Abstract
There is a paucity of species-specific antibodies available for feline haematopoietic conditions. The purpose of this study was to broaden the panel of antibodies available for use in the immunophenotypic characterisation of feline haematopoietic cells by testing clones of anti-human monoclonal antibodies (mAbs) on normal, neoplastic and cultured feline haematopoietic progenitors to determine cross-reactivity to feline counterparts. In this study, 24 clones of anti-human mAbs were tested on normal or neoplastic feline bone marrow and peripheral blood cells. Six of these mAbs, including anti-cluster of differentiation (CD)61, anti-CD18, anti-CD14, anti-CD235a, anti-CD41 and anti-CD29, cross-reacted with normal feline bone marrow cells, whereas anti-CD33 and anti-CD117 cross-reacted with the blast cells in the bone marrow of two cats with myelodysplastic syndrome, and anti-CD71, anti-235a, anti-41 and anti-42 cross-reacted with immature erythroid cells in a cat with erythroleukaemia. In a feline immunodeficiency virus-positive cat, bone marrow cells were labelled with anti-CD33, anti-14 and anti-45. Anti-CD18, anti-CD14, anti-CD41 and anti-CD61 also reacted with the peripheral blood cells of the healthy cats. The feline haematopoietic progenitors formed colonies in the methylcellulose-based semisolid medium with significant enrichment of colony-forming unit-granulocyte, monocyte and burst-forming unit-erythroid. A panel of six anti-feline mAbs (anti-CD21-like, anti-T lymphocytes, anti-CD172a, anti-granulocyte, anti-CD45-like and anti-CD18) and eight anti-human antibodies (anti-CD71, anti-CD33, anti-CD235a, anti-CD41, anti-CD61, anti-CD117, anti-CD38 and anti-CD34) were used for the immunophenotypic characterisation of the feline bone marrow progenitors. CD45, CD33, CD235a and CD18 were expressed by the feline haematopoietic progenitor cells, with the highest expression level for CD45.
Introduction
The cat is an important companion animal, but also serves as an appropriate animal model 1 of haematopoietic disease, particularly bone marrow-related leukaemias.2–6 In human medicine, there are numerous monoclonal antibodies (mAbs) available to phenotype bone marrow haematopoietic cells, and the flow cytometric analysis of marrow cells is commonly performed in healthy and neoplastic conditions.7,8 There is a dearth of similar species-specific mAbs available for feline haematopoietic conditions. There is also a deficiency in both species of mAbs that can accurately identify erythroid precursors.
The purpose of this study was to broaden the number of mAbs available for use in the study of feline haematopoietic diseases by testing clones of anti-human cluster of differentiation (CD) antibodies to determine cross-reactivity to feline counterparts. This study employed clones of commercially-available anti-human mAbs on normal, neoplastic and cultured feline haematopoietic progenitors.
Materials and methods
Animals
To test the cross-reactivity of anti-human mAbs with feline haematopoietic cells and to develop a reference interval for the expression of CD molecules by normal marrow cells, bone marrow and peripheral blood samples were harvested from 12 clinically healthy cats [determined by history, clinical examination, complete blood count (CBC) and biochemical profile] and four cats with haematopoietic disorders (these four cats were client-owned). All of the procedures performed on the animals were in accordance with the guidelines published in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication 85–23, revised 1996). The study protocol was approved by the institutional Animal Care and Use Committee of the University of Tehran.
Sample collection
A 16-gauge bone marrow needle connected to a syringe containing either ethylenediamine tetra-acetic acid (EDTA) solution (Merck) or 3000 units of heparin (for cell culture) was used to collect 5 ml of bone marrow aspirate. Marrow aspiration was performed from the iliac crest. Bone marrow smears were prepared and stained with Giemsa stain (Merck) for cytological examination. Blood was collected from the saphenous vein into tubes with or without EDTA to perform a CBC (Vet Hema-Screen 18; Hospitex Diagnostics) and to separate the serum to perform a feline leukaemia virus (FeLV)/feline immunodeficiency virus (FIV) ELISA test (Idexx). All the samples were immediately sent to the laboratory and processed within a 24-h period.
Flow cytometric immunophenotyping
The flow cytometric analysis was performed with a panel of 24 mAbs (Table 1). The cells were incubated with positive and negative control mAbs. One hundred microlitres of bone marrow or peripheral blood cells, containing approximately 106 nucleated cells, was mixed with 5–10 μl of each antibody in a plastic tube and incubated in the dark for 30 mins at room temperature (20°C), as recommended by the manufacturers (Table 1). Two millilitres of lysing solution (DAKO) was then added to the cells and incubated for 15 mins at room temperature. Thereafter, the tubes were centrifuged at 250 g for 5 mins to pellet the cells. The supernatant was subsequently discarded, and the cells were washed in 100 μl of phosphate-buffered saline (PBS) (0.01 M, pH 7.2) and re-suspended in 100 μl paraformaldehyde (2.5% w/v in PBS). The cells were kept at 4°C in the dark before flow cytometric analysis. 9 The flow cytometric analysis was performed using a fluorescence-activated cell sorting (FACS) Calibur Flow Cytometer (BD Biosciences). Data were collected for 10,000 cells. The cells were first displayed on the forward scatter (FSC-H) and side scatter (SSC-H) in a linear mode. Fluorescent markers [fluorescein isothiocyanate (FITC) or phycoerythrin (PE)] were then displayed in a log mode. For each marker, fluorescence intensity above that of the relevant isotype control was assessed to indicate the cross-reactivity of that mAb with feline cells. 1
Table 1.
