zimmer T, et al. Characterization of the major sialyl-Lex-positive mucins present in colon, colon carcinoma, and sera of patients with colorectal cancer. Cancer Res 1995; 55: 928–933. [PubMed] [Google Scholar]
- 34. Nakagoe T, Sawai T, Tsuji T, et al. Predictive factors for preoperative serum levels of sialy Lewis(x), sialyl Lewis(a) and sialyl Tn antigens in gastric cancer patients. Anticancer Res 2002; 22: 451–458. [PubMed] [Google Scholar]