Abstract
The aim of this study was to evaluate the efficacy and safety of electrochemotherapy (ECT) with bleomycin for treatment of squamous cell carcinoma (SCC) in cats. Between March 2008 and October 2011, 11 cats with 17 superficial SCC nodules in different clinical stages (ranging from Tis to T4), located on nasal planum (6/11), pinnae (3/11) and both locations (2/11), were included in a prospective non-randomised study. Sixteen of 17 SCC nodules were treated with ECT (15/16 with single session and in one case with two sessions); one nodule was surgically removed. Altogether, complete response (CR) was achieved for 81.8% (9/11) cats and 87.5% (14/16) nodules, lasting from 2 months up to longer than 3 years. Only 2/9 cats in which CR was initially observed, had recurrence 2 and 8 months after the ECT procedure. In the remaining two cats with highly infiltrative spread into adjacent tissues, progression of the disease was observed, despite ECT, and both were euthanased 4 and 5 months after the procedure. ECT in cats was well tolerated and no evident local or systemic side effects were observed. The results of this study suggest that ECT is a highly effective and safe method of local tumour control of feline cutaneous SCCs. It should be considered as an alternative treatment option, especially when other treatment approaches are not acceptable by the owners, owing to their invasiveness, mutilation or high cost.
Introduction
Squamous cell carcinoma (SCC) is one of the most frequent malignant tumours in cats, accounting for approximately 15% of feline cutaneous tumours and 60–75% of oral tumours in cats.1,2 SCC affects mostly older animals with a mean age of 12 years. 1
Cutaneous SCCs are usually found in unpigmented or lightly pigmented skin, and are most frequently located on the nasal planum (80–90%), 3 pinnae (especially margins) (50%), eyelids (20%) and lip, presumably due to higher ultraviolet exposure of these locations.1,3 Furthermore, infection with papillomavirus has been proposed as a possible contributing factor to development of the disease.4,5 In addition, SCC could be diagnosed as a consequence of chronic dermatitis, lupus erythematosus, or cutaneous and follicular cysts. Cutaneous SCCs are usually present either as a proliferative lesion or as an ulcerative poorly healing wound, mostly in more advanced stages of the disease.1,3
SCCs of cats are locally invasive with late metastatic potential, predominantly in regional lymph nodes. 3 Generally, lesions with lower degree of local invasion (Tis and T1) respond more favourably to the treatment than lesions with significant invasion (T2–T4).1,3
A number of treatment modalities have been applied for treatment of cutaneous and oral SCCs, with generally good outcomes if the lesions are treated in the initial stage of the disease. Surgery and cryosurgery are the most commonly used treatment modalities, 1 although some reports of radiotherapy6,7 and photodynamic therapy have been published.8–10 In general, the recommended aggressive surgical treatment (1–3 cm safety margins) is the easiest to perform in lesions located on the pinnae, resulting in 100% complete response with long-term control of the disease (>1.5 years) in the majority of cases.11–13 Similarly, the response rate in studies using cryosurgery was nearly 100% for lesions located on pinnae and eyelids; however, the response rate of only 70% was achieved in lesions located on nasal planum. 14 Different radiotherapy protocols have been applied in treatment of feline SCCs, resulting in a response rate of 52–100%, depending on the stage of the disease, type of radiation protocol, concomitant treatment used, etc.8,15–18 Strontium plesiotherapy, a form of superficial radiotherapy, has provided long-term control with a high complete response rate (88%) in 49 treated cats with nasal planum SCCs. 6 A similar response was achieved using photodynamic therapy in superficial lesions, with a complete response obtained in 85% of the animals treated. 9
Chemotherapy has only limited use in the treatment of feline SCCs. Different chemotherapeutic protocols utilising either monotherapy or combinations of cytotoxic agents (eg, mitoxantrone, actinomycin D, doxorubicin/cyclophosphamide combinations, bleomycin, carboplatin) have been investigated, applied either intralesionally or systemically, but generally with poor success and short-lived duration of therapeutic response.19–21 However, chemotherapy could be beneficial as an adjuvant therapy following surgery or radiotherapy for higher-grade lesions. 1
Electrochemotherapy (ECT) is a combined use of chemotherapeutic drugs such as bleomycin or cisplatin in combination with high-voltage electric pulses that cause reversible permeabilisation of cell membranes, enabling the entry of drugs into the cells. ECT using either of these cytotoxic drugs, different injection routes and different parameters of electric pulses has been shown to be an alternative treatment option for local control of tumours of different histological types in different animal species 22 and is also used in humans in more than 100 oncological centres throughout Europe.23–25