Panel of anti-human monoclonal antibodies used to test the cross-reactivity with feline bone marrow cells
| CD | Clone * |
|---|---|
| CD5 | DK23 |
| CD10 | SS2/36 |
| CD11b | 2LPM |
| CD14 | TÜK4 |
| CD19 | HD37 |
| CD20 | Bly1 |
| CD33 | WM-54 |
| CD34 | BIRMA-K3 |
| CD45 | T29/33 |
| CD117 | 104D2 |
| CD235a | JC159 |
| CD2 | MT910 |
| CD3 | UCHT1 |
| CD13 | WM-47 |
| CD29 | K20 |
| CD38 | AT13/5 |
| CD41 | 5B12 |
| CD56 | MOC-1 |
| CD61 | Y2/51 |
| CD71 | Ber-T9 |
| CD64 | 10.1 |
| CD42 | AN51 |
| CD18 | MHM23 |
| Iso Ctrl |
Clones were from DAKO
Methyl cellulose colony-forming assay
Bone marrow was aspirated from the iliac crest of seven clinically healthy cats (determined by history, clinical examination, CBC and blood chemistry) after ketamine–diazepam anaesthesia (0.11 mg/kg, 22 mg/kg). Bone marrow mononuclear cells were isolated as previously described. 10 Briefly, heparinised bone marrow was diluted with PBS in a 1:2 ratio before it was layered over 3 ml of Ficoll (Sigma-Aldrich) and centrifuged at 400 g for 30 mins at room temperature. Afterwards, the mononuclear cell layer was removed, transferred to a sterile 15 ml tube and washed twice with Iscove’s Modified Dulbecco’s Medium (IMDM; GIBCO-Invitrogen) containing 2% fetal bovine serum (FBS) (Invitrogen). The cell pellet was then re-suspended in 2 ml IMDM + 2% FBS, and cell counting was performed with a haemacytometer. The cells were diluted with IMDM + 2% FBS to a final concentration of 5 × 104 cells per ml. Additionally, 0.3 ml of diluted cells was then added to 3 ml MethoCult medium M3334 (Stem Cell Technologies) in a tube for a duplicate assay according to the manufacturer’s instructions. After vortexing the tube, 1.1 ml of MethoCult mixture containing cells were dispensed into 35 mm culture dishes (SPL, Life Science). All the experiments were performed in duplicate. The culture dishes were incubated at 37°C, in 5% CO2, with 95% humidity for 2 weeks. At days 7 and 14 of incubation, the entire dish was scanned on low power (2× objective) to assess the colonies based on the standard morphological criteria. Colony-forming unit–erythroid (CFU-E), burst-forming unit–erythroid (BFU-E), colony-forming unit–granulocyte, macrophage (CFU-GM) and colony-forming unit–granulocyte, erythroid, macrophage, megakaryocyte (CFU-GEMM) colonies were scored according to the manufacturer’s instructions. To evaluate the expression of cell surface markers by feline haematopoietic progenitors, cooled PBS was added to MethoCult medium containing progenitor cells. The cell mixture was then harvested, transferred to a 15 ml conical tube and centrifuged twice for 5 mins at 250 g. Cell suspensions were stained with a panel of anti-feline antibodies [anti-CD21-like (Cr-B1), anti-T lymphocyte subpopulation, anti-CD172a, anti-granulocyte, anti-pan leukocytes (CD45-like) and anti-CD18], as well as eight anti-human antibodies (anti-CD71, anti-CD38, anti-CD41, anti-CD33, anti-CD34, anti-CD235a, anti-CD117 and anti-CD61) and analysed for surface-marker expression using a FACS caliber system (Becton Dickinson). Anti-human antibodies were used on the progenitor cells harvested from the cell culture, as was described above. The anti-feline antibodies used for the labelling of the haematopoietic progenitors were primary unconjugated antibodies (all from VMRD). In order to evaluate the anti-feline markers, 100 μl of cell suspension were aliquoted into 10 (12 × 75 mm) polystyrene tubes (Becton Dickinson). Each primary antibody or its isotype control was added to each tube (50 μl of antibody from a 10 μg/ml stock) and incubated at room temperature for 15 mins. Afterwards, the secondary antibody (FITC-conjugated goat anti-mouse IgG and PE-labelled goat anti-mouse IgM; Serotec) was added to each tube and incubated at 4°C in the dark for 20 mins. Finally, the cells were washed twice with PBS and analysed. Collected events were analysed using Flow Jo software version 7.6.5.
