Abstract
A case of a stillborn Norwegian Forest kitten characterised in the course of anatomopathological and genetic examination is reported. The hydatidiform mole was diagnosed by delayed development, low birth weight of the kitten and abnormal placental development. Anatomopathological diagnosis was confirmed in genetic tests based on the amplification of highly heterozygous microsatellite sequences located on the X (FCA311) and autosomal chromosomes (FCA506, FCA532 and FCA178), as well as the sex-specific Sry and amelogenin (Amel) genes. The presence of two microsatellite alleles of paternal origin and one allele of maternal origin was observed in all analysed tissues (kidney, liver, brain, heart and spleen) of the stillborn kitten. The kitten’s sex was diagnosed by the presence of the paternal Sry gene, and maternal and paternal products of Amel, as well as one maternal and one paternal X chromosome FCA311 microsatellite allele. The results thus confirmed that the haploid egg was fertilised by two sperm, yielding a triploid karyotype. In summary, the successful application of genetic markers in postnatal diagnosis of this condition in the cat confirms considerable usefulness of these techniques, especially in cases where cytogenetic diagnosis is insufficient or impossible.
Case Report
Hydatidiform moles (HM) (hydatid mole, mola hydatidosa) are excessively oedematous placentae characterised by massive fluid accumulation within the villous parenchyma, formation of microcysts within the villi and an absence of fetal blood vessels. 1 The etymology is derived from hydatisia (meaning ‘a drop of water’ in Greek), referring to the watery contents of the cysts, and mole (from the Latin ‘mola’, meaning millstone/false conception). The aetiology of this placental abnormality in animals is not well known. 2 In human-based morphology, HM can be divided into two types: complete moles, in which all the chorionic villi are vesicular, and no sign of embryonic or fetal development is present, and partial moles, where some villi are vesicular, whereas others appear more normal, and embryonic or fetal development may be seen, but the fetus is always malformed and is never viable. 3 A complete mole is caused by a single (90%) or two (10%) sperm combining with an egg that has lost its DNA. 4 In the first case, the genotype is diploid with XX sex chromosomes (owing to subsequent mitosis of the fertilising sperm) and in the second case it is diploid with XY sex chromosomes 46, XY (diploid). 4 In contrast, a partial mole occurs when an egg is fertilised by one sperm or by two sperm, which can reduplicate itself yielding triploid genotypes with XXY sex chromosomes and tetraploid genotypes with XXXY sex chromosomes.4,5 Molar changes of the placenta are exceptionally rare in animals and there are only a few descriptions of moles in cows, dogs and swine. 2 However, in the available literature there is no information about HM in cats.
The aim of this study was to describe the case of a partial HM in a cat.
Material for histopathology examination was fixed in 4% buffered formalin, embedded in paraffin and stained routinely with haematoxylin and eosin.
Amplification of microsatellites located on the X (FCA311) and autosomal chromosomes (FCA506—F2 chromosome; FCA532—A2 chromosome; FCA178—A1 chromosome) and fragments of the Sry and amelogenin (Amel) genes was conducted on DNA extracted from the fetus’s internal organs (kidney, liver, brain, heart and spleen), as well as the placenta and mother’s hair. Polymerase chain reaction (PCR) mix and thermal profiles of amplification were prepared independently for each microsatellite and Sry/Amel genes (Table 1). The fluorescent PCR products (labelled with indodicarbo-cyanine-Cy5) of microsatellite amplification were separated and analysed using an Automated Laser Fluorescent (ALFexpress) DNA Sequencer. PCR products of Sry and Amel gene amplification were separated in 2% agarose gel and visualised with a Molecular Imager FX (Bio-Rad).
Table 1.