Bleomycin is the chemotherapeutic agent indicated for treatment of some types of carcinomas, including SCC, mostly in combination with other drugs or modalities, and at a recommended dosage of 2 mg/m2 weekly or 125–200 mg of total cumulative dosage. It may cause acute toxicity with increased body temperature, anorexia, vomiting and allergic reaction, and, rarely, myelosupression. Chronic toxicity may be presented with dermatological alterations (alopecia, rashes), stomatitis, pneumonitis and pulmonary fibrosis.26,27 In one study, a single administration of bleomycin (Soviet bleomycin, bleomycetin) had no significant effects on arterial pressure, respiration, electrocardiogram characteristics and elements of the vegetative nervous system in narcotised cats. 28
Results obtained in a preliminary study, published by Spugnini et al 29 in 2009 suggest that ECT with intralesional administration of bleomycin, followed by biphasic electric pulses, could be a safe and effective option for SCCs in cats. Nine cats with T2–T4 SCC received two sessions of ECT 1 week apart, and 7/9 cats (77.7%) had a complete response lasting up to 3 years. 29
The aim of our prospective non-randomised pilot study was to establish the safety and efficacy of ECT, using intravenous administration of bleomycin, followed by local application of standard parameters of electric pulses in treatment of subcutaneous feline SCCs.
Materials and methods
Patients
Between March 2008 and October 2011, 11 cats with cutaneous superficial SCCs were included in the prospective non-randomised pilot study conducted in accordance with protocols for ECT that were based on previous experience in human and animal clinical studies (Table 1).30–32 Written informed consent was obtained from each owner before beginning treatment.
Table 1.
Clinical dates, treatment used and outcome in cats with cutaneous squamous cell carcinoma included in the study
| Cat | Age (years) | Sex | Number of nodules | Location | Staging | Tx | Outcome (1 month after ECT) | Disease-free interval (months) |
Notes |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 12.0 | F | 1 | P | T1 | ECT | CR | 13 | |
| 2 | 12.0 | M | 2 | P | T2 | ECT | CR | 8 | Sx of both pinnae after recurrence |
| P | T2 | ECT | CR | 8 | |||||
| 3 | 8.0 | F | 2 | P | T4 | ECT | CR | 13 | |
| P | T1 | ECT | CR | 13 | |||||
| 4 | 10.0 | F | 1 | N | Tis | ECT | CR | 36 | |
| 5 | 2.5 | M | 1 | N | T2 | ECT | CR | 42 | |
| 6 | 14.0 | F | 1 | N | T2 | ECT | CR | 13 | |
| 7 | 14.5 | F | 1 | N | T4 | 2 × ECT | 1st ECT: CR 2nd ECT: PR |
2 7 |
|
| 8 | 13.0 | M | 1 | N | T4 | ECT | PD | – | Infiltrative growth into nasal and oral cavity |
| 9 | 12.0 | F | 1 | N | T3 | ECT | PD | – | Big lesion causing facial distortion |
| 10 | 12.0 | M | 2 | N | T1 | ECT | CR | 13 | |
| P | T4 | Sx | CR | 13 | |||||
| 11 | 10.5 | M | 4 | N | T2 | ECT | CR | 28 | |
| P | T2 | ECT | CR | 28 | |||||
| P | T2 | ECT | CR | 28 | |||||
| E | T2 | ECT | CR | 28 |
Tx = therapy; ECT = electrochemotherapy; F = female; M = male; P = pinna; N = nasal planum; E = eye canthus; T1 = <2 cm and superficial; T2 = 2–5 cm or minimal invasion, regardless diameter; T4 = invasion in subcutis and other tissues, including fascia, muscle, cartilage or bone; 33 Tis = carcinoma in situ; T3 = >5 cm or invasion in subcutis regardless of diameter; Sx = surgery; CR = complete response; PR = partial response; PD = progressive disease
The study cohort comprised of five males and six females of different breeds; their age ranged from 2.5 to 14.5 years (median 12 years) (Table 1). Inclusion criteria for the study comprised of at least one cutaneous superficial tumour lesion, cytologically confirmed as SCC; good general health status of the animal with the basic haematology and biochemistry profile within reference limits; normal renal and cardiovascular function; and an expected survival time of at least 3 months. The diagnosis was made based on examination of fine-needle aspiration biopsies taken from lesions, finding cytological criteria consistent with SCC. Clinical staging was performed using tumor, nodes, metastasis (TNM) adapted classification. 33
Treatment protocol
Cats were premedicated with a combination of medetomidine (0.02 mg/kg Domitor; Pfizer Animal Health) and ketamine (2 mg/kg Bioketan; Vetoquinol). General anaesthesia was induced with propofol (1 mg/kg Diprivan; Zeneca) and maintained with isoflurane (Forane; Abbott Laboratories). During anaesthesia, animals received Hartmann’s solution (B Braun Melsungen) at a rate of 10 ml/kg/h. Where it was applicable, two perpendicular sizes (a, b) of ulcerative lesions were measured in cats under general anaesthesia and the surface of lesions was calculated using the formula: area = a × b.