Results
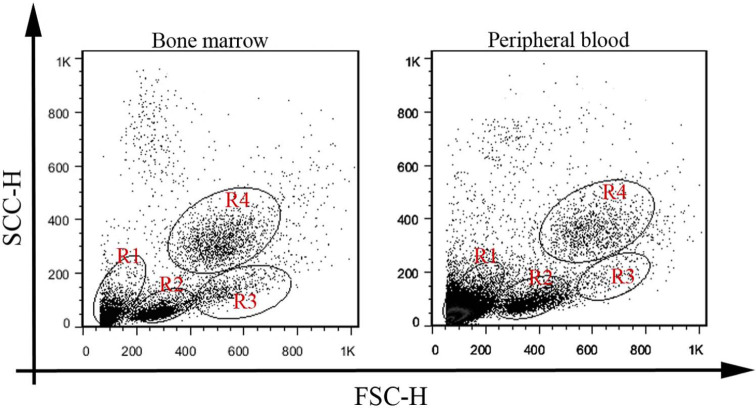
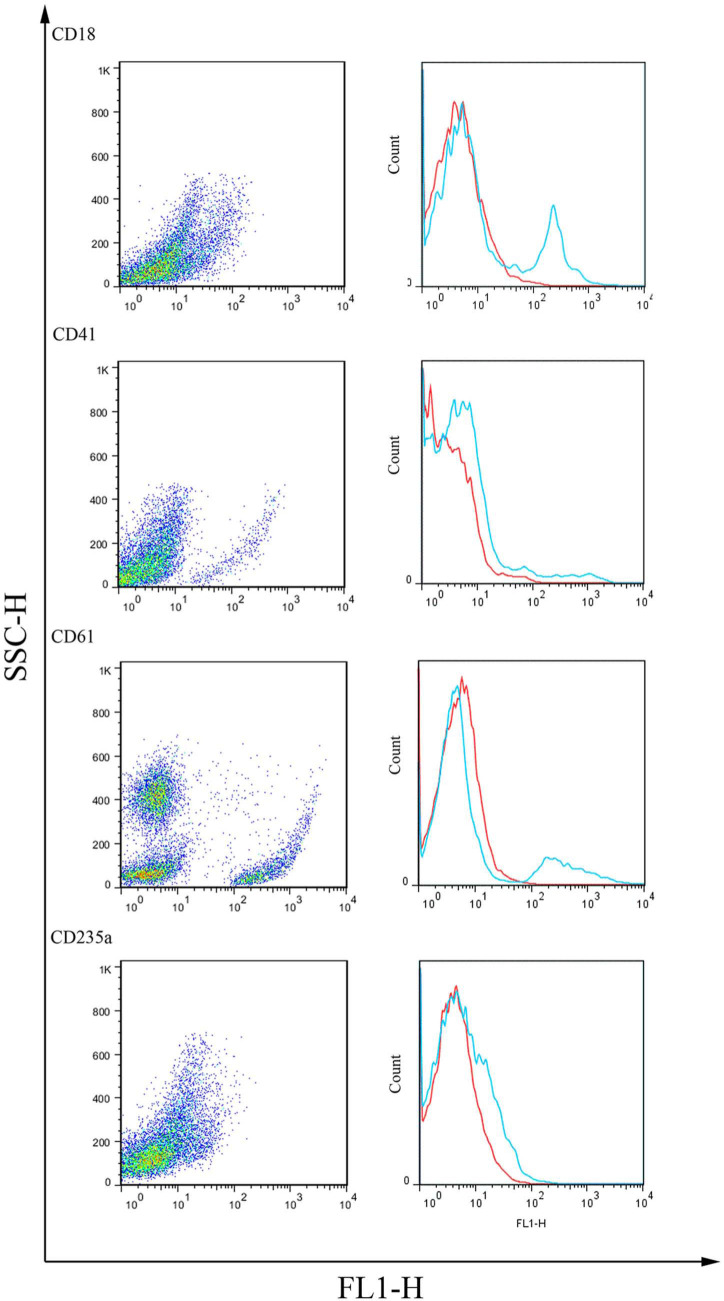
After labelling the bone marrow or peripheral blood with mAbs, the cells were first displayed as FSC versus SSC to determine the scatter-plot profile. Here, four cellular subsets were consistently distinguished in the bone marrow and the peripheral blood (Figure 1). A gate was then placed around each cellular subset (Figure 1). To test cross-reactivity, 24 mAbs against various CD molecules were tested on the bone marrow cells from the normal cats. The flow cytometric analysis of the normal feline bone marrow cells revealed positive reactivity for six (CD61, CD18, CD14, CD235a, CD41 and CD29) of the 24 anti-human mAbs (Figure 2). The bone marrow cells from all 12 healthy cats were reproducibly labelled with five of these anti-human antibodies, including CD61, CD18, CD14, CD41 and CD29, whereas five healthy cats were positively labelled with CD235a with a weak reaction for the cells in gate R2. The peripheral blood cells from all healthy cats were labelled with anti-CD18, anti-CD14, anti-CD41 and anti-CD61. Data analysis on the bone marrow and peripheral blood cells revealed that the cells positive for CD18 antigen had become enriched in gates R4, R3 and R2 in descending order. Gate R3 possessed the highest percentage of CD14-positive cells, consistent with the enrichment of monocytic lineage in this gate. The highest percentages of CD61 and CD41 were seen in gate R1. CD29 was positive in gate R2 of the bone marrow.
Figure 1.
Forward versus side scatter plots of bone marrow and peripheral blood cells from normal, healthy cats. Forward (FSC-h)/side scatter (SCC-H) dot plots with four cellular subsets, categorised into four gate regions (R1–R4), are shown for the bone marrow and peripheral blood
Figure 2.
Reactivity of the human monoclonal antibodies anti-cluster of differentiation (CD)18, anti-CD41, anti-CD61 and anti-CD235a (glycophorin A) with feline bone marrow haematopoietic cells compared with the isotype control. The y-axis indicates the relative numbers of cells, and the x-axis indicates the fluorescence intensity (FL1-H) on a logarithmic scale. Blue line = CD markers; red line = isotype control. SSC-H = side scatter
A panel of mAbs, including anti-CD71, anti-CD235a, anti-CD117, anti-CD34, anti-CD33, anti-CD2, anti-CD19, anti-CD10, anti-CD20, anti-CD5, anti-CD10, anti-CD41, anti-CD61 and anti-CD11b, was also used for the flow cytometric immunophenotyping of the bone marrow cells from the feline cases with haematopoietic disorders.