Length [base pairs (bp)] of microsatellite alleles of maternal and paternal origin (in bold), AmelX and AmelY gene isoforms, and Sry gene in the tissues of a stillborn fetus
| Microsatellite/Gene | Mother | Father | Stillborn fetus |
Placenta | ||||
|---|---|---|---|---|---|---|---|---|
| Kidney | Liver | Brain | Heart | Spleen | ||||
| FCA178 | 198/224 | 208/216 | 198/208/216 | 198/208/216 | 198/208/216 | 198/208/216 | 198/208/216 | 198/224208/216 |
| FCA532 | 202/218 | 220/238 | 202/220/238 | 202/220/238 | 202/220/238 | 202/220/238 | 202/220/238 | 202/218220/238 |
| FCA506 | 182/242 | 190/220 | 242/190/220 | 242/190/220 | 242/190/220 | 242/190/220 | 242/190/220 | 182/242190/220 |
| FCA311 | 122/174 | 114/156 | 174/156 | 174/156 | 174/156 | 174/156 | 174/156 | 122/174156 |
| Amel (206 bp)* | 206/206 | 206/182 | 206/206/182 | 206/206/182 | 206/206/182 | 206/206/182 | 206/206/182 | 206/206/182 |
| Sry | None | 613 | 613 | 613 | 613 | 613 | 613 | 613 |
The maternal and paternal origin of the fragment of AmelX isoform cannot be distinguished in the stillborn fetus and placenta
The placenta of the kitten was thickened, swollen and abnormally developed (Figure 1). It consisted of a number of differently sized, thin-walled, grape-like, friable cysts. A part of the placenta resembled normal villi, but other parts showed villous dimorphism and/or abnormal focal villous trophoblast hyperplasia within the villous edema. The lack of anatomopathological changes in internal organs, with the exception of extra-medullary haemopoiesis in the liver, was observed. The birth weight of the affected fetus (78 g) was lower than other newborn kittens of this breed (90–110 g).
Figure 1.
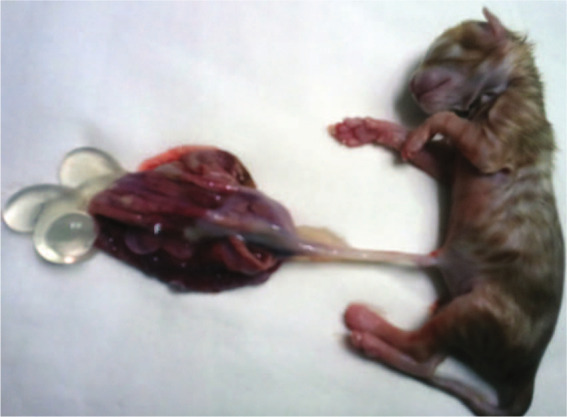
Norwegian Forest kitten with changed placenta
During the examination of segments of embryonic membranes and placentae we found vesicular chorion villi distension with oedema and focal mucous stromal degeneration. Total avascularity of villose and a different intensity growth of the trophoblast epithelium was noticed, accompanied by a considerable atypism of cells. There was also focally visible cytoplasmic vacuolation of the trophoblast cytoplasm (Figure 2). Based upon these histopathological findings, a diagnosis of a partial HM was made.
Figure 2.
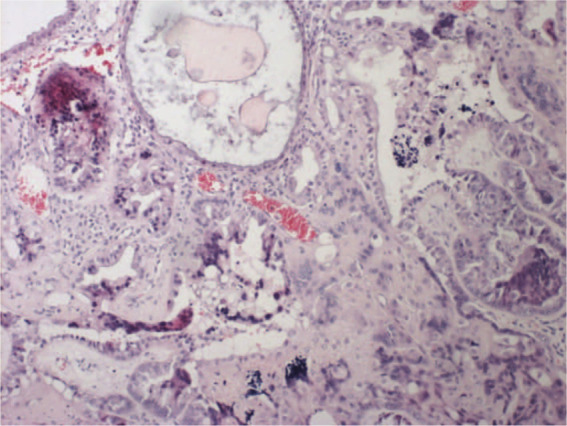
Partial hydatidiform mole — hypertrophy and atypia of trophoblast (haematoxylin and eosin, 10×)
The presence of two paternal alleles of FCA506, FCA532 and FCA178 microsatellites and one allele of maternal origin (FCA506, FCA532 and FCA178) was observed in all analysed tissues (kidney, liver, brain, heart and spleen) isolated from the stillborn kitten (Table 1). The results thus confirm that the haploid egg was fertilised by two sperm yielding a triploid karyotype. The molecular analysis determining the kitten’s sex yielded a positive result concerning the presence of the paternal Sry gene (613 base pair fragment), and maternal and paternal products of Amel, as well as one maternal and one paternal X chromosome FCA311 microsatellite allele (Table 1). This result provides evidence that one X chromosome was inherited from the mother and two chromosomes (X and Y) were of paternal origin.