The dosage of bleomycin was determined according to the body surface area (BSA), calculated from the bodyweight (BW) mass with the formula: BSA (in m2) = (BW in g)2/3 × 10–3. ECT consisted of intravenous injection of bleomycin (3 mg/ml Blenoxane; Bristol-Myers) at a dosage of 30 mg/m2 BSA.
Ten mins after injection of bleomycin, electroporation of the tumour was performed with the electric pulse generator (Cliniporator; Igea) using plate electrodes (two parallel stainless steel electrodes 8 mm apart and with a length of 30 mm). Good contact between the electrodes and the skin was assured by depilation and application of a conductive gel to the treatment area. Eight electric pulses were delivered with 1300 V/cm amplitude to electrode distance ratio, a duration of 100 µs and a frequency of 5000 Hz.
Additionally, all animals were provided with oral analgesia using the non-steroidal anti-inflammatory drug meloxicam (Loxicom; Norbrook Laboratories) at a dose of 0.1 mg/kg/day for three consecutive days.
Treatment evaluation
In order to evaluate treatment effectiveness and possible local (during and after electroporation) and systemic (body temperature, respiratory and cardiac rate) side effects, the cats were examined during the first 4 h after drug administration, every 2 weeks for the first month and monthly thereafter. At each visit the treated tumours were measured and photographed. Response to treatment was evaluated based on size and clinical appearance of the surface area of treated tumours. Complete response (CR) was defined as complete eradication of the treated tumour with re-epithelialisation of skin. Partial response (PR) was defined as a decrease of >50% of the largest perpendicular diameters of measurable lesions. No change was defined as a reduction of <50% and an increase of <25% of the above-mentioned measurements. Progressive disease was defined by an increase of >25%. In cases where it was not possible to obtain measurements because tumours were ulcerated or covered with crusts, they were rated as non-evaluable. The number of objective responses was determined by combining the number of CR and PR. Observation time was calculated as the interval between the date of the first treatment and the date of the last examination of the patient. All data and parameters of the treatment procedure were stored in an electronic database storing the electronic case record forms (CRF). 34 The electronic CRF included measurements and photographs of the treated tumours before and after the treatment, and reports on side effects that were evaluated according to Veterinary Cooperative Oncology Group Common Terminology Criteria for Adverse Events (VCOG–CTCAE) toxicity scale on a weekly basis for the first month, then monthly for 6 months and 3–6 monthly thereafter until the end of the follow up. 35 Beside clinical evaluation, complete haematology analyses and serum biochemical parameters (urea, creatinine, alanine aminotransferase, alkaline phosphatase, total proteins, albumin, phosphate, sodium, potassium and chloride concentration) were performed at each examination.