Case 1
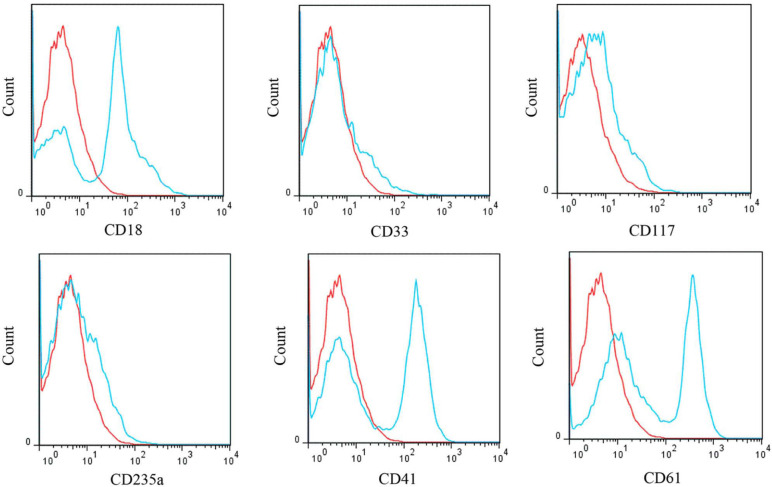
A 3-year-old male domestic shorthair cat was presented with a history of anorexia, lethargy, weakness and weight loss. On admission, the cat was recumbent. On physical examination, pale mucous membranes, dehydration, harsh lung sounds, and massive splenomegaly and hepatomegaly were found. Hepatosplenomegaly was confirmed by radiographic evaluation. This cat was categorised as having myelodysplastic syndrome with excess blasts (MDS-EB). Peripheral blood was characterised by severe macrocytic, normochromic, non-regenerative anaemia and thrombocytopenia with circulating myeloid precursors and giant platelets. Additionally, moderate toxic changes in neutrophils, together with hyposegmentation and hypersegmentation of neutrophils, were also noted. The cat was positive for FeLV. Bone marrow from this cat exhibited an altered myeloid:erythroid (M:E) (13.8) ratio with 60.8% of the nucleated cells belonging to the myeloid series. Blast cells comprised 15% of all nucleated cells. By flow cytometry, 52% of the cells were positive for CD18, whereas CD33, CD117 and CD235a (glycophorin A) showed a weak reactivity. A significant degree of positive reaction to megakaryocytic lineage markers, including CD41 and CD61, was observed (Figure 3). No reactivity was observed for CD10 and CD34.
Figure 3.
Flow cytometric analysis of bone marrow cells from a cat with myelodysplastic syndrome with excess blasts. Flow cytometric histograms show the isotype control and cells with positive reactivity with some human monoclonal antibodies. Fifty-two percent of the cells are positive for cluster of differentiation (CD)18, whereas immunophenotyping with anti-CD33, anti-CD117 and anti-CD235a antibodies shows a weak reactivity. A significant degree of positive reaction to CD41 and CD61 is demonstrated. Blue line = CD markers; red line = isotype control
Case 2
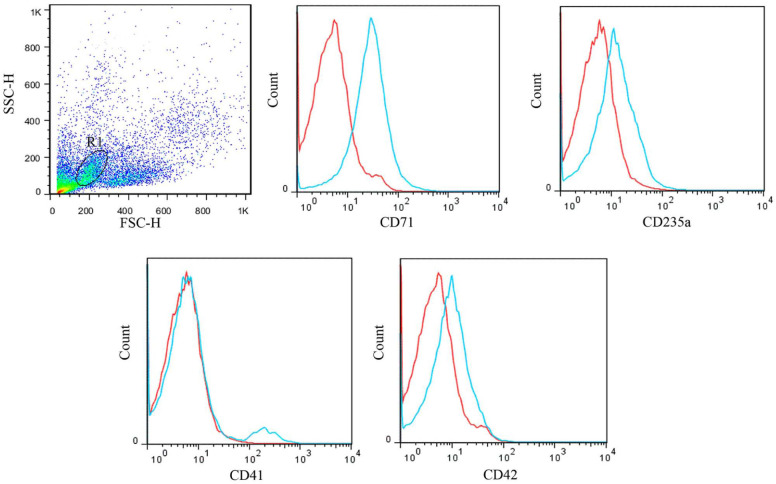
A 2-year-old female domestic shorthair cat was referred with a history of anorexia, weakness and petechiae. Physical examination revealed pale mucous membranes and marked hepatosplenomegaly, which caused abdominal distension. This case had a diagnosis of erythroleukaemia. Peripheral blood was characterised by severe anaemia and thrombocytopenia. A few circulating erythroid precursors, including rubriblasts, rubricytes and metarubricytes, were found. By cytological analysis of the bone marrow aspirate, the erythroid series was found to comprise 82% of the nucleated cells, with 67% dysplastic morphology in erythroid cells and an M:E of 0.19. The rubriblasts constituted 18.6% of all the nucleated cells and 22.4% of the erythroid cells. The forward/side scatter dot plot in flow cytometry revealed a different pattern of cell distribution than the normal bone marrow (Figure 4), with an increased population of immature cells, which were positive for erythroid markers (CD71 and CD235a) and megakaryocytic markers (CD41 and CD42) (Figure 4). The bone marrow cells were negative for CD19 and CD34.
Figure 4.