In the placenta we identified two maternal and two paternal alleles of FCA506, FCA532 and FCA178 microsatellites originating from the autosomal chromosome, as well as both maternal and paternal products of the Amel gene and FCA311 microsatellite located on the X chromosome. Moreover, PCR amplification of the Sry gene originating from the Y chromosome was also positive. Such results indicate the participation of both fetal and maternal components in the development of the placenta.
Several studies have described complete or partial HM in humans. However, the biological factors that contribute to the development of partial or complete HM are not completely understood, mainly owing to the lack of good animal models for such studies. Potential risk factors may include defects in the egg, abnormalities within the uterus or nutritional deficiencies (low in protein, folic acid or carotene3,6,7).
In our research we identified a case of partial HM in a stillborn Norwegian Forest kitten. To our knowledge this is the first case of HM described in a cat. The presence of two microsatellite alleles of paternal origin and one allele of maternal origin in all analysed tissues, together with the presence of the paternal Sry gene, and maternal and paternal products of Amel, as well as one maternal and one paternal X chromosome FCA311 microsatellite allele confirm that the haploid egg was fertilised by two sperm yielding a triploid karyotype.
Most partial moles are triploid (three chromosome sets). The nucleus contains one maternal set of genes and two paternal sets obtained by reduplication of the paternal haploid set from a single sperm. However, our results indicate a consequence of dispermic fertilisation of the egg. The aetiology of this case of HM is not completely understood. Parents of the described kitten with HM were housed in normal conditions and fed with a balanced commercial diet. Therefore, nutritional deficiencies as a potential risk factor for HM can be omitted. Another risk factor, such as morphological abnormalities in the uterus, was also not observed. The parents were not rebred because of the possible risk of additional affected offspring.
We concluded that the partial HM in the stillborn Norwegian Forest kitten may have been caused by chromosomal abnormalities predisposing to HM.
Conclusions
This case report increases the veterinary knowledge of the condition of partial HM in cats. The successful application of genetic markers in postnatal diagnosis of this condition in the cat confirms considerable usefulness of these techniques, especially in cases where cytogenetic diagnosis is insufficient or impossible. We showed that the haploid egg was fertilised by two sperm, yielding a triploid karyotype. Further genetic tests leading to the analysis of gene expression and gene polymorphism may bring more substantial evidence of the involvement of the heritable predisposition in the phenomenon of partial HM.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this case report.
The authors do not have any potential conflicts of interest to declare.
Accepted: 9 June 2013
References
- 1. Benirschke K, Kaufmann P. Molar pregnancies. In: Kaufmann P. (ed). Pathology of the human placenta. New York: Springer Verlag, 1990, pp 782–815. [Google Scholar]
- 2. Meinecke B, Kuiper H, Drogemuller C, et al. Mola hydatidosa coexistent with a foetus in a bovine Freemartin pregnancy. Placenta 2002; 24: 107–112. [DOI] [PubMed] [Google Scholar]
- 3. Petignat P, Billieux MH, Blouin JL. Is genetic analysis useful in the routine management of hydatidiform mole? Hum Reprod 2003; 18: 243–249. [DOI] [PubMed] [Google Scholar]
- 4. Kumar T, Vinay P. Pathologic basis of disease. 8th ed. Philadelphia: Saunders Elsevier, 2010, pp 1057–1058. [Google Scholar]
- 5. Fukunaga M. Early partial hydatidiform mole: prevalence, histopathology, DNA ploidy, and persistence rate. Virchows Arch 2000; 437: 180–184. [DOI] [PubMed] [Google Scholar]
- 6. King V, Goodfellow PN, Wilkerson AJ, et al. Evolution of the male-determining gene SRY within the cat family Felidae. Genetics 2007: 175; 1855–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paoloni-Giacobino A. Epigenetics in reproductive medicine. Pediatr Res 2007; 61: 51–57. [DOI] [PubMed] [Google Scholar]