Results
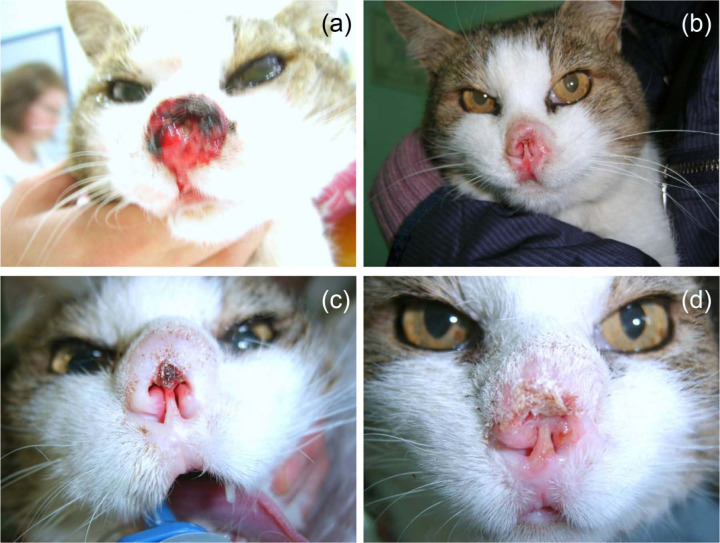
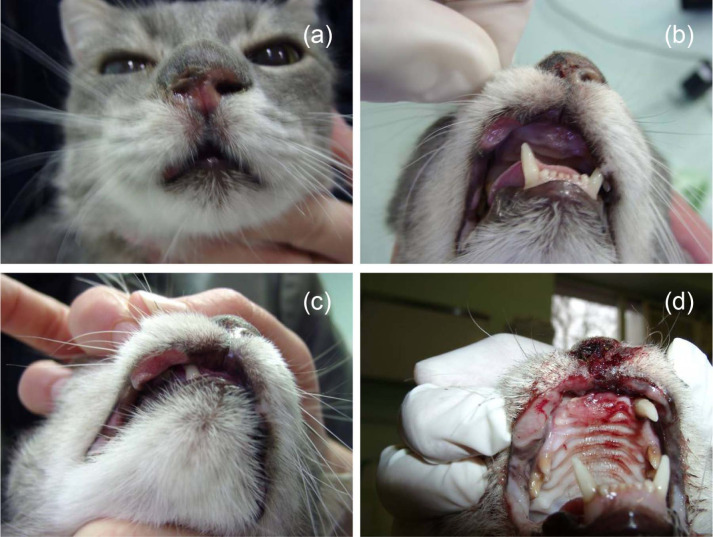
Eleven cats with 17 cytologically confirmed cutaneous SCCs were enrolled in our study, with tumour stages ranging from Tis to T4 (Table 1). Six cats had lesions involving nasal planum, in three cats one or both pinnae were involved and two had multiple nodules involving nasal planum, pinnae and/or eye canthus (Table 1). Two of the lesions on nasal planum had infiltrated into the nasal mucosa (cat 7, Figure 1; cat 8, Figure 2) and two of the lesions involving pinnae had infiltrated into auricular cartilage (cats 3 and 10).
Figure 1.
Cat 7. Squamous cell carcinoma on nose; stage T4, two sessions of electrochemotherapy (ECT) separated by 2.5 months. Complete response by 2 months/partial response by 7 months; owner decision for euthanasia 9 months after treatment. (a) Before ECT; (b) 4 weeks after ECT; (c) 2 months after ECT and second ECT performed; (d) 1 week after second ECT session
Figure 2.
Cat 8. Squamous cell carcinoma with infiltrative growth into nasal and oral cavity: (a) nose; (b) maxilla; (c) labia, stage T4, one session of electrochemotherapy; (d) progressive disease and euthanasia after 5 months
Altogether, 16/17 SCC nodules were treated with ECT; one nodule was surgically removed. All cats received a single ECT session except cat 7 (Figure 1), in which recurrence of tumour growth was observed after the first ECT session; therefore, one additional ECT was applied to the recurrent nodule 2 months after the first ECT session (Table 1). In 9/11 cats ECT was performed as a single therapy of SCC. In cat 10, which had two lesions, the low-grade tumour (T1) involving nasal planum was treated with ECT and, at the same time, the higher-grade tumour (T4) involving the pinna was surgically removed. In one patient with recurrent disease on the pinna (cat 2), surgical resection of the pinna was performed 8 months after ECT.
Altogether, CR was achieved in 9/11 cats (81.8%) or in 14/16 nodules (87.5%), lasting from 2 months up to longer than 3 years. Two of these cats in which CR was initially observed had recurrence before the end of observation period, 2 and 8 months after the ECT procedure (Table 1), and the other seven are still alive without any signs of recurrent tumour growth. In the remaining two cats with two nodules displaying highly infiltrative spread into adjacent tissues, progression of the disease was observed despite ECT and both were euthanased 4 and 5 months after the procedure.