Flow cytometric analysis of bone marrow cells from a cat with erythroleukaemia. A distinct population of the neoplastic cells was gated (R1) in the forward scatter (FSC)–side scatter (SSC) plot. The histograms for cluster of differentiation (CD)71, CD235a, CD41 and CD42 expression by the cells in gate R1 are shown. Blue line = CD markers; red line = isotype control
Case 3
A 6-month-old male domestic shorthair cat with a history of anorexia, lethargy and weakness was presented. The cat was classified as having MDS. Haematological findings in the peripheral blood included leukocytosis, macrocytic hypochromic anaemia and thrombocytopenia. The bone marrow cells were dysplastic in both myeloid and erythroid series with an M:E ratio of 7.3. Also, 19% of the myeloid cells and 13% of the erythroid cells exhibited dysplastic features. Serological assay for anti-FIV antibody was positive. The bone marrow cells in this cat were weakly positive for CD33, CD14, and CD45, and negative for CD11b, CD117, CD20, CD34, CD19, CD5 and CD10.
Case 4
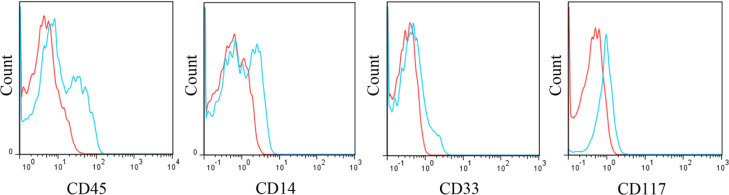
A domestic shorthair cat with a history of anorexia, lethargy, pale mucous membranes and lateral recumbency was diagnosed with MDS. Peripheral blood was characterised by severe, refractory anaemia; leukopenia; and marked thrombocytopenia. The cat was positive for FeLV. Remarkable dysplastic morphologies were notified in erythroid, myeloid and megakaryocytic lineages by the cytological analysis of the bone marrow aspirate. The M:E ratio was 8.3. The flow cytometric analysis of the bone marrow cells showed a significant positivity for CD45 and CD14, and a weak positivity for CD33 and CD117 (Figure 5). The bone marrow cells in this cat were negative for CD64 and CD11b.
Figure 5.
Flow cytometric analysis of bone marrow cells from a cat with myelodysplastic syndrome. The histograms for cluster of differentiation (CD)45, CD14, CD33 and CD117 are shown. Blue line = CD markers; red line = isotype control
Culture of feline haematopoietic progenitors in methylcellulose-based medium
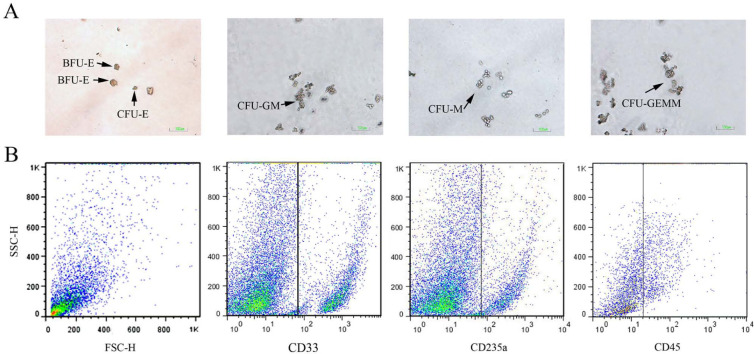
By culturing the bone marrow cells in methylcellulose-based medium, we increased the number of cells with an immature phenotype. CFU-E, BFU-E, CFU-GM, colony-forming unit-granulocyte (CFU-G), colony-forming unit–macrophage (CFU-M) and CFU-GEMM colonies were detected in the methylcellulose assays (Figure 6A). The cultured marrow cells were subsequently counted (Table 2). CFU-GM and BFU-E were the most frequent colonies outgrown from the feline bone marrow cells, whereas CFU-M was the least frequent progenitor in the feline bone marrow (Figure 6; Table 2).
Figure 6.
(A) Representative images of the feline haematopoietic progenitor cell colonies. Colony-forming unit–erythroid (CFU-E): a cell cluster containing fewer than 100 haemoglobinised erythroblasts. Burst-forming unit–erythroid (BFU-E): two small BFU-E colonies with more than 200 cells. Individual cells are characterised by a small size with a reddish colour. Colony-forming unit–granulocyte, macrophage (CFU-GM) colony: two distinct cell clusters are distinguishable. The monocyte/macrophage cells are larger, whereas the granulocyte lineage cells are smaller and more regular in shape. Colony-forming unit–macrophage (CFU-M): the colony is characterised by large, colourless cells. Colony-forming unit–granulocyte, erythroid, macrophage, megakaryocyte (CFU-GEMM): mixture of erythroid clusters and granulocyte and macrophage cells. (B) Forward scatter (FSC-H) versus side scatter (SSC-H) plot along with dot plots of cluster of differentiation (CD)33 (anti-human), CD235a (anti-human) and CD45 (anti-feline) expression in the feline haematopoietic progenitor cells cultured in the methylcellulose-based medium. The vertical line is placed to the right of the cells labelled with isotype control
Table 2.
Feline haematopoietic progenitor cell colonies in the methylcellulose-based colony forming cell assay and the percentage expression of cluster of differentiation (CD) markers on the progenitor cells. Data are expressed as mean ± SD
| Colony |
% CD-positive progenitor cells |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| CFU-GM | BFU-E | CFU-E | CFU-G | CFU-M | CFU-GEMM | CD45 | CD33 | CD235a | CD18 |
| 85 ± 17 | 82 ± 40 | 55 ± 19 | 15 ± 3 | 12 ± 3 | 1.8 ± 0.8 | 18.3 ± 8.5 | 14.9 ± 7.3 | 11.5 ± 3.2 | 9.2 ± 5.2 |
CFU-GM = colony-forming unit–granulocyte, macrophage; BFU-E = burst-forming unit–erythroid; CFU-E = colony-forming unit–erythroid; CFU-G = colony-forming unit-granulocyte; CFU-M = colony-forming unit-macrophage; CFU-GEMM = colony-forming unit–granulocyte, erythroid, macrophage, megakaryocyte
A panel of six anti-feline antibodies (anti-CD21-like, anti-T-lymphocytes, anti-CD172a, anti-granulocyte, anti-CD45-like and anti-CD18) and eight anti-human antibodies (anti-CD71, anti-CD33, anti-CD235a, anti-CD41, anti-CD6, anti-CD117, anti-CD38 and anti-CD34) were used on the feline bone marrow progenitors cultured on MethoCult’s methylcellulose-based medium to probe the cell-surface antigens expressed by the feline haematopoietic progenitor cells. The flow cytometric immunophenotyping of the colony cells revealed that these cells were negative for CD21-like, T-lymphocyte, CD172a, granulocyte, CD41 and CD61, but were positive for pan leukocytes (CD45-like), CD18, CD33, CD71 and CD235a (glycophorin A) (Figure 6B). Also, 18.33 ± 8.49, 14.9 ± 7.34, 11.57 ± 3.20 and 9.16 ± 5.24% of the total progenitor cells harvested from the cell culture were CD45-, CD33-, CD235a- and CD18-positive, respectively (Table 2). CD133 tested in a single culture showed 4% positivity. As CD133 is a marker that is frequently expressed by multipotent progenitor cells, the expression of this antigen on the cultured feline bone marrow cells indicated the enrichment of the immature haematopoietic progenitor cells.
Discussion
Six human-specific mAbs, including anti-CD61, anti-CD18, anti-CD14, anti-CD235a, anti-CD41 and anti-CD29 antibodies, showed cross-reactions with the feline antigens. In humans, CD235a (glycophorin A) appears at the erythroblast stage and rapidly reaches its maximum expression level in the early normoblast stage. 11 Once glycophorin reaches maximum expression, the amount of expression is constant and does not change during further maturation. 11 In this study, the expression of glycophorin was detected on the feline bone marrow erythroid cells. We found an increased expression of CD235a in a cat with MDS. Consistent with this study, an increased expression of CD235a (glycophorin A) has been reported in a cat with a diagnosis of acute erythroid leukaemia with multi-lineage dysplasia. 6 Deregulation of glycophorin A gene expression has recently been reported to occur in dysplastic erythroblasts. 12 This marker appears to be useful in supporting a diagnosis of MDS affecting the erythroid series, a common form of MDS in the cat. 13 Anti-CD235a antibody has also been reported to have cross-reactivity with canine erythroid cells. 14
In human medicine, a limited number of antibodies, including anti-CD71 (transferrin receptor) and anti-glycophorin antibodies, are currently available for evaluating erythroid cells. 15 The expression of CD71 on erythroid cells precedes the expression of glycophorin. 11 Human erythroid colony-forming cells (CFU-E and BFU-E) often express low-to-intermediate amounts of CD71. 11 We found the expression of CD71 by feline haematopoietic CFUs on the methylcellulose medium. The blast cells in the cat with erythroleukaemia showed positivity for CD71, which is consistent with the erythroid nature of the blasts. Although the bone marrow cells were positive for glycophorin A, consistent with an increase in the number of the neoplastic cells of the erythroid origin, the blasts were negative for CD117. It has been reported that despite the high sensitivity for myeloid detection, CD117 expression is usually absent in cases with megakaryocytic or erythroid involvement. 16
In this study, we found anti-CD41 and anti-CD61 antibodies to cross-react with feline antigens. Human CD41 is expressed on platelets and megakaryocytes. Meister et al 1 showed that a small percentage of CD41-positive cells are likely the result of adherent platelets or platelet fragments. Anti-CD61 antibody detects platelets in the peripheral blood and bone marrow, and also reacts with megakaryocytes and megakaryoblasts. This antibody is of value in the diagnosis of megakaryoblastic leukaemia. 17 Anti-human CD61 has been reported to cross-react with canine and caprine cells.14,18,19 The clone Y2/51 of anti CD61 antibody, which we used in the present study, reacted with the feline platelets in a manner similar to that of human platelets. In addition, M7057 clone of anti-CD41, M753 clone of anti-CD61 and MCA1095 clone of anti-CD41/61 have also been reported to cross-react with feline platelets. 20 Strong positivity for CD41 and CD61 in the cat with MDS-EB indicates the involvement of the megakaryocytic lineage.
CD14 antigen is a receptor with a high affinity for the complex of lipopolysaccharide (LPS) and LPS-binding protein and is expressed on myeloid-derived cells. 21 CD14 is primarily expressed on monocytes and macrophages. The antibody against CD14 is of value in the detection of normal and neoplastic cells of the monocytic lineage. It is an antibody in the standard panel of antibodies recommended for the immunophenotyping of all human patients being evaluated for leukaemia. Clone CAM36A anti-CD14 mAb has previously been demonstrated to be able to label feline monocytes and granulocytes, but not lymphocytes. 1 In our study, the low percentage of CD14-positive cells in the normal healthy cats indicates that this antibody labels only monocytic cells, which comprise a low percentage of bone marrow cells.