Safety evaluation
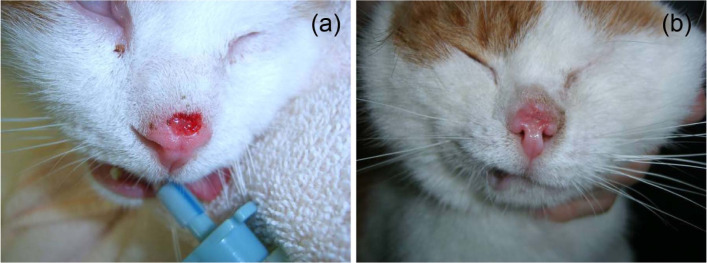
Cats tolerated the treatment well and no evident local or systemic side effects were observed. Muscle contractions were observed after the application of electric pulses. Only one contraction occurred as a result of the high repetition frequency of applied electric pulses (5 kHz) and it was instantaneous, disappearing immediately at the end of the applied electric pulses. Treatment with bleomycin administered intravenously did not result in any local or systemic toxicity. All haematological and biochemical parameters were within the reference intervals at each examination during the whole follow-up period (data not presented). In some cases, we noticed partial necrosis of the tumours after 1 week with formation of a superficial scab (grade 1 VCOG–CTCAE), which fell off within 4 weeks, while in some cases the scab was not formed (Figure 3). After treatment, none of the patients suffered from a local or systemic infection.
Figure 3.
Cat 4. Squamous cell carcinoma on nose; stage T2, one electrochemotherapy session. Complete response (CR) after 4 weeks without scab formation, CR >3 years. (a) Before treatment; (b) 4 weeks after treatment
Discussion
This study evaluated the efficacy and safety of ECT in the treatment of feline SCC, and demonstrated that this treatment option is an efficient and safe procedure, especially in cutaneous forms of the disease, resulting in a response rate of up to 87.5% of the treated nodules.
The clear drawback of this study is that it was not a randomised study within a planned clinical trial with appropriate statistical analysis comparing ECT to other standard treatments of SCC. The slow recruitment–although most of the referred patients to our clinic were treated with ECT – has enabled an observational study, providing data about the efficacy and safety of ECT in 11 cats with 17 tumours.
SCCs involving facial skin in the cats can be extremely locally invasive. Response to therapy depends on the degree of invasiveness and it is much better for lower-stage lesions (eg, Tis and T1) than for more advanced tumours. 1 Although radical surgical excision can result in an excellent response rate, it is crucial to achieve clean surgical margins in order to gain good local control of the tumour. Wide surgical margins are not always applicable owing to the anatomical locations of these tumours, especially in lesions located on nasal planum, eyelid or intra-orally. Radiotherapy and photodynamic therapy, despite being very successful in achieving local tumour control and prolongation of survival, are not widely available in veterinary medicine and are usually associated with considerable costs. Therefore, a new therapeutic modality would be appreciated, which would combine both good anti-tumour and cosmetic effects at low cost, while being less invasive than radical surgery.
Experiences with ECT in treatment of different tumours in both human and veterinary medicine show that this novel tumour treatment meets the majority of these criteria.22,32 ECT combines either intra-tumoural or systemic application of the chemotherapeutic drug (bleomycin or cisplatin) with local application of electric pulses to the tumour, which facilitate uptake of the chemotherapeutic drug into the cells of tumours and thus enhance the killing of these cells.
In our study, ECT with systemic application of bleomycin was used, resulting in an excellent local anti-tumour effect. Complete response was achieved in 87.5% of treated tumours. In comparison, other non-surgical modalities, which resulted in similar complete response rates of 85–90% (55 cats), are photodynamic therapy 9 and strontium plesiotherapy (49 and 15 cats).6,36 However, the disadvantage of phototherapy compared with ECT procedure is the length of treatment protocol — in a study of photodynamic therapy using photosensitising agent 5-aminolaevulinic acid, the cream was applied to the lesion every 30 mins for 6–8 h with cats being sedated during the whole period, followed by a 30-min illumination procedure under general anaesthesia. 9 In pleisotherapy, the major drawback is the fact that the procedure is limited only to very superficial lesions early in the course of the disease, as the dose of radiation decreases significantly below depths of 2 mm. 36 Other different types of radiotherapy of cutaneous SCCs resulted in varying therapeutic responses in treated cats with CR rates of 25 tumours in 15 cats ranging from 40% using a hypofractionated protocol 7 to 100% with a multimodality protocol utilising a combination of intralesional carboplatin and superficial radiotherapy performed on six cats. 16 The majority of curative radiation protocols are performed with multiple fractions, often up to 12, 16 which require that cats undergo multiple general anaesthesia procedures, which can carry considerable risks for older animals. This, along with the cost of such treatment, can be a significant deterrent for owners, despite relatively good efficiency of the treatment.
The only other report on using ECT for treatment of feline SCCs utilised intralesional application of bleomycin and local delivery of biphasic electric pulses. 29 Two consecutive ECT sessions delivered 1 week apart resulted in a slightly lower CR rate (77%, 7/9 cats) compared with 81.8% (9/11 cats) CR in the single ECT session employed in our study. Long-term remissions lasting longer than 1 year in the study by Spugnini et al 29 were reached in 55% of treated nodules. In comparison, in our study, remission longer than 1 year was achieved in 12/16 (75%) of the treated nodules.
The majority of small, low-grade lesions usually respond favourably to most types of treatment with the general rule that pinnal nodules are easier to treat than nasal planum lesions owing to their better accessibility.1,14 In our study, location did not affect outcome of therapy — both nasal planum (Figures 3 and 4) and pinnal tumours responded favourably. The only exceptions were two cats with advanced disease involving extremely big lesions with infiltrative growth into adjacent tissue (eg, nasal and oral cavity) (Figure 2). The prognosis of oral SCC in cats is much graver compared with cutaneous lesions, with very poor response rates and survival times after the majority of treatment modalities. 1 With clinical stage being one of the most important prognostic factors for treatment of feline SCCs it came as no surprise that the two cats with disease stage T3 and T4 and highly invasive growth responded poorly to ECT.
Figure 4.
Cat 6. Squamous cell carcinoma on nose; stage T2, one ECT session. Complete response (CR) after 8 weeks with superficial scab formation and good functional and cosmetic effect. CR > 1 year. (a) Before treatment; (b) 2 weeks after treatment; (c) 4 weeks after treatment; (d) 8 weeks after treatment
The highest therapeutic value of this new treatment procedure can be seen in feline SCCs involving nasal planum, where, owing to the anatomical location, the radical surgical excision necessitates a highly invasive procedure requiring nosectomy, often with an unacceptable cosmetic effect for the owners. 37 In our study, non-invasive forms of SCCs located on the nasal planum responded very well to ECT, with recurrence of tumour growth in only one of five patients with lesions located at this site. Furthermore, the cosmetic effect of therapy was very favourable.
The safety of this therapy for SCC in cats was demonstrated by the lack of change in haematological and biochemical parameters, as well as absence of clinical signs of bleomycin toxicity, and the absence of side effects associated with application of electric pulses. The safety of ECT has already been reported in human clinical trials, 38 as well as in our previous study on dogs with perianal tumours employing ECT and bleomycin. 30 Application of electric pulses induces muscle contractions that are painful, especially on sensitive locations. The pain dissipates immediately after application of the pulses, but the number of sensations can be reduced to one by increasing the frequency of the pulse delivery to 5 kHz. Pain in humans is controlled by local or general anaesthesia, as shown by virtual pain score of 35 (local) or 10 (general), 2 days after therapy. Analogous to human clinical trial reports, we can presume that cats undergoing ECT under general anaesthesia do not sense pain, short- or long-term, and that ECT can be generally considered safe.
Conclusions
The results of this observational, prospective, non-randomised pilot study suggest that ECT is a highly effective and safe method of local tumour control of feline cutaneous SCC, even in advanced clinical stages, with anti-tumour efficacy comparable with other conventional types of therapy. Therefore, ECT could be considered as an alternative treatment for SCC in cats, especially when other treatment approaches are not acceptable by the owners, mainly owing to their invasiveness, mutilation potential or high cost.
Footnotes
Funding: The study was supported by the Slovenian Research Agency (Projects P3-0003, J3-7044, P4-0053 and P4-0092).
The authors do not have any potential conflicts of interest to declare.
Accepted: 7 September 2013
References
- 1. Vail DM, Withrow SJ. Tumors of the skin and subcutaneous tissues. In: Withrow SJ, Vail DM. (eds). Small animal clinical oncology. 4th ed. St Louis, MO: Saunders, 2001, pp 375–401. [Google Scholar]
- 2. Marconato L. Tumori di cavo orale. In: Marconato L, Del Piero F. (eds). Oncologia medica dei piccoli animali. 1st ed. Milan: Poletto editore, 2005, pp 236–269. [Google Scholar]
- 3. Albanese F, Marconato L. Tumori di cute e sottocute. In: Marconato L, Del Piero F. (eds). Oncologia medica dei piccoli animali. 1st ed. Milan: Poletto editore, 2005, pp 176–235. [Google Scholar]
- 4. Favrot C, Welle M, Heimann M, et al. Clinical, histologic, and immunohistochemical analyses of feline squamous cell carcinoma in situ. Vet Pathol 2009; 46: 25–33. [DOI] [PubMed] [Google Scholar]
- 5. O’Neill SH, Newkirk KM, Anis EA, et al. Detection of human papillomavirus DNA in feline premalignant and invasive squamous cell carcinoma. Vet Dermatol 2011; 22: 68–74. [DOI] [PubMed] [Google Scholar]
- 6. Hammond GM, Gordon IK, Theon AP, et al. Evaluation of strontium Sr 90 for the treatment of superficial squamous cell carcinoma of the nasal planum in cats: 49 cases (1990–2006). J Am Vet Med Assoc 2007; 231: 736–741. [DOI] [PubMed] [Google Scholar]
- 7. Cunha SC, Carvalho LA, Canary PC, et al. Radiation therapy for feline cutaneous squamous cell carcinoma using a hypofractionated protocol. J Feline Med Surg 2010; 12: 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buchholz J, Wergin M, Walt H, et al. Photodynamic therapy of feline cutaneous squamous cell carcinoma using a newly developed liposomal photosensitizer: preliminary results concerning drug safety and efficacy. J Vet Intern Med 2007; 21: 770–775. [DOI] [PubMed] [Google Scholar]
- 9. Bexfield NH, Stell AJ, Gear RN, et al. Photodynamic therapy of superficial nasal planum squamous cell carcinomas in cats: 55 cases. J Vet Intern Med 2008; 22: 1385–1389. [DOI] [PubMed] [Google Scholar]
- 10. Ferreira I, Rahal SC, Rocha NS, et al. Hematoporphyrin-based photodynamic therapy for cutaneous squamous cell carcinoma in cats. Vet Dermatol 2009; 20: 174–178. [DOI] [PubMed] [Google Scholar]
- 11. Atwater SW, Powers BE, Straw RC, et al. Squamous cell carcinoma of the pinna and nasal planum: 54 cats (1980–1991). Proceed Vet Cancer Soc 1991; 11: 35–36. [Google Scholar]
- 12. Lana SE, Ogilvie GK, Withrow SJ, et al. Feline cutaneous squamous cell carcinoma of the nasal planum and the pinnae: 61 cases. J Am Anim Hosp Assoc 1997; 33: 329–332. [DOI] [PubMed] [Google Scholar]
- 13. Schmidt K, Bertani C, Martano M, et al. Reconstruction of the lower eyelid by third eyelid lateral advancement and local transposition cutaneous flap after ‘en bloc’ resection of squamous cell carcinoma in five cats. Vet Surg 2005; 34: 78–82. [DOI] [PubMed] [Google Scholar]
- 14. Clarke RE. Cryosurgical treatment of feline cutaneous squamous cell carcinoma. Aust Vet Pract 1991; 21: 148–153. [Google Scholar]
- 15. Théon AP, Madewell BR, Shearn VI, et al. Prognostic factors associated with radiotherapy of squamous cell carcinoma of the nasal plane in cats. J Am Vet Med Assoc 1995; 206: 991–996. [PubMed] [Google Scholar]
- 16. De Vos JP, Burm AG, Focker BP. Results from the treatment of advanced stage squamous cell carcinoma of the nasal planum in cats, using a combination of intralesional carboplatin and superficial radiotherapy: a pilot study. Vet Comp Oncol 2004; 2: 75–81. [DOI] [PubMed] [Google Scholar]
- 17. Melzer K, Guscetti F, Rohrer Bley C, et al. Ki67 reactivity in nasal and periocular squamous cell carcinomas in cats treated with electron beam radiation therapy. J Vet Intern Med 2006; 20: 676–681. [DOI] [PubMed] [Google Scholar]
- 18. Fidel J, Lyons J, Tripp C, et al. Treatment of oral squamous cell carcinoma with accelerated radiation therapy and concomitant carboplatin in cats. J Vet Intern Med 2011; 25: 504–510. [DOI] [PubMed] [Google Scholar]
- 19. Théon AP, VanVechten MK, Madewell BR. Intratumoral administration of carboplatin for treatment of squamous cell carcinomas of the nasal plane in cats. Am J Vet Res 1996; 57: 205–210. [PubMed] [Google Scholar]
- 20. Kisseberth WC, Vail DM, Yaissle J, et al. Phase I clinical evaluation of carboplatin in tumor-bearing cats: a Veterinary Cooperative Oncology Group study. J Vet Intern Med 2008; 22: 83–88. [DOI] [PubMed] [Google Scholar]
- 21. Martinez-Ruzafa I, Dominguez PA, Dervisis NG, et al. Tolerability of gemcitabine and carboplatin doublet therapy in cats with carcinomas. J Vet Intern Med 2009; 23: 570–577. [DOI] [PubMed] [Google Scholar]
- 22. Cemazar M, Tamzali Y, Sersa G, et al. Electrochemotherapy in veterinary oncology. J Vet Intern Med 2008; 22: 826–831. [DOI] [PubMed] [Google Scholar]
- 23. Miklavcic D, Sersa G, Brecelj E, et al. Electrochemotherapy: technological advancements for efficient electroporation-based treatment of internal tumors. Med Biol Eng Comput 2012; 50: 1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mali B, Jarm T, Snoj M, et al. Antitumor effectiveness of electrochemotherapy: a systematic review and meta-analysis. Eur J Surg Oncol 2013; 39: 4–16. [DOI] [PubMed] [Google Scholar]
- 25. Mali B, Miklavcic D, Campana LG, et al. Tumor size and effectiveness of electrochemotherapy. Radiol Oncol 2013; 47: 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Plumb DC. Veterinary drug handbook. 7th ed. Stockholm, WI: PharmaVet, 2011, pp 117–118. [Google Scholar]
- 27. Marconato L. Chemioterapici utilizzati in medicina veterinaria. In: Marconato L, Del Piero F. (eds). Oncologia medica dei piccoli animali. 1st ed. Milan: Poletto editore, 2005, pp 108–144. [Google Scholar]
- 28. Goldberg LE, Kunrat IA, Stepanova ES, et al. Toxicity, pharmacokinetics and pharmacodynamics of the Soviet bleomycin, bleomycetin, in a single administration to laboratory animals. Antibiotiki 1979; 24: 363–368. [PubMed] [Google Scholar]
- 29. Spugnini EP, Vincenzi B, Citro G, et al. Electrochemotherapy for the treatment of squamous cell carcinoma in cats: a preliminary report. Vet J 2009; 179: 117–120. [DOI] [PubMed] [Google Scholar]
- 30. Tozon N, Kodre V, Sersa G, et al. Effective treatment of perianal tumors in dogs with electrochemotherapy. Anticancer Res 2005; 25: 839–845. [PubMed] [Google Scholar]
- 31. Sersa G. The state-of-the-art of electrochemotherapy before the ESOPE study; advantages and clinical uses. Eur J Cancer Suppl 2006; 4: 52–59. [Google Scholar]
- 32. Sersa G, Miklavcic D, Cemazar M, et al. Electrochemotherapy in treatment of tumours. EJSO 2008; 34: 232–240. [DOI] [PubMed] [Google Scholar]
- 33. Owen LM. Classification of tumors in domestic animals. Geneva: World Health Organization, 1980. [Google Scholar]
- 34. Pavlovic I, Miklavcic D. Web-based electronic data collection system to support electrochemotherapy clinical trial. IEEE Trans Inf Technol Biomed 2007; 11: 222–230. [DOI] [PubMed] [Google Scholar]
- 35. Veterinary Cooperative Oncology Group. Veterinary Cooperative Oncology Group – Common Terminology Criteria for Adverse Events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.0. Vet Comp Oncol 2004; 2: 194–213.19379293 [Google Scholar]
- 36. Goodfellow M, Hayes A, Murphy S, et al. A retrospective study of (90)Strontium plesiotherapy for feline squamous cell carcinoma of the nasal planum. J Feline Med Surg 2006; 8: 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Withrow SJ. Tumors of the respiratory system. In: Withrow SJ, Vail DM. (eds). Small animal clinical oncology. 4th ed. St Louis, MO: Saunders, 2001, pp 511–539. [Google Scholar]
- 38. Marty M, Serša G, Garbay JR, et al. Electrochemotherapy – an easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. EJC Suppl 2006; 4: 3–13. [Google Scholar]