In humans, CD34 is considered as a marker of progenitor cells, but the antibody clone that we used in this study failed to label the feline bone marrow cells obtained from the healthy cats or the cats with haematopoietic abnormalities. Furthermore, it did not label the feline haematopoietic colonies cultured on the methylcellulose-based medium.
CD33 did not express on the bone marrow cells from the healthy cats, whereas the bone marrow cells from the two cats with dysplastic myeloid cells (cases 1 and 3) expressed CD33. In humans, CD33 is found on cells committed to the myeloid lineage, and on leukaemic blasts of myeloid origin, but not on normal multipotent haematopoietic stem cells or on lymphoid cells.22,23 CD33 is expressed in all stages of granulocytic maturation, but its expression decreases as the cells mature. 24 Human leukaemia studies have demonstrated that in AML, neoplastic cells are more likely to be CD33+ than cells in MDS because their expression is decreased with the maturity of the myeloid cells. 23 However, CD33 surface intensity significantly differs with the type of disease. 25 For example, in MDS the blasts are less likely to be CD33+. 23
Culturing the feline bone marrow cells in the methylcellulose-based medium resulted in the outgrowth of haematopoietic progenitors with significant enrichment of more immature cells, CFU-GM and BFU-E. BFU-E and CFU-GM progenitors of the feline bone marrow can also be enriched by counterflow centrifugal elutriation and subsequent treatment of the fractionated cells with the soybean agglutinin lectin. 26 In humans, CFU-GM cells are characterised by the cell surface markers CD13, CD33, CD34 and human leukocyte antigen (HLA)-DR. BFU-E is characterised by the expression of several cell surface markers, such as CD33, CD34 and HLA-DR. CD45, CD33, CD235a and CD18 were found to have been expressed on the feline bone marrow progenitor cells, with the highest expression level of CD45, which is in line with the enrichment of CFU-GM and BFU-E, as has been previously described for human myeloid and erythroid progenitors.27–29 In a previous study using the mouse monoclonal anti-CD45, WC45a, the authors demonstrated that this marker was expressed on the surface of all feline leukocyte populations tested, including granulocytes, monocytes, and T and B lymphocytes. 30 CD33 expression has also been reported in human myeloid progenitors, as well as in BFU-E, but not in CFU-E. 29 The methylcellulose-based medium used in this study did not support the growth of megakaryocytic lineage. It seems that, similar to that seen in humans, a megakaryocytic-specific medium is required for culturing megakaryocytic cells in cats. 31 The colony-forming cell assay based on the ability of feline haematopoietic progenitors to form colonies in the methylcellulose-based semisolid medium, however, can be used to study haematopoiesis and leukaemogenesis in cats.
Conclusions
In this study, we employed new clones of commercially-available anti-human antibodies on normal, neoplastic and cultured feline haematopoietic progenitors. We demonstrated that a panel of anti-human antibodies can be used for the recognition and differentiation of feline bone marrow haematopoietic cells. A number of the antibodies studied have potential application in the recognition of feline blasts cells and the diagnosis of haematopoietic disorders.
Acknowledgments
We wish to thank Mrs L Haghighi for technical assistance.
Footnotes
Funding: This study was supported by a research grant from Iran National Science Foundation (INSF) (grant 89002374) and a grant from the University of Tehran.
The authors do not have any potential conflicts of interest to declare.
Accepted: 22 August 2013
References
- 1. Meister RK, Taglinger K, Haverson K, et al. Progress in the discovery and definition of monoclonal antibodies for use in feline research. Vet Immunol Immunopathol 2007; 119: 38–46. [DOI] [PubMed] [Google Scholar]
- 2. Breuer W, Hermanns W, Thiele J. Myelodysplastic syndrome (MDS), acute myeloid leukaemia (AML) and chronic myeloproliferative disorder (CMPD) in cats. J Comp Pathol 1999; 121: 203–216. [DOI] [PubMed] [Google Scholar]
- 3. Hutson C, Rideout B, Pedersen N. Neoplasia associated with feline immunodeficiency virus infection in cats of southern California. J Am Vet Med Assoc 1991; 199: 1357 –1362. [PubMed] [Google Scholar]
- 4. Jain NC. Classification of myeloproliferative disorders in cats using criteria proposed by the Animal Leukaemia Study Group: A retrospective study of 181 cases (1969–1992). Comp Haematol Int 1993; 3: 125–134. [Google Scholar]
- 5. Sharifi H, Nassiri SM, Esmaelli H, Khoshnegah J. Eosinophilic leukaemia in a cat. J Feline Med Surg 2007; 9: 514–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shirani D, Nassiri SM, Aldavood SJ, et al. Acute erythroid leukemia with multilineage dysplasia in a cat. Can Vet J 2011; 52: 389–393. [PMC free article] [PubMed] [Google Scholar]
- 7. Comazzi S, Gelain ME, Riondato F, Paltrinieri S. Flow cytometric expression of common antigens CD18/CD45 in blood from dogs with lymphoid malignancies: a semi-quantitative study. Vet Immunol Immunopathol 2006; 112: 243–252. [DOI] [PubMed] [Google Scholar]
- 8. Reggeti F, Bienzle D. Flow cytometry in veterinary oncology. Vet Pathol 2011; 48: 223–235. [DOI] [PubMed] [Google Scholar]
- 9. Tasca S, Carli E, Caldin M, et al. Hematologic abnormalities and flow cytometric immunophenotyping results in dogs with hematopoietic neoplasia: 210 cases (2002–2006). Vet Clin Pathol 2009; 38: 2–12. [DOI] [PubMed] [Google Scholar]
- 10. Nassiri SM, Khaki Z, Soleimani M, et al. The similar effect of transplantation of marrow-derived mesenchymal stem cells with or without prior differentiation induction in experimental myocardial infarction. J Biomed Sci 2007; 14: 745–755. [DOI] [PubMed] [Google Scholar]
- 11. Loken M, Shah V, Dattilio K, Civin C. Flow cytometric analysis of human bone marrow: I. Normal erythroid development. Blood 1987; 69: 255–263. [PubMed] [Google Scholar]
- 12. Frisan E, Vandekerckhove J, de Thonel A, et al. Defective nuclear localization of Hsp70 is associated with dyserythropoiesis and GATA-1 cleavage in myelodysplastic syndromes. Blood 2012; 119: 1532–1542. [DOI] [PubMed] [Google Scholar]
- 13. McManus PM. Classification of myeloid neoplasms: a comparative review. Vet Clin Pathol 2005; 34: 189–212. [DOI] [PubMed] [Google Scholar]
- 14. Schuberth H-J, Kucinskiene G, Chu R-M, Faldyna M. Reactivity of cross-reacting monoclonal antibodies with canine leukocytes, platelets and erythrocytes. Vet Immunol Immunopathol 2007; 119: 47–55. [DOI] [PubMed] [Google Scholar]
- 15. Sharma A, Buxi G, Yadav R, et al. Childhood acute erythroleukemia diagnosis by flow cytometry. Indian J Pathol Microbiol 2011; 54: 173–175. [DOI] [PubMed] [Google Scholar]
- 16. Nomdedéu JF, Mateu R, Altès A, et al. Enhanced myeloid specificity of CD117 compared with CD13 and CD33. Leukemia Res 1999; 23: 341–347. [DOI] [PubMed] [Google Scholar]
- 17. Mercher T, Raffel GD, Moore SA, et al. The OTT-MAL fusion oncogene activates RBPJ-mediated transcription and induces acute megakaryoblastic leukemia in a knockin mouse model. J Clin Invest 2009; 119: 852–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Davis WC, Drbal K, Mosaad AAE, et al. Use of flow cytometry to identify monoclonal antibodies that recognize conserved epitopes on orthologous leukocyte differentiation antigens in goats, llamas, and rabbits. Vet Immunol Immunopathol 2007; 119: 123–130. [DOI] [PubMed] [Google Scholar]
- 19. Ameri M, Wilkerson MJ, Stockham SL, et al. Acute megakaryoblastic leukemia in a German Shepherd dog. Vet Clin Pathol 2010; 39: 39–45. [DOI] [PubMed] [Google Scholar]
- 20. Brodersen R, Bijlsma F, Gori K, et al. Analysis of the immunological cross reactivities of 213 well characterized monoclonal antibodies with specificities against various leucocyte surface antigens of human and 11 animal species. Vet Immunol Immunopathol 1998; 64: 1–13. [DOI] [PubMed] [Google Scholar]
- 21. Steinberg JD, Olver CS, Davis WC, et al. Acute myeloid leukemia with multilineage dysplasia in an alpaca. Vet Clin Pathol 2008; 37: 289–297. [DOI] [PubMed] [Google Scholar]
- 22. Jilani I, Estey E, Huh Y, et al. Differences in CD33 intensity between various myeloid neoplasms. Am J Clin Pathol 2002; 118: 560–566. [DOI] [PubMed] [Google Scholar]
- 23. Abdool A, Yeh C-H, Kantarjian H, et al. Circulating CD33 and its clinical value in acute leukemia. Exp Hematol 2010; 38: 462–471. [DOI] [PubMed] [Google Scholar]
- 24. Gorczyca W, Sun ZY, Cronin W, et al. Immunophenotypic pattern of myeloid populations by flow cytometry analysis. Methods Cell Biol 2011; 103: 221–266. [DOI] [PubMed] [Google Scholar]
- 25. Florian S, Sonneck K, Hauswirth AW, et al. Detection of molecular targets on the surface of CD34+/CD38− stem cells in various myeloid malignancies. Leukemia Lymphoma 2006; 47: 207–222. [DOI] [PubMed] [Google Scholar]
- 26. Gengozian N. Development of monoclonal antibodies to erythroid progenitors in feline bone marrow. Vet Immunol Immunopathol 1998; 64: 299–312. [DOI] [PubMed] [Google Scholar]
- 27. Lansdorp PM, Sutherland HJ, Eaves CJ. Selective expression of CD45 isoforms on functional subpopulations of CD34+ hemopoietic cells from human bone marrow. J Exp Med 1990; 172: 363–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oertel J, Oertel B, Schleicher J, Huhn D. Immunotyping of blasts in human bone marrow. Ann Hematol 1996; 72: 125–129. [DOI] [PubMed] [Google Scholar]
- 29. Papayannopoulou T, Brice M, Broudy VC, Zsebo KM. Isolation of c-kit receptor-expressing cells from bone marrow, peripheral blood, and fetal liver: functional properties and composite antigenic profile. Blood 1991; 78: 1403–1412. [PubMed] [Google Scholar]
- 30. Hunt P, Else RW, McConnell I, Hopkins J. Identification of CD45 (leucocyte common antigen) in the domestic cat. Res Vet Sci 1995; 59: 201–204. [DOI] [PubMed] [Google Scholar]
- 31. Blair A, Baker CL, Pamphilon DH, Judson PA. Ex vivo expansion of megakaryocyte progenitor cells from normal bone marrow and peripheral blood and from patients with hematological malignancies. Br J Haematol 2002; 116: 912–919. [DOI] [PubMed] [Google Scholar